Abstract
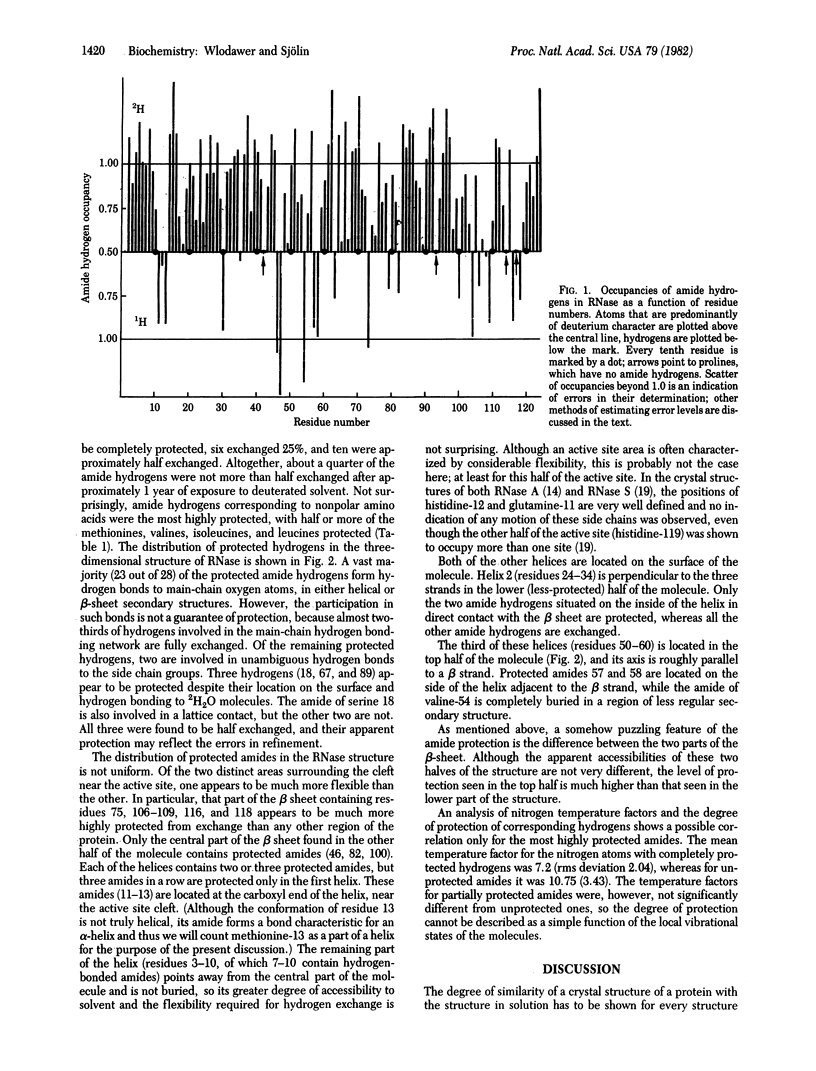
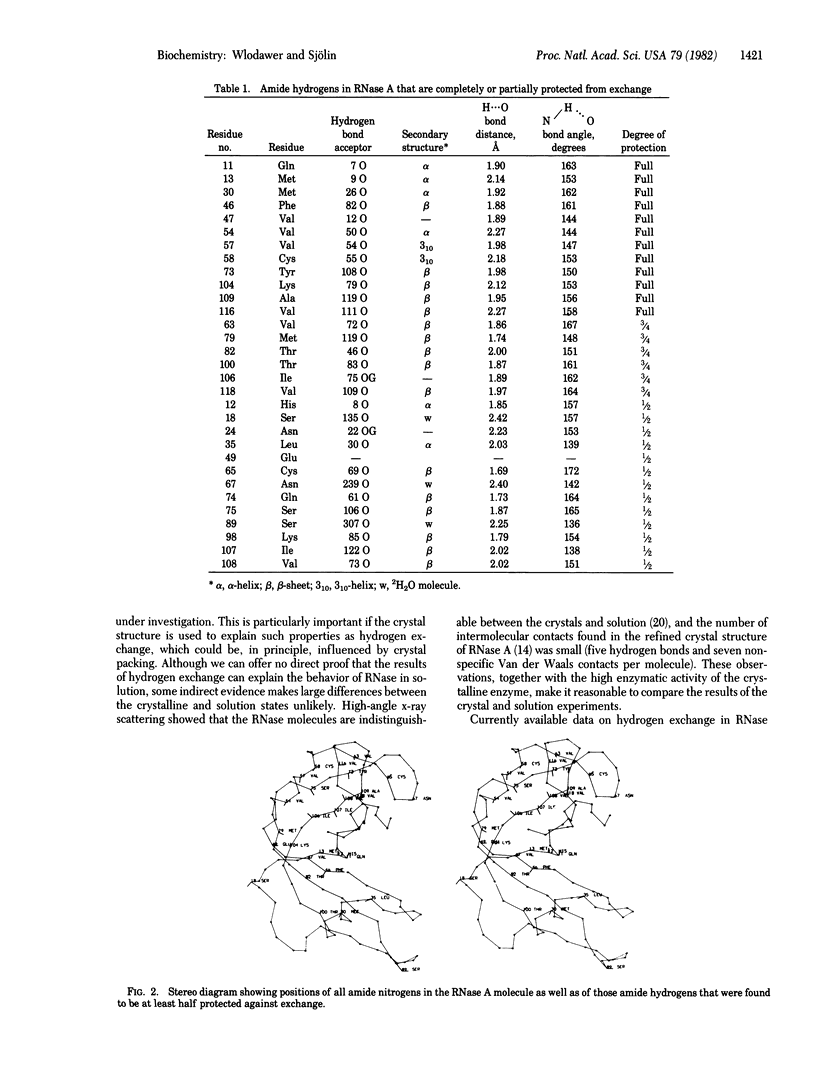
Hydrogen exchange has been studied in a single crystal of RNase A [ribonuclease (pancreatic), EC 3.1.27.5] in the course of a neutron structure investigation. Refinement of the occupancies of amide hydrogens provided information about the kind of isotope present in each site and also provided estimates of the errors associated with the measurement. Twenty-eight of the 120 peptide amide hydrogens were found to be at least partially protected from exchange during approximately 1 year required for crystal preparation and data collection. Most of the protected hydrogens were involved in hydrogen bonds with main-chain carbonyl groups. A contiguous region of the beta-sheet containing residues 75, 106--109, 116, and 118 had a large number of protected hydrogens, indicating its low flexibility and the lack of accessibility to solvent. Residues 11--13 from the alpha-helix near the amino terminus were protected, in good agreement with a model of cooperative unwinding of this helix, starting from the free (amino) end.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artymiuk P. J., Blake C. C., Grace D. E., Oatley S. J., Phillips D. C., Sternberg M. J. Crystallographic studies of the dynamic properties of lysozyme. Nature. 1979 Aug 16;280(5723):563–568. doi: 10.1038/280563a0. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Rolfe A. Hydrogen exchange studies of respiratory proteins. 3. Structural and free energy changes in hemoglobin by use of a difference method. J Biol Chem. 1973 Jul 10;248(13):4852–4861. [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- HVIDT A., LINDERSTRØM-LANG K. Exchange of hydrogen atoms in insulin with deuterium atoms in aqueous solutions. Biochim Biophys Acta. 1954 Aug;14(4):574–575. doi: 10.1016/0006-3002(54)90241-3. [DOI] [PubMed] [Google Scholar]
