The amino acid profile and the secretory responses of glutamate dehydrogenase (GDH)-deficient β-cells are characterized. This study shows that GDH is essential for both insulin release and net glutamate synthesis evoked by glucose. Adding cellular glutamate restored the full development of glucose-stimulated insulin secretion, showing the requirement for permissive glutamate levels.
Abstract
In pancreatic β-cells, glutamate dehydrogenase (GDH) modulates insulin secretion, although its function regarding specific secretagogues is unclear. This study investigated the role of GDH using a β-cell–specific GDH knockout mouse model, called βGlud1−/−. The absence of GDH in islets isolated from βGlud1–/– mice resulted in abrogation of insulin release evoked by glutamine combined with 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid or l-leucine. Reintroduction of GDH in βGlud1–/– islets fully restored the secretory response. Regarding glucose stimulation, insulin secretion in islets isolated from βGlud1–/– mice exhibited half of the response measured in control islets. The amplifying pathway, tested at stimulatory glucose concentrations in the presence of KCl and diazoxide, was markedly inhibited in βGlud1–/– islets. On glucose stimulation, net synthesis of glutamate from α-ketoglutarate was impaired in GDH-deficient islets. Accordingly, glucose-induced elevation of glutamate levels observed in control islets was absent in βGlud1–/– islets. Parallel biochemical pathways, namely alanine and aspartate aminotransferases, could not compensate for the lack of GDH. However, the secretory response to glucose was fully restored by the provision of cellular glutamate when βGlud1–/– islets were exposed to dimethyl glutamate. This shows that permissive levels of glutamate are required for the full development of glucose-stimulated insulin secretion and that GDH plays an indispensable role in this process.
INTRODUCTION
Glutamate dehydrogenase (GDH) is a highly conserved mitochondrial enzyme encoded by GLUD1 (Michaelidis et al., 1993) that catalyzes the reversible reaction α-ketoglutarate + NH3 + NADH ↔ glutamate + NAD+ (Hudson and Daniel, 1993). In eukaryotes, GDH exhibits complex allosteric regulation by leucine, pyridine, adenine, and guanine nucleotides (Fisher, 1985; Smith et al., 2001). GDH is mainly expressed in brain, kidney, liver, pancreas, and lymph nodes. While it catalyzes the same reaction in every tissue, its function regarding metabolic homeostasis and its preferred directional flux varies according to specific organs, nutrient state, allosteric regulation, and redox and energy states of mitochondria (Frigerio et al., 2008). In pancreatic β-cells, the importance of GDH in insulin secretion was recognized long ago (Sener and Malaisse, 1980). Activating mutations of GDH have been associated with hypoglycemia in infants and young children (Stanley et al., 1998). GDH can play an anaplerotic role, generating α-ketoglutarate (α-KG) to feed the tricarboxylic acid (TCA) cycle, or a cataplerotic role, generating glutamate at the expense of α-ketoglutarate (Owen et al., 2002). Therefore GDH might play a role in a glucose-induced amplifying pathway through generation of glutamate (Maechler and Wollheim, 1999; Hoy et al., 2002) and/or as an amino acid sensor triggering insulin release upon glutamine stimulation in conditions of GDH allosteric activation (Sener et al., 1981b; Fahien et al., 1988; Li et al., 2006).
The preferential flux direction of GDH within the β-cell is still debated. Most studies have investigated GDH function through increasing activity using the allosteric activator leucine or its nonmetabolized analogue 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (BCH; Sener et al., 1982; Panten et al., 1984; Fahien et al., 1988). Alternatively, GDH activity can be increased by means of overexpression of the enzyme, an approach that has been combined with allosteric activation of the enzyme (Carobbio et al., 2004). In other studies, an activating mutation of GDH associated with a hyperinsulinism syndrome (Stanley et al., 1998) has been expressed in β-cells of transgenic mice, rendering their islets glutamine-responsive in terms of insulin secretion (Li et al., 2006). Taken together, these gain-of-function approaches demonstrated that glutamine can be turned into a secretagogue upon enhanced GDH activity, although glucose-stimulated insulin secretion is not significantly modified (Sener et al., 1982; Carobbio et al., 2004; Li et al., 2006).
In a limited number of studies, GDH activity was reduced in insulin-secreting cells using inhibitors (Bryla et al., 1994; Yang et al., 2003) or an antisense approach (Maechler et al., 2006). Green tea polyphenols were also shown to inhibit GDH and to reduce insulin release when islets were stimulated with glutamine plus BCH, although not upon glucose stimulation (Li et al., 2006). Moreover, GDH activity can be reduced when the enzyme is ADP-ribosylated by the mammalian Sir2 homologue SIRT4, thereby inhibiting insulin secretion (Haigis et al., 2006; Ahuja et al., 2007).
In this study, we investigated the role of GDH in pancreatic islets isolated from β-cell–specific GDH knockout mice, called βGlud1−/− (Carobbio et al., 2009). These mice develop normally, and they exhibit normoglycemia, whereas their insulin levels are reduced (Carobbio et al., 2009). The aim of the present study was to characterize the insulin secretory pattern of βGlud1−/− islets, thereby determining the putative role of GDH in the amplifying pathway and its requirement in the responses to glucose versus glutamine stimulations. Moreover, we investigated the complex equilibrium between closely associated amino acids and contribution of related biochemical routes.
RESULTS
Kinetics of glucose-stimulated insulin secretion in βGlud1–/– islets
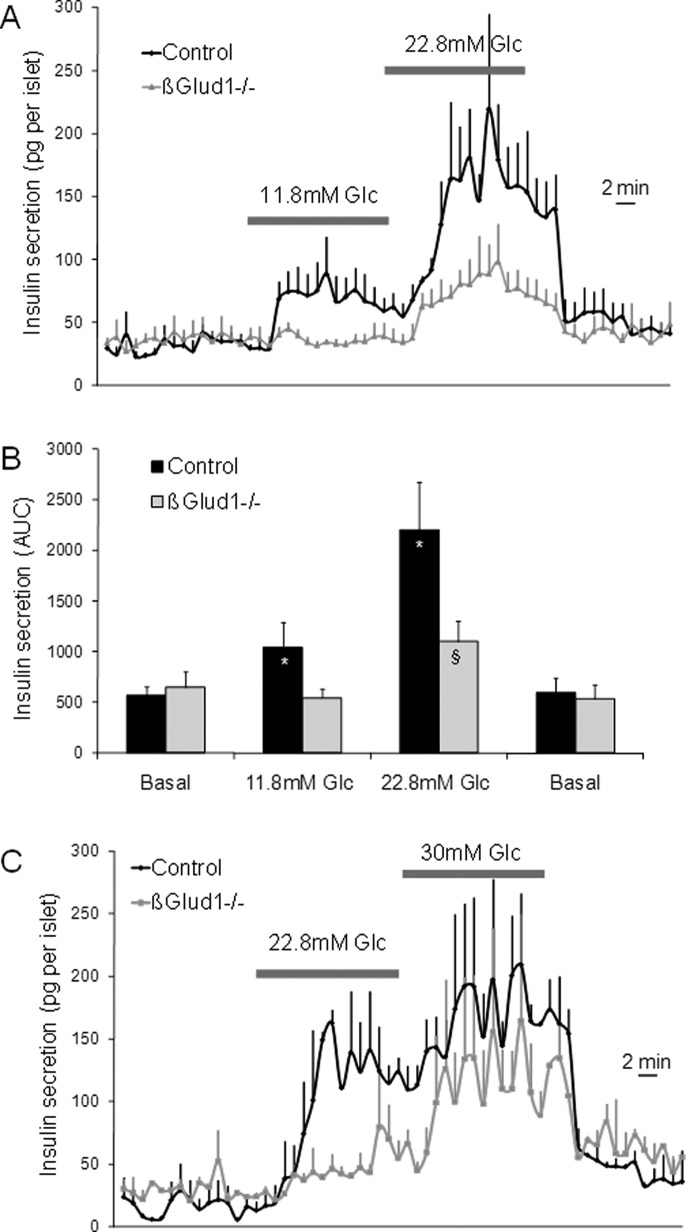
In control islets, 11.8 and 22.8 mM glucose stimulated insulin secretion 1.8-fold and 3.9-fold over basal release, respectively (Figure 1A). In GDH knockout islets isolated from βGlud1−/− mice, the secretory responses were reduced by 48% at 11.8 mM glucose and by 50% at 22.8 mM glucose compared with control islets (Figure 1, A and B). Basal insulin release at 2.8 mM glucose was not affected by the lack of GDH, and total islet insulin contents were similar between the two groups (Supplemental Figure S1). These data demonstrate that GDH is required for the full development of glucose-stimulated insulin secretion.
FIGURE 1:
Kinetics of glucose-stimulated insulin secretion in βGlud1−/− islets. After an overnight culture in RPMI-1640 medium, islets isolated from control and βGlud1−/− knockout mice were handpicked and perifused with KRBH at 2.8 mM glucose (Basal) before 15-min stimulations with the indicated glucose (Glc) concentrations. (A) Islets were stimulated with 11.8 and 22.8 mM glucose. (B) Quantification of insulin secretion shown in (A) expressed as AUC. (C) Islets were stimulated with 22.8 and 30 mM glucose. Values are means ± SE of four independent experiments. *, p < 0.05 vs. basal of corresponding genotype; §, p < 0.05 vs. control under corresponding stimulation condition.
We then tested whether βGlud1−/− islets were intrinsically unable to develop a complete secretory response. To this end, glucose was first directly switched from basal 2.8 mM to stimulatory 22.8 mM, showing again a secretory response reduced by half in βGlud1−/− islets compared with controls (Figure 1C; area under the curve [AUC]: 832 ± 37 vs. 1171 ± 311 ng/15 min, respectively; −53%; p < 0.02). Then glucose was raised further to supraphysiological concentrations (30 mM). Under such extreme conditions, βGlud1−/− islets could secrete insulin to levels approaching those of control islets (AUC: 2557 ± 346 vs. 1761 ± 348 ng/15 min, respectively; NS). This suggests that GDH-independent, glucose-derived additive factors could partially compensate for the absence of this enzyme at very high glucose concentrations.
Glutamine-induced insulin secretion in βGlud1−/− islets rescued by GDH ectopic expression
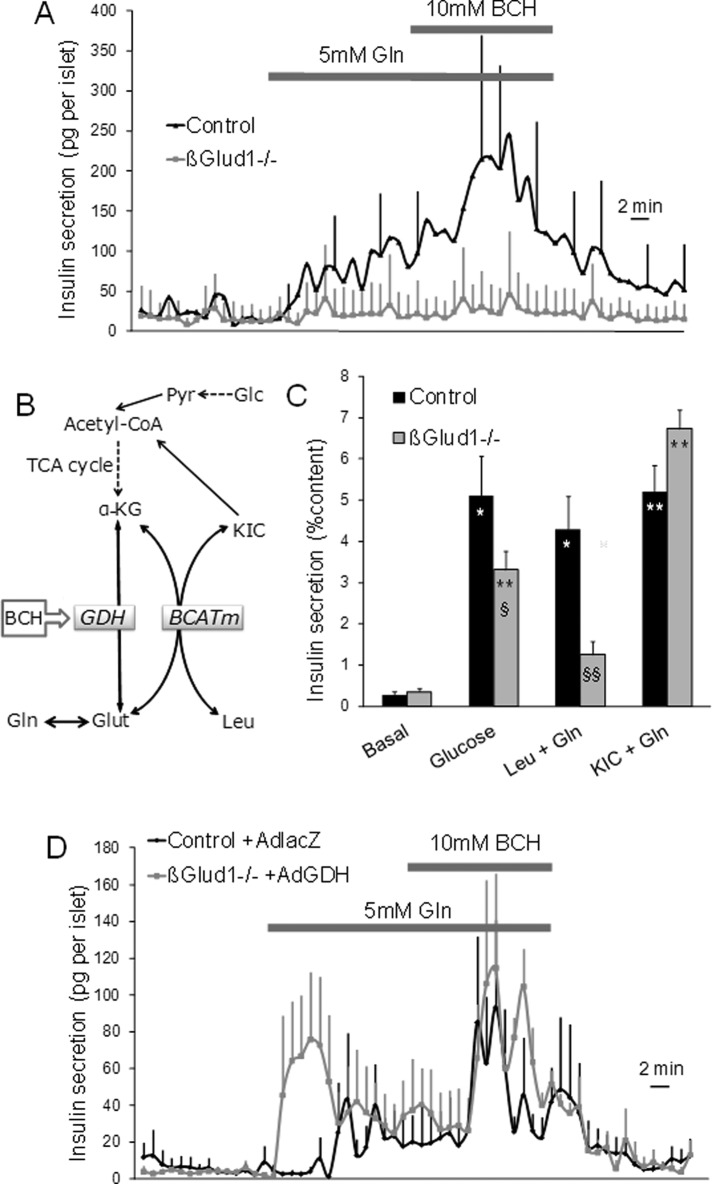
On its own, glutamine is not an efficient secretagogue, unless it is combined with an allosteric activator of GDH, such as BCH. Such an experimental maneuver allows complete glutamine oxidation with NH4+ release (Sener et al., 1981b) and elevation of the necessary Ca2+ signal (Gao et al., 1999). As shown in Figure 2A, insulin secretion was poorly increased in control islets in the presence of 5 mM glutamine, whereas further addition of 10 mM BCH resulted in a robust sevenfold secretory response versus basal release (AUC: 2321 ± 905 vs. 330 ± 72 ng/15 min, respectively; p < 0.01; Figure S2). In βGlud1−/− islets, the absence of GDH resulted in complete abrogation of glutamine responses, both in the absence and in the presence of BCH (Figure 2A). The glutamine response was also tested in a static insulin secretion assay in the presence of leucine. Leucine exhibits dual effects on mitochondrial activation, since it is a natural GDH allosteric activator and is additionally metabolized, feeding the TCA cycle with its own carbons (Figure 2B). These properties of leucine can be tested separately by using either its nonmetabolized analogue BCH for GDH activation (see above) or its deamination product α-ketoisocaproate (KIC) for direct metabolic contribution. It is noteworthy that KIC tested alone fails to stimulate insulin release (Figure S3), unless it is combined with another metabolite, for instance, glutamine (Sener et al., 1981a). In control islets, 10 mM leucine plus 2 mM glutamine induced a 15.3-fold response, whereas a poor 3.6-fold increase in insulin release was observed in βGlud1−/− islets, corresponding to 71% inhibition (p < 0.01; see Figure 2C). The response to 10 mM KIC plus 2 mM glutamine was 18.5-fold in control islets and 19.2-fold in βGlud1−/− islets. These data show that the KIC response was fully preserved in βGlud1−/− islets, incidentally demonstrating that, beside GDH abrogation, the TCA cycle machinery was functional.
FIGURE 2:
Requirement of GDH for secretory responses of glutamine combined with l-leucine and derivatives in control and βGlud1−/− islets. After an overnight culture in RPMI-1640 medium, islets isolated from control and βGlud1−/− mice were handpicked and perifused with KRBH at 2.8 mM glucose (Basal). (A) Islets were sequentially stimulated for 15 min with 5 mM of glutamine (Gln) and Gln plus 10 mM BCH. (B) l-Leucine (Leu) is deaminated to KIC by BCATm, transferring the amino group to α-KG and thereby producing glutamate (Glut). Glutamate can also be produced by deamidation of Gln. KIC can generate acetyl-CoA, the latter being also produced by pyruvate (Pyr), a cytosolic product of glucose (Glc) catabolism. GDH can be allosterically activated by l-leucine or its nonmetabolized analogue BCH. (C) Secretory responses of βGlud1−/− islets to l-leucine and KIC. Insulin secretion was tested in islets over a 1-h incubation period at basal and 22.8 mM glucose, and at basal plus 2 mM glutamine with either 10 mM l-leucine or KIC. (D) Islets isolated from control and βGlud1−/− mice were transduced with Ad-lacZ and Ad-GDH adenoviruses, respectively. After overnight culture, islets were perifused with KRBH at 2.8 mM glucose (Basal) and then stimulated for 15 min with 5 mM Gln and 15 min with Gln plus 10 mM BCH. (B and C) Values are means ± SE of three independent experiments for each group. *, p < 0.05, **, p < 0.01 vs. basal of corresponding genotype; §, p < 0.05, §§, p < 0.01 control under corresponding stimulation condition; ##, p < 0.01 vs. 22.8 mM glucose of corresponding genotype.
Transduction of βGlud1−/− islets with an adenovirus encoding for GDH (Ad-GDH) fully restored the secretory response to glutamine combined with BCH (Figure 2D). Of note, βGlud1−/− islets transduced with Ad-GDH became responsive to glutamine alone, an effect attributed to the resulting overexpression of GDH, as reported previously (Carobbio et al., 2004). These data show that the glutamine response is completely dependent on GDH, without compensatory pathways.
We also measured insulin secretion in the in situ pancreatic perfusion preparation (Figure S4). This model confirmed abrogation of the secretory response evoked by the combination of 5 mM glutamine plus 10 mM BCH in βGlud1−/− mice versus controls (−64%; p = 0.03).