- Karplus S., Snyder G. H., Sykes B. D. A nuclear magnetic resonance study of bovine pancreatic trypsin inhibitor. Tyrosine titrations and backbone NH groups. Biochemistry. 1973 Mar 27;12(7):1323–1329. doi: 10.1021/bi00731a012. [DOI] [PubMed] [Google Scholar]
- Masson A., Wüthrich K. Proton magnetic resonance investigation of the conformational properties of the basic pancreatic trypsin inhibitor. FEBS Lett. 1973 Apr 1;31(1):114–118. doi: 10.1016/0014-5793(73)80086-9. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. M., VITHAYATHIL P. J. The preparation of subtilisn-modified ribonuclease and the separation of the peptide and protein components. J Biol Chem. 1959 Jun;234(6):1459–1465. [PubMed] [Google Scholar]
- Rosa J. H., Richards F. M. Hydrogen exchange from identified regions of the S-protein component of ribonuclease as a function of temperature, pH, and the binding of S-peptide. J Mol Biol. 1981 Feb 5;145(4):835–851. doi: 10.1016/0022-2836(81)90318-1. [DOI] [PubMed] [Google Scholar]
- Rosa J. J., Richards F. M. An experimental procedure for increasing the structural resolution of chemical hydrogen-exchange measurements on proteins: application to ribonuclease S peptide. J Mol Biol. 1979 Sep 25;133(3):399–416. doi: 10.1016/0022-2836(79)90400-5. [DOI] [PubMed] [Google Scholar]
- Schreier A. A., Baldwin R. L. Concentration-dependent hydrogen exchange kinetics of 3H-labeled S-peptide in ribonuclease S. J Mol Biol. 1976 Aug 15;105(3):409–426. doi: 10.1016/0022-2836(76)90101-7. [DOI] [PubMed] [Google Scholar]
- Timchenko A. A., Ptitsyn O. B., Dolgikh D. A., Fedorov B. A. The structure of ribonuclease in solution does not differ from its crystalline structure. FEBS Lett. 1978 Apr 1;88(1):105–108. doi: 10.1016/0014-5793(78)80618-8. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Sjölin L. Orientation of histidine residues in RNase A: neutron diffraction study. Proc Natl Acad Sci U S A. 1981 May;78(5):2853–2855. doi: 10.1073/pnas.78.5.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]