The amplifying pathway tested in βGlud1–/– islets
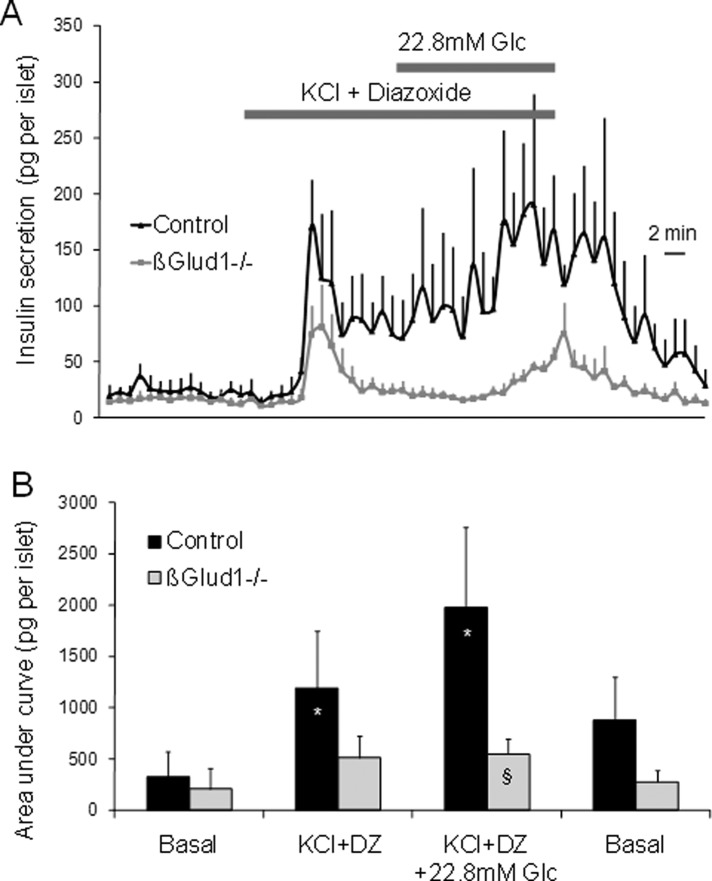
The classical experimental procedure to reveal a glucose-evoked amplifying pathway requires clamping of [Ca2+]i at permissive levels; this is achieved by depolarization of β-cells with KCl in the presence of diazoxide, which holds KATP channels open (Gembal et al., 1992). At basal glucose, Ca2+-induced insulin release stimulated by KCl was observed both in control and in βGlud1−/− islets, while the amplitude was slightly reduced in knockout islets (p = 0.05; Figure 3, A and B). In control islets, further addition of 22.8 mM glucose induced amplification of the Ca2+ signal with strong and sustained secretory response. The amplifying pathway was not induced in βGlud1−/− islets, which secreted much less insulin during the stimulation period versus controls (−72%; p = 0.01; Figure 3, A and B). This shows that GDH is required for the development of the amplifying pathway.
FIGURE 3:
The amplifying pathway of the secretory response tested in the absence of GDH in βGlud1−/− islets. (A) After an overnight culture in RPMI-1640 medium, islets isolated from control and βGlud1−/− mice were handpicked and perifused with KRBH at 2.8 mM glucose (Basal). Then islets were stimulated for 15 min with 30 mM KCl plus 250 μM diazoxide (DZ) before elevation of glucose (Glc) concentration to 22.8 mM for another 15-min period. (B) Quantification of insulin secretion shown in (A) expressed as AUC. Values are means ± SE of three independent experiments. *, p< 0.05 vs. basal of corresponding genotype; §, p < 0.05 vs. control under corresponding stimulation condition.
Ca2+ levels and glutamate sensitivity of βGlud1–/– islets
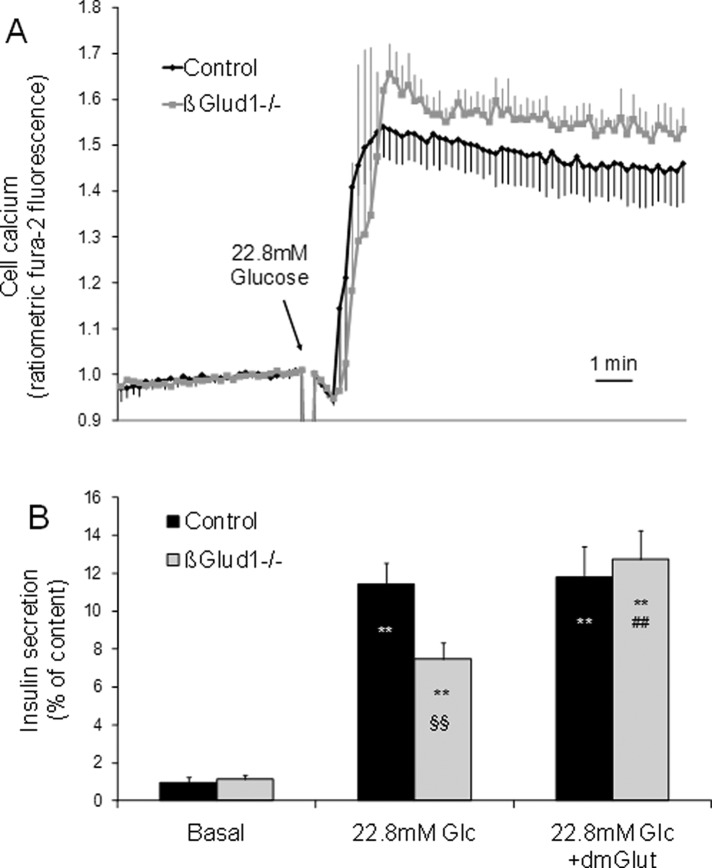
Elevation of cytosolic Ca2+ is required for insulin exocytosis, although it is not sufficient for the full development of the glucose response. To determine whether the reduction in glucose-stimulated insulin secretion observed in βGlud1−/− islets was associated with modifications of the [Ca2+]i response, we measured this parameter using fura-2 fluorescence. As shown in Figure 4A, control and βGlud1−/− islets responded similarly to 22.8 mM glucose stimulation, both in terms of kinetic and amplitude of the responses.
FIGURE 4:
Cellular calcium changes and glutamate sensitivity of βGlud1−/− islets. Islets were isolated from control and βGlud1−/− mice and kept in culture before experiments. (A) Cellular calcium levels were monitored in islets loaded with fura-2 and placed in KRBH with 2.8 mM glucose before stimulation with 22.8 mM glucose. (B) Insulin secretion was tested in islets over a 1-h incubation period at 2.8 mM (Basal) and 22.8 mM (Glc) glucose supplemented with 5 mM dimethyl glutamate (dmGlut) where indicated. Values are means ± SE of six independent mice for each group. **, p < 0.01 vs. basal of corresponding genotype; §§, p < 0.01 vs. control under corresponding stimulation condition; ##, p < 0.01 vs. 22.8 mM Glc of corresponding genotype.
On glucose stimulation, both ATP and Ca2+ increases are preserved in βGlud1−/− islets (see Carobbio et al. [2009] and Figure 4A, respectively), indicating that the triggering pathway does not rely on GDH activity. Conversely, the amplifying pathway is deficient in GDH knockout β-cells (Figure 3A). We then tested whether a lack of glutamate, secondary to GDH deletion, could explain the reduced secretory response in βGlud1−/− islets. Islets were stimulated with 22.8 mM glucose in the absence or the presence of dimethyl glutamate, a cell membrane–permeable glutamate precursor (Figure 5A). In control islets, insulin secretion was stimulated 12-fold by 22.8 mM glucose, while the response was 55% lower in βGlud1−/− islets (p < 0.01; Figure 4B). Addition of dimethyl glutamate fully restored the secretory response of βGlud1−/− islets, whereas it did not exhibit additive effects in controls.
FIGURE 5:
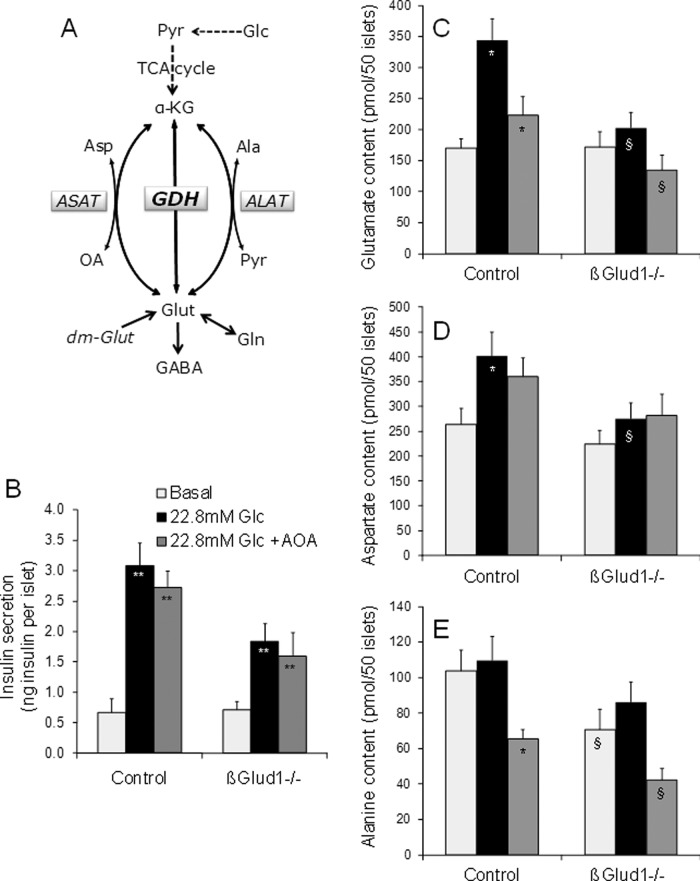
Respective roles of GDH and aminotransferases in glucose-stimulated insulin secretion. (A) GDH and aminotransferases (ASAT and ALAT) connect glutamate (Glut) and the TCA cycle intermediate α-KG. ASAT uses either oxaloacetate (OA) or aspartate (Asp) as a cosubstrate and ALAT uses either pyruvate (Pyr) or alanine (Ala), along with Glut or α-KG, respectively. Glutamate can also arise from glutamine (Gln) deamidation. (B–E) After an overnight culture in RPMI-1640 medium, islets isolated from control and βGlud1−/− mice were handpicked and preincubated for 1 h in glucose- and glutamine-free RPMI-1640 medium. Then islets were incubated for 1 h at 2.8 mM (Basal) and 22.8 mM (Glc) glucose in the absence or presence of 2 mM AOA. At the end of the assay period, supernatants were collected to measure insulin secretion (B) and islets were collected in 5% (wt/vol) 5-sulfosalicylic acid before determination of amino acid concentrations by HPLC (C–E). Values are means ± SE of six independent mice for each group. *, p < 0.05, **, p < 0.01 vs. basal of corresponding genotype; §, p < 0.05, §§, p < 0.01 vs. control under corresponding stimulation condition; #, p < 0.01 vs. 22.8 mM Glc of corresponding genotype.
Respective roles of GDH and aminotransferases in glucose-stimulated insulin secretion
Because impaired glucose-stimulated insulin secretion in βGlud1−/− islets could be rescued by provision of cellular glutamate, we measured concentrations of glutamate and related metabolites (Figure 5A) in response to glucose. In control islets, four amino acids were increased by 22.8 mM glucose stimulation: glutamate (twofold) and its amidation product glutamine (3.7-fold), aspartate (1.5-fold), and taurine (1.8-fold); see Table 1. In βGlud1−/− islets, glucose stimulation failed to increase these amino acids, resulting in lower islet contents of glutamate (−41%) and aspartate (−32%) versus controls. Glutamate decarboxylation catalyzed by glutamate decarboxylase (GAD) forms γ-aminobutyric acid (GABA). Interestingly, the GABA/glutamate ratio was about twofold higher in glucose-stimulated βGlud1−/− islets versus controls, in both the absence and presence of glutamine (Table 1). This suggests that, in the absence of GDH, there was increased production of GABA via GAD using glutamate as a substrate.
TABLE 1:
Amino acid levels in islets from control and βGlud1–/– mice stimulated with glucose and glutamine.
| Amino acid | Group | Basal 2.8 mM glucose | Glucose 22.8 mM | Glc 22.8 mM + AOA | Basal + 1 mM glutamine | Glc 22.8 mM + glutamine |
|---|---|---|---|---|---|---|
| Alanine (Ala) | Control | 103.8 ± 11.5 | 109.6 ± 13.5 | 65.3 ± 5.3** | 203.1 ± 45.4** | 147.5 ± 20.8 |
| βGlud1−/− | 70.6 ± 11.5* | 86.2 ± 11.2 | 42.3 ± 6.3* | 92.4 ± 14.7* | 137.6 ± 28.1 | |
| Arginine (Arg) | Control | 125.3 ± 31.0 | 86.8 ± 22.4 | 30.4 ± 5.5 | 222 ± 59.5 | 189.9 ± 60.6 |
| βGlud1−/− | 82.8 ± 20.6 | 78.3 ± 19.1 | 30.3 ± 3.9 | 148.6 ± 21.5 | 151.2 ± 20.0 | |
| Aspartate (Asp) | Control | 263.7 ± 32.8 | 402.0 ± 47.2** | 359.6 ± 38.0 | 776.2 ± 211.9** | 453.6 ± 146.7 |
| βGlud1−/− | 224.6 ± 27.1 | 275.5 ± 31.5* | 282.1 ± 42.2 | 407.0 ± 128.6* | 428.6 ± 80.2 | |
| γ-aminobutyric acid (GABA) | Control | 78.9 ± 10.9 | 101.3 ± 18.0 | 87.7 ± 12.7 | 210.9 ± 73.1** | 62.2 ± 11.7*** |
| βGlud1−/− | 105.2 ± 21.6 | 108 ± 16.6 | 67.4 ± 15.8 | 169.6 ± 33.7 | 146.9 ± 29.0* | |
| Glutamate (Glu) | Control | 170.3 ± 15.1 | 343.8 ± 34.7** | 223.2 ± 30.6** | 1848.0 ± 215.2** | 1592.9 ± 167.6** |
| βGlud1−/− | 172.2 ± 24.6 | 202.9 ± 24.6* | 134.4 ± 24.4* | 1389.3 ± 232.3** | 1881.2 ± 256.5** | |
| Glutamine (Gln) | Control | 41.3 ± 16.2 | 151.2 ± 63.1** | 35.4 ± 2.7 | 22246 ± 1730** | 24192 ± 2689** |
| βGlud1−/− | 29.3 ± 9.7 | 98.0 ± 36.2 | 15.1 ± 3.0* | 17221 ± 2888** | 20798 ± 1910** | |
| Glycine (Gly) | Control | 264.6 ± 76.5 | 356.9 ± 99.9 | 96.2 ± 9.7 | 942.0 ± 382.4** | 594 ± 106.3 |
| βGlud1−/− | 221.9 ± 59.5 | 271.4 ± 68.1 | 89.3 ± 23.3 | 456.3 ± 114.1 | 334.7 ± 63.6 | |
| Histidine (His) | Control | 24.8 ± 2.3 | 31.9 ± 7.5 | 18.9 ± 3.5 | 74.5 ± 43.7** | 19 ± 5.5 |
| βGlud1−/− | 24.4 ± 5.3 | 22.2 ± 4.2 | 20.1 ± 12.8 | 25.3 ± 1.3* | 44.5 ± 12.3 | |
| Serine (Ser) | Control | 217.8 ± 25.6 | 254.4 ± 54.9 | 129.5 ± 20.5 | 446.0 ± 120.5** | 334.7 ± 26.8 |
| βGlud1−/− | 196.2 ± 35.1 | 200.2 ± 32.5 | 146.3 ± 67.7 | 252.6 ± 29.5 | 371.2 ± 103.6 | |
| Taurine (Tau) | Control | 314.1 ± 37.5 | 571.4 ± 66.4** | 468.1 ± 24.8** | 626.1 ± 184 | 450.2 ± 138.3 |
| βGlud1−/− | 318.6 ± 74.0 | 398.7 ± 61.0 | 333.7 ± 53.4 | 406.3 ± 94.3 | 433.8 ± 56.1 | |
| Tyrosine (Tyr) | Control | 34.6 ± 3.3 | 86.8 ± 22.4 | 20.9 ± 4.4 | 32.1 ± 10 | 23.3 ± 4.0 |
| βGlud1−/− | 29.6 ± 5.6 | 78.3 ± 19.1 | 20.3 ± 6.2 | 16.9 ± 2.6 | 15.4 ± 3.5 |
After an overnight culture, isolated islets were preincubated for 1 h in glucose- and glutamine-free RPMI-1640 medium. Then islets were incubated for 1 h at 2.8 mM (basal) and 22.8 mM glucose with or without 2 mM AOA or 1 mM glutamine. Values (in picomoles per 50 islets) are means ± SE of six independent mice. *, p < 0.05 vs. control under corresponding stimulation condition; **, p < 0.05 vs. basal of corresponding phenotype; ***, p < 0.05 vs. basal plus glutamine of corresponding phenotype.
Glutamate can be formed from the TCA cycle intermediate α-ketoglutarate through GDH. Alternatively, glutamate can arise from glutamine deamidation or from transamination of α-ketoglutarate, with alanine and aspartate as amino group donors, generating pyruvate and oxaloacetate, respectively (Figure 5A). To test the putative contribution of the respective alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT) to cellular glutamate levels, we inhibited these enzymes with aminooxyacetate (AOA). As shown in Figure 5B, stimulation with 22.8 mM glucose increased insulin secretion 4.6-fold in control islets, while the response was 44% lower in βGlud1−/−. Glutamate concentrations were increased twofold in control islets stimulated with 22.8 mM glucose (Figure 5C). In βGlud1−/− islets, glutamate levels at 22.8 mM glucose were 41% lower compared with controls, a magnitude similar to the reduced insulin release measured in the same islets (Figure 5B). The presence of 2 mM AOA did not modify the secretory response to glucose stimulation in either control or βGlud1−/− islets. AOA partially reduced the glucose-induced elevation of islet glutamate contents, indicating some contribution of aminotransferases in this process (Figure 5C). Of note, glutamate synthesis from α-ketoglutarate by GDH requires ammonia as a cosubstrate, and ammonia was not added in the stimulation buffer. However, the increment in cellular glutamate measured in control islets upon glucose stimulation would require only hundreds of picomoles of ammonia, probably contributed by the cellular milieu.
Regarding aspartate levels, there was a 1.5-fold increase upon glucose stimulation in control islets (Figure 5D). GDH knockout impaired glucose-induced increases in aspartate levels as observed in βGlud1−/− islets compared with controls (−32%). Measurements of alanine revealed that glucose stimulation did not modify concentrations of this amino acid in control islets, whereas addition of AOA decreased islet alanine contents by 40% (Figure 5E). In βGlud1−/− islets kept at nonstimulatory 2.8 mM glucose, alanine was 32% lower compared with control islets, possibly indicating the use of this amino acid for maintenance of the basal glutamate pool in GDH-null islets through a transamination reaction.
Contribution of glucose-derived carbons for de novo glutamate and aspartate synthesis
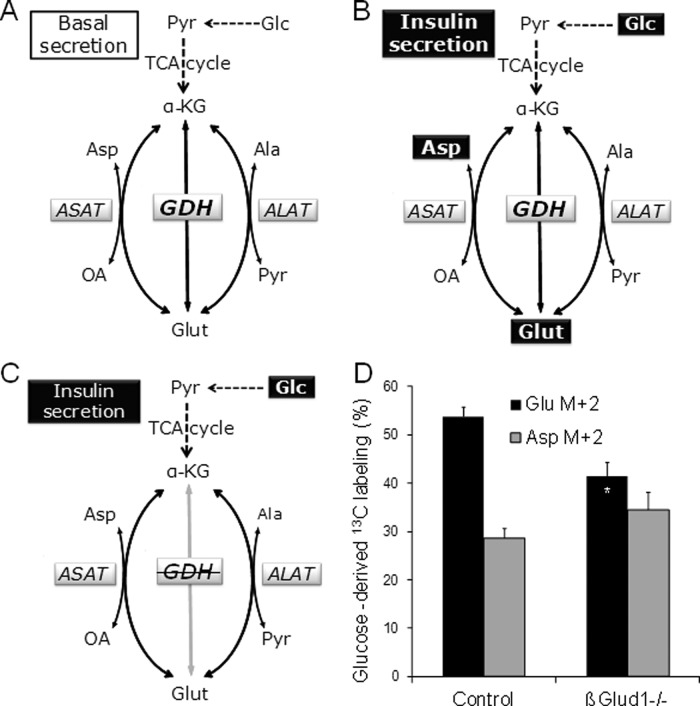
Overall, measurements of islet amino acid contents (Table 1) pointed to GDH as an important player contributing to glutamate and aspartate levels upon glucose stimulation (Figure 6, A–C).To further investigate the contribution of GDH to the active synthetic pathway of glutamate and aspartate evoked by glucose, we incubated isolated islets in medium containing 22.8 mM [U-13C]-glucose. [U-13C]glucose is metabolized to [1,2-13C]acetyl-coenzyme A (CoA) which may condense with oxaloacetate. After successive steps in the TCA cycle [4,5-13C]α-ketoglutarate is formed, which may undergo reductive amination by GDH to generate [4,5-13C]glutamate (metabolite with two labeled carbons, M+2). Alternatively, [4,5-13C]α-ketoglutarate can be further metabolized in the TCA cycle to oxaloacetate, which may be transaminated to double-labeled (M+2) aspartate. We observed that the net synthesis of glutamate from α-ketoglutarate was reduced by 23% in βGlud1−/− islets, while aspartate labeling was similar to controls (Figure 6D).
FIGURE 6:
Contribution of glucose and GDH for glutamate and aspartate synthesis. (A–C) A model for the role of GDH in glutamate-related pathways. GDH and aminotransferases (ASAT and ALAT) are three enzymes connecting glutamate (Glut) to the TCA cycle intermediate α-KG. Depending on its flux direction, ASAT uses either oxaloacetate (OA) or aspartate (Asp) as a cosubstrate and ALAT uses either pyruvate (Pyr) or alanine (Ala), along with Glut or α-KG, respectively. (A) Basal insulin release is observed at 2.8 mM glucose (Glc), accompanied by basal levels of glutamate, aspartate, and alanine. (B) A level of 22.8 mM glucose (Glc) stimulates insulin secretion and results in increased levels of both glutamate and aspartate. (C) In the absence of GDH (GDH), glucose stimulation fails to increase glutamate and aspartate, and the secretory response is blunted. (D) Isolated islets were incubated in medium containing 22.8 mM [U-13C]glucose for 1 h. Next islets were extracted, lyophilized, reconstituted, and derivatized before GC-MS analysis of isotopic enrichment in glutamate and aspartate. The quantification of double labeling (M+2) was calculated as percent of the pool of unlabeled metabolite. Values are means ± SD, n = 6. *, p < 0.05 vs. Glu M+2 of corresponding genotype.
Effects of glutamine on glutamate levels and insulin secretion in βGlud1−/− islets
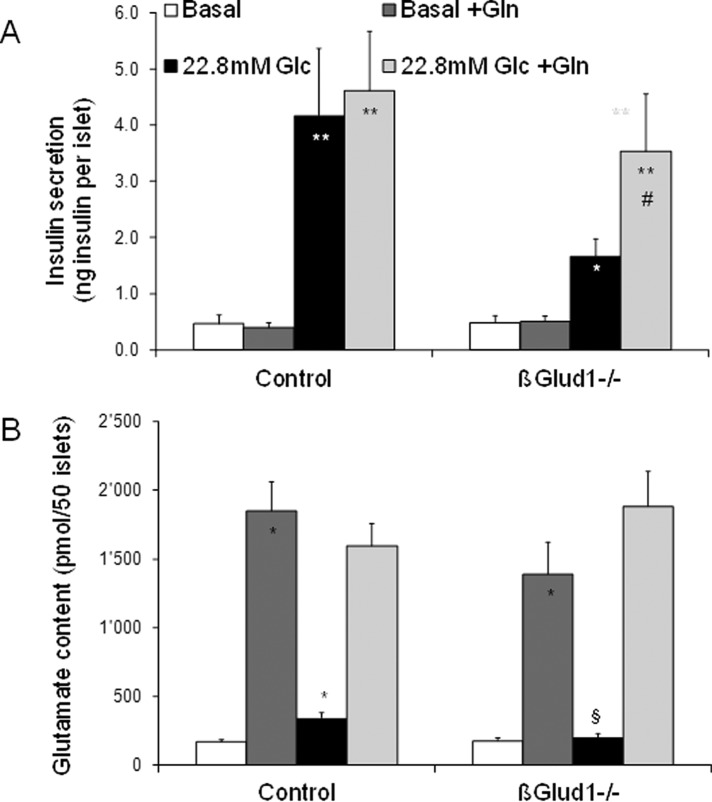
Glutamine can enter into β-cells, in which it is efficiently converted to glutamate via glutaminase. However, further catabolism requires allosteric activation of GDH in order to feed the TCA cycle. Consequently, glutamine-induced elevation of cellular glutamate is not sufficient to promote insulin secretion (Bertrand et al., 2002; Fernandez-Pascual et al., 2004). This is shown here by the addition of 1 mM glutamine at basal glucose, which did not stimulate insulin release (Figure 7A), unlike the slight response evoked by 5 mM glutamine tested in islet perifusion system (Figure 2A). Glucose stimulation of βGlud1−/− islets resulted in a blunted secretory response and an absence of a glutamate increase (Figure 7, A and B). Exposure of βGlud1−/− islets to glutamine markedly increased glutamate concentrations and restored glucose-stimulated insulin secretion to levels similar to those of control islets. In these conditions, glucose contributed to the rise in Ca2+ (Figure 4A), while glutamine provided elevated glutamate (Figure 7B). Therefore both glutamine and dimethyl glutamate (Figure 4B) can serve as a glutamate precursor to restore glutamate pools and insulin secretion in glucose-stimulated GDH-deficient islets.
FIGURE 7:
Effects of glutamine on glutamate levels and insulin secretion in βGlud1−/− islets. After an overnight culture in RPMI-1640 medium, islets isolated from control and βGlud1−/− mice were handpicked and preincubated for 1 h in glucose- and glutamine-free RPMI-1640 medium. Then, islets were incubated for 1 h at 2.8 mM (Basal) and 22.8 mM (Glc) glucose in the absence or presence of 1 mM glutamine (Gln). At the end of the assay period, supernatants were collected to measure insulin secretion (A), and islets were collected in 5% (wt/vol) 5-sulfosalicylic acid before determination of amino acid concentrations by HPLC (B). Values are means ± SE of six independent mice for each group. *, p < 0.05, **, p < 0.01 vs. basal of corresponding genotype; §, p < 0.05, §§, p < 0.01 vs. control under corresponding stimulation condition; #, p < 0.05 vs. 22.8 mM glucose of corresponding genotype.
In control islets exposed to glutamine at basal glucose, aspartate and alanine levels were increased 2.9-fold and twofold, respectively (Table 1). In βGlud1−/− islets, the same glutamine and glucose conditions failed to significantly increase aspartate and alanine levels, indicating that GDH is required for such pathways.
DISCUSSION
The present study characterized the amino acid profile and the secretory responses of GDH-deficient β-cells in islets isolated from βGlud1−/− mice. This offered the opportunity of investigating the role of GDH in the secretory response of different secretagogues and its putative requirement for the development of the amplifying pathway. First, we found evidence that secretion induced by a rise in intracellular Ca2+ evoked by KCl was not significantly modified in βGlud1−/− islets compared with controls, although we observed a trend toward lower insulin release. Second, glucose-stimulated insulin secretion was reduced by half in GDH-null β-cells, correlating with impaired glutamate formation, while the rise in Ca2+ was preserved. In the absence of GDH, the amplifying pathway failed to develop upon glucose stimulation in βGlud1−/− islets. Finally, insulin secretion induced by glutamine required both expression and allosteric activation of GDH, as demonstrated in βGlud1−/− islets treated with BCH or leucine. Collectively these data show that GDH differently and specifically contributes to secretory responses of the main secretagogues.
Regarding leucine, we also tested its deamination product KIC in order to discriminate between GDH allosteric activation properties (tested with BCH), as well as leucine's contribution to the TCA cycle activation with its own carbons (tested with KIC; see Figure S3). In the presence of glutamine, preservation of the KIC response, but not that of leucine, in βGlud1−/− islets suggested that GDH is required for transamination of leucine to KIC by mitochondrial branched-chain aminotransferase (BCATm). BCATm is necessary for insulin secretion evoked by KIC and glutamine, as shown in islets from mice lacking this enzyme (Zhou et al., 2010). In the BCATm reaction, the TCA cycle intermediate α-ketoglutarate is used as an acceptor of the amino group transferred from leucine, thereby forming glutamate. To refill the TCA cycle, GDH might serve as anaplerotic enzyme in these specific conditions, completing a futile cycle enabling efficient leucine deamination. This might explain the weak leucine response in GDH-deficient islets, while combination of KIC plus glutamine maintained a robust secretory response.
Unlike glucose, glutamine alone does not stimulate insulin secretion under normal conditions (Malaisse et al., 1980). Glutamine becomes a secretagogue only when GDH is allosterically activated, for instance, by BCH (Panten et al., 1984), or in the case in which the enzyme carries an activating mutation (Li et al., 2006) responsible for a hyperinsulinism syndrome (Stanley et al., 1998). The present study demonstrates that the secretory response evoked by the combination of glutamine plus BCH (or leucine) is totally GDH-dependent and there is no alternative compensatory pathway. Although glutamine deamidation produces glutamate, further catabolism through GDH is required for induction of insulin secretion. Therefore elevation of intracellular glutamate levels is not sufficient to promote insulin exocytosis without the necessary Ca2+ increase to permissive levels, as shown previously in permeabilized insulin-secreting cells (Maechler and Wollheim, 1999). Elevation of cytosolic Ca2+ requires ATP generation secondary to mitochondrial metabolism through TCA cycle activation, an effect achieved by glucose alone, but not by glutamine, unless it is combined with GDH allosteric activators (Sener et al., 1981b). As opposed to glutamine, glucose stimulation promotes elevation of both cytosolic Ca2+ (Figure 4A) and cellular glutamate levels (Figure 5C). Of note, glutamine has been reported to remain a weak secretagogue, even upon KCl depolarization (Bertrand et al., 2002; Fernandez-Pascual et al., 2004), suggesting that other signals are still missing in these experimental conditions. Alternatively, glutamine-derived glutamate might be preferentially converted to GABA, in accordance with observations in rat islets showing that in the absence of any other substrates, glutamine is dose-dependently decarboxylated independently of mitochondrial metabolism (Fernandez-Pascual et al., 2004).
Failure of glutamate generation upon glucose stimulation in GDH-deficient islets could be rescued by exposure to glutamate precursors. Indeed, provision of cellular glutamate to βGlud1−/− islets, adding either dimethyl glutamate or glutamine, fully restored the secretory response to glucose (Figures 4B and 7A, respectively). This is in agreement with replenishment of cytosolic glutamate in β-cells down-regulating the mitochondrial glutamate carrier GC1 and thereby restoring insulin secretion (Casimir et al., 2009a). Recently, bioinformatic analysis of gene expression in mouse islets versus other murine tissues identified ornithine aminotransferase as a new “disallowed gene” in pancreatic islets (Pullen et al., 2010). This enzyme, which converts glutamate to ornithine, is selectively down-regulated in β-cells, preventing dissipation of the glutamate pool (Pullen et al., 2010). Altogether, these observations established that permissive levels of glutamate are necessary for the full development of the secretory response to glucose stimulation. In other words, intracellular glutamate would render insulin granules exocytosis-competent. The cytosolic target of glutamate might be the insulin granule itself, as several studies by different groups have shown the requirement of glutamate uptake by secretory vesicles for insulin exocytosis (Maechler and Wollheim, 1999; Hoy et al., 2002; Eto et al., 2003; Storto et al., 2006; Gammelsaeter et al., 2011). Present data point to GDH as the enzyme in charge of a glucose-induced increase in cellular glutamate, with aminotransferases playing no significant role in this process. Moreover, βGlud1−/− islets reveal that GDH is required for development of the amplifying pathway.
On the basis of present data, we designed a model for glutamate-related pathways (Figure 6). When control islets are stimulated with glucose, glutamate and aspartate levels are increased along with insulin secretion (Figure 6B). Measurements of carbon fluxes using [U-13C]glucose labeling and gas chromatography–mass spectrometry (GC-MS) analysis revealed that GDH contributes to the net synthesis of glutamate from α-ketoglutarate upon glucose stimulation. The lower glutamate enrichment in glucose carbons observed in βGlud1−/− islets was not due to lower transaminase activity, since the labeling was not reduced in aspartate. In contrast, the slight tendency toward an increased labeling of aspartate in βGlud1−/− islets may illustrate that 13C labeling was retained in the TCA cycle when the flow of carbons toward glutamate was hindered by the lack of GDH.
Inhibition of transaminases does not change the secretory response, but it lowers aspartate levels in control islets, indicating that ALAT works in the direction of α-ketoglutarate formation upon glucose stimulation. Significantly, our study shows increased aspartate and unchanged alanine levels upon glucose stimulation, while others reported a decrease and an increase, respectively (Li et al., 2003, 2006). These discrepancies might be explained by preincubation conditions resulting in different amino acid pools when glucose stimulation is initiated. For instance, in studies by Li et al. (2003, 2006) mouse islets were preincubated with 10 mM glutamine, thereby filling the cellular glutamine/glutamate pool before stimulation. We opted for fuel depletion, that is, both glucose- and glutamine-free preincubation medium, before stimulation with one or the other metabolite. Discrepancies might also be explained by species specificities. Indeed, using the rat-derived insulin-secreting cell line INS-1E, we previously reported decreased aspartate and increased alanine upon glucose stimulation (Carobbio et al., 2004). More recently, no change in aspartate levels was recorded in glucose-stimulated rat islets when preincubated in the absence of nutrients (Pizarro-Delgado et al., 2009). It is intriguing that these two amino acids, aspartate in particular, exhibit high variability between studies. Aspartate participates in both amino acid metabolism and the glucose-induced NADH mitochondrial shuttle (Casimir et al., 2009b). This might render its cellular levels highly sensitive to nutrient states. Of note, while 4 mM AOA was shown to inhibit glucose-stimulated insulin secretion in rat islets (Casimir et al., 2009b), we did not observe such an effect at 2 mM AOA in mouse islets. This is consistent with previous studies reporting stronger effects of AOA in rat (MacDonald, 1982; Malaisse et al., 1982) versus mouse (Eto et al., 1999; Ravier et al., 2000) islets. Thus the present results confirm that glucose-stimulated insulin secretory response of mouse islets is not affected by AOA at 2 mM (Figure 5B), while at 5 mM there are either no (Eto et al., 1999) or marginal (Ravier et al., 2000) inhibitory effects. The reason for such a species difference is unknown.
The fact that the rise in glutamate evoked by glucose is not affected by transaminase inhibition suggests that this amino acid is mainly contributed by an alternative enzyme connecting the TCA cycle to glutamate, namely GDH. In the absence of GDH, glucose stimulation can no longer increase glutamate and aspartate (Figure 7D). This observation further points to GDH as the key enzyme for the generation of glutamate. Moreover, transaminases are not able to compensate for the lack of GDH in βGlud1−/− islets. Data show that GDH is also required for aspartate formation during glucose stimulation. The “GABA-shunt,” active at high glucose levels, is an alternative pathway for glutamate metabolism. According to this model (Pizarro-Delgado et al., 2009), glutamate exported out of mitochondria is decarboxylated to GABA. Next importing GABA back into mitochondria results in α-ketoglutarate–dependent transamination, favoring the formation of the TCA cycle intermediate succinate (Pizarro-Delgado et al., 2010). Alternatively, GABA can be released from the β-cell and exert an autocrine positive feedback loop, as observed in human islets (Braun et al., 2010). However, rodent insulin-secreting cells do not exhibit similar sensitivity, as shown in rat and mouse islets (Gilon et al., 1991) and insulinoma INS-1E cells (Rubi et al., 2001).
According to previous and present results, we favor the following model for GDH regulation in β-cells: 1) at basal glucose, a low energy state might induce moderate glutamate consumption to maintain basal ATP levels; 2) upon glucose stimulation inducing insulin secretion, GDH would work in the cataplerotic direction, generating glutamate from α-ketoglutarate enriched by glucose-derived carbons; 3) in the pathological situation of hyperinsulinism syndrome, the GDH-activating mutation renders the enzyme anaplerotic and the β-cell glutamine-responsive, thereby triggering inappropriate insulin release even at low glucose.
In conclusion, this study demonstrates that GDH is essential for sustained insulin release evoked by glucose. The lack of GDH prevents glucose-induced glutamate generation, with transaminases failing to play a compensatory role. Provision of cellular glutamate is sufficient to restore the full development of glucose-stimulated insulin secretion, indicating that permissive levels of glutamate are required in the amplifying pathway.
MATERIALS AND METHODS
Generation and genotyping of β-cell–specific GDH knockout mouse (βGlud1–/–)
Female transgenic mice containing the exon 7 of Glud1 flanked by two lox/P sites, previously generated in the lab (Carobbio et al., 2009), were crossed with male mice expressing Cre recombinase under the control of the rat insulin promoter (Rip-Cre mice; Herrera, 2000), in order to achieve the recombination of GDH specifically within β-cells. Animals were maintained on a mixed (C57BL/6J × 129/Sv) genetic background. Both male and female animals were studied for some representative parameters, and control islets were isolated from nonknockout mice from the same litters. Mouse breeding and handling was carried out in our local certified animal facility according to procedures that were approved by the animal care and experimentation authorities of the Canton of Geneva. Transgenic animals were genotyped by PCR on genomic DNA extracted from tail biopsies (Carobbio et al., 2009).
Pancreatic islet isolation and adenoviral treatment
Mouse pancreatic islets were isolated by collagenase digestion (collagenase P; Roche, Rotkreuz, Switzerland), as described previously (Pralong et al., 1994), and were cultured free-floating in RPMI-1640 medium supplemented with 5% (vol/vol) heat-inactivated fetal calf serum, 2 mM glutamine, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 50 μM 2-mercaptoethanol. For rescue experiments, control islets were infected with Ad-lacZ, and ectopic expression of hGLUD1 was achieved by transducing islets isolated from βGlud1−/− mice with the recombinant adenovirus overexpressing GDH (pAd-GDH), as detailed previously (Carobbio et al., 2004). Transductions were done by infection of islets on the day of isolation with 1 μl of purified virus/1 ml of media for 2 h (corresponding to approximately 40 plaque-forming units per cell), and islets were used the next day for secretion assays. Transient correction of GDH deficiency did not induce apparent changes in islet morphology.
Insulin secretion and measurements
For static incubations, mouse islets were maintained for 2 h in glucose-free and glutamine-free RPMI-1640 medium and then washed in Krebs Ringer bicarbonate HEPES buffer (KRBH, containing [in mM]: 135 NaCl, 3.6 KCl, 10 HEPES, pH 7.4, 5 NaHCO3, 0.5 NaH2PO4, 0.5 MgCl2, 1.5 CaCl2, 0.1% bovine serum albumin) containing basal (2.8 mM) glucose concentration. Then batches of 40–50 islets were handpicked and incubated for 1 h at 37°C with the indicated secretagogues. At the end of the assay period, islets were put on ice, and the supernatant was collected to measure insulin secretion by radioimmunoassay (Linco, St. Charles, MO). Values were expressed per number of islets.
For islet perifusions, 10 handpicked islets were each put in a 250-μl chamber and thermostated at 37°C (Brandel, Gaithersburg, MD). The flux was set at 0.5 ml/min, and fractions were collected every min after a 30-min washing period at basal 2.8 mM glucose. Islets were then stimulated for 15 min with the indicated secretagogues. At the end of the assay period, supernatants were collected to measure insulin. All compounds used for insulin secretion were obtained from Sigma-Aldrich (Buchs, Switzerland).
For in situ pancreatic perfusion, the pancreas was perfused ex vivo in anesthetized mice as described previously (Maechler et al., 2002) with a 1.5 ml/min perfusion rate.
Measurement of amino acid levels
After stimulation with the different secretagogues to measure insulin secretion, islets were resuspended in 35% (wt/vol) 5-sulfosalicylic acid to extract amino acids. Following overnight incubation at 4°C, samples were frozen at −80°C, and amino acid measurements were done by reverse-phase high-performance liquid chromatography (HPLC) after derivatization with O-phthalaldehyde (Bustamante et al., 2001).
[U-13C]glucose incubations
Islets were isolated, cultured, and preincubated as described above. Batches of 300 islets were then transferred to new tubes containing KRBH-BSA buffer with 22.8 mM [U-13C]glucose and incubated for 1 h at 37°C. The incubation was terminated by putting islet tubes in ice-cold water before removal of media and islet extraction using 70% vol/vol ethanol. The extracted islets were lyophilized, reconstituted in water, and derivatized with N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide (Sigma-Aldrich) in the presence of 15% N′-N-dimethyl formamide modified after the method of Mawhinney et al. (1986) before GC-MS analysis of isotopic enrichment in glutamate and aspartate. The GC-MS system consisted of a Shimadzu GC-2010 gas chromatograph linked to a Shimadzu GC–MS-Q2010plus mass spectrometer (Shimadzu Corporation, Tokyo, Japan). The percent labeling was corrected for natural abundance of the isotope by subtracting the mass distribution of a standard containing the relevant metabolites. The quantification of double labeling (M+2) was calculated as percent of the pool of metabolite being unlabeled.
Ca2+ measurements
After isolation, mouse islets were kept overnight in RPMI-1640 medium before being transferred onto glass coverslips; this was followed by another overnight culture to let islets settle and adhere. Islets were then loaded with 3 μM Fura2-AM (Molecular Probes, Eugene, OR) for 1 h in 2.8 mM glucose KRBH at 37°C and washed before transfer to the microscope (Nikon Eclipse Ti, Egg, Switzerland). Ratiometric measurements of fura-2 fluorescence were performed with 340/380-nm filters for excitation and a 510-nm filter for emission, and the signal was acquired using MetaMorph system software (Molecular Devices, Downingtown, PA).
Statistical analysis
Statistics were done using the SPSS 15.0 statistical package (SPSS, Chicago, IL). Unless indicated, data are represented as the means ± SE for at least three independent experiments performed in triplicate. Differences between βGlud1−/− and control were assessed by the two-tailed umpaired t test for single comparison or by one-way analysis of variance analysis, using a post hoc multiple comparison procedure (Fischer's least-significant difference method). Results were considered statistically significant at p < 0.05.
Supplementary Material
Acknowledgments
We are grateful to Clarissa Bartley and Gaelle Chaffard for technical assistance. This work was supported by the Swiss National Science Foundation (310030B-135704 to P.M.), the State of Geneva, the Ministerio de Ciencia e Innovación (SAF2009-12671, Madrid), and the Hjelt Diabetes Foundation (fellowship to S.P.).
Abbreviations used:
- α-KG
α-ketoglutarate
- βGlud1−/−
β-cell–specific GDH knockout mice
- Ad-GDH
adenovirus encoding for GDH
- ALAT
alanine aminotransferase
- AOA
aminooxyacetate
- ASAT
aspartate aminotransferase
- AUC
area under the curve
- BCATm
mitochondrial branched-chain aminotransferase
- BCH
2-aminobicyclo[2.2.1]heptane-2-carboxylic acid
- CoA
coenzyme A
- GABA
γ-aminobutyric acid
- GAD
glutamate decarboxylase
- GC-MS
gas chromatography–mass spectrometry
- GDH
glutamate dehydrogenase
- HPLC
high-performance liquid chromatography
- KIC
α-ketoisocaproate
- KRBH
Krebs Ringer bicarbonate HEPES buffer
- TCA
tricarboxylic acid
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-08-0676) on August 8, 2012.
REFERENCES
- Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–33592. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- Bertrand G, Ishiyama N, Nenquin M, Ravier MA, Henquin JC. The elevation of glutamate content and the amplification of insulin secretion in glucose-stimulated pancreatic islets are not causally related. J Biol Chem. 2002;277:32883–32891. doi: 10.1074/jbc.M205326200. [DOI] [PubMed] [Google Scholar]
- Braun M, Ramracheya R, Bengtsson M, Clark A, Walker JN, Johnson PR, Rorsman P. γ-Aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic β-cells. Diabetes. 2010;59:1694–1701. doi: 10.2337/db09-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryla J, Michalik M, Nelson J, Erecinska M. Regulation of the glutamate dehydrogenase activity in rat islets of Langerhans and its consequence on insulin release. Metabolism. 1994;43:1187–1195. doi: 10.1016/0026-0495(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Bustamante J, Lobo MV, Alonso FJ, Mukala NT, Gine E, Solis JM, Tamarit-Rodriguez J, Martin Del Rio R. An osmotic-sensitive taurine pool is localized in rat pancreatic islet cells containing glucagon and somatostatin. Am J Physiol Endocrinol Metab. 2001;281:E1275–E1285. doi: 10.1152/ajpendo.2001.281.6.E1275. [DOI] [PubMed] [Google Scholar]
- Carobbio S, et al. Deletion of glutamate dehydrogenase in β-cells abolishes part of the insulin secretory response not required for glucose homeostasis. J Biol Chem. 2009;284:921–929. doi: 10.1074/jbc.M806295200. [DOI] [PubMed] [Google Scholar]
- Carobbio S, Ishihara H, Fernandez-Pascual S, Bartley C, Martin-Del-Rio R, Maechler P. Insulin secretion profiles are modified by overexpression of glutamate dehydrogenase in pancreatic islets. Diabetologia. 2004;47:266–276. doi: 10.1007/s00125-003-1306-2. [DOI] [PubMed] [Google Scholar]
- Casimir M, Lasorsa FM, Rubi B, Caille D, Palmieri F, Meda P, Maechler P. Mitochondrial glutamate carrier GC1 as a newly identified player in the control of glucose-stimulated insulin secretion. J Biol Chem. 2009a;284:25004–25014. doi: 10.1074/jbc.M109.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimir M, Rubi B, Frigerio F, Chaffard G, Maechler P. Silencing of the mitochondrial NADH shuttle component aspartate-glutamate carrier AGC1/Aralar1 in INS-1E cells and rat islets. Biochem J. 2009b;424:459–466. doi: 10.1042/BJ20090729. [DOI] [PubMed] [Google Scholar]
- Eto K, et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- Eto K, Yamashita T, Hirose K, Tsubamoto Y, Ainscow EK, Rutter GA, Kimura S, Noda M, Iino M, Kadowaki T. Glucose metabolism and glutamate analog acutely alkalinize pH of insulin secretory vesicles of pancreatic β-cells. Am J Physiol Endocrinol Metab. 2003;285:E262–E271. doi: 10.1152/ajpendo.00542.2002. [DOI] [PubMed] [Google Scholar]
- Fahien LA, MacDonald MJ, Kmiotek EH, Mertz RJ, Fahien CM. Regulation of insulin release by factors that also modify glutamate dehydrogenase. J Biol Chem. 1988;263:13610–13614. [PubMed] [Google Scholar]
- Fernandez-Pascual S, Mukala-Nsengu-Tshibangu A, Martin Del Rio R, Tamarit-Rodriguez J. Conversion into GABA (γ-aminobutyric acid) may reduce the capacity of l-glutamine as an insulin secretagogue. Biochem J. 2004;379:721–729. doi: 10.1042/BJ20031826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher HF. l-Glutamate dehydrogenase from bovine liver. Methods Enzymol. 1985;113:16–27. doi: 10.1016/s0076-6879(85)13006-5. [DOI] [PubMed] [Google Scholar]
- Frigerio F, Casimir M, Carobbio S, Maechler P. Tissue specificity of mitochondrial glutamate pathways and the control of metabolic homeostasis. Biochim Biophys Acta. 2008;1777:965–972. doi: 10.1016/j.bbabio.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Gammelsaeter R, Coppola T, Marcaggi P, Storm-Mathisen J, Chaudhry FA, Attwell D, Regazzi R, Gundersen V. A role for glutamate transporters in the regulation of insulin secretion. PLoS One. 2011;6:e22960. doi: 10.1371/journal.pone.0022960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZY, Li G, Najafi H, Wolf BA, Matschinsky FM. Glucose regulation of glutaminolysis and its role in insulin secretion. Diabetes. 1999;48:1535–1542. doi: 10.2337/diabetes.48.8.1535. [DOI] [PubMed] [Google Scholar]
- Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992;89:1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C, Henquin JC. The influence of γ-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology. 1991;129:2521–2529. doi: 10.1210/endo-129-5-2521. [DOI] [PubMed] [Google Scholar]
- Haigis MC, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Hoy M, Maechler P, Efanov AM, Wollheim CB, Berggren PO, Gromada J. Increase in cellular glutamate levels stimulates exocytosis in pancreatic β-cells. FEBS Lett. 2002;531:199–203. doi: 10.1016/s0014-5793(02)03500-7. [DOI] [PubMed] [Google Scholar]
- Hudson RC, Daniel RM. L-glutamate dehydrogenases: distribution, properties and mechanism. Comp Biochem Physiol B. 1993;106:767–792. doi: 10.1016/0305-0491(93)90031-y. [DOI] [PubMed] [Google Scholar]
- Li C, et al. Effects of a GTP-insensitive mutation of glutamate dehydrogenase on insulin secretion in transgenic mice. J Biol Chem. 2006;281:15064–15072. doi: 10.1074/jbc.M600994200. [DOI] [PubMed] [Google Scholar]
- Li C, Najafi H, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Matschinsky FM, Stanley CA. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J Biol Chem. 2003;278:2853–2858. doi: 10.1074/jbc.M210577200. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ. Evidence for the malate aspartate shuttle in pancreatic islets. Arch Biochem Biophys. 1982;213:643–649. doi: 10.1016/0003-9861(82)90594-x. [DOI] [PubMed] [Google Scholar]
- Maechler P, Carobbio S, Rubi B. In beta-cells, mitochondria integrate and generate metabolic signals controlling insulin secretion. Int J Biochem Cell Biol. 2006;38:696–709. doi: 10.1016/j.biocel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Maechler P, Gjinovci A, Wollheim CB. Implication of glutamate in the kinetics of insulin secretion in rat and mouse perfused pancreas. Diabetes. 2002;51:S99–S102. doi: 10.2337/diabetes.51.2007.s99. [DOI] [PubMed] [Google Scholar]
- Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402:685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ, Malaisse-Lagae F, Sener A. The stimulus-secretion coupling of glucose-induced insulin release: effect of aminooxyacetate upon nutrient-stimulated insulin secretion. Endocrinology. 1982;111:392–397. doi: 10.1210/endo-111-2-392. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ, Sener A, Carpinelli AR, Anjaneyulu K, Lebrun P, Herchuelz A, Christophe J. The stimulus-secretion coupling of glucose-induced insulin release. XLVI. Physiological role of l-glutamine as a fuel for pancreatic islets. Mol Cell Endocrinol. 1980;20:171–189. doi: 10.1016/0303-7207(80)90080-5. [DOI] [PubMed] [Google Scholar]
- Mawhinney TP, Robinett RS, Atalay A, Madson MA. Analysis of amino acids as their tert.-butyldimethylsilyl derivatives by gas-liquid chromatography and mass spectrometry. J Chromatogr. 1986;358:231–242. doi: 10.1016/s0021-9673(01)90333-4. [DOI] [PubMed] [Google Scholar]
- Michaelidis TM, Tzimagiorgis G, Moschonas NK, Papamatheakis J. The human glutamate dehydrogenase gene family: gene organization and structural characterization. Genomics. 1993;16:150–160. doi: 10.1006/geno.1993.1152. [DOI] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- Panten U, Zielmann S, Langer J, Zunkler BJ, Lenzen S. Regulation of insulin secretion by energy metabolism in pancreatic β-cell mitochondria. Studies with a non-metabolizable leucine analogue. Biochem J. 1984;219:189–196. doi: 10.1042/bj2190189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Delgado J, Braun M, Hernandez-Fisac I, Martin-Del-Rio R, Tamarit-Rodriguez J. Glucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in β-cells. Biochem J. 2010;431:381–389. doi: 10.1042/BJ20100714. [DOI] [PubMed] [Google Scholar]
- Pizarro-Delgado J, Hernandez-Fisac I, Martin-Del-Rio R, Tamarit-Rodriguez J. Branched-chain 2-oxoacid transamination increases GABA-shunt metabolism and insulin secretion in isolated islets. Biochem J. 2009;419:359–368. doi: 10.1042/BJ20081731. [DOI] [PubMed] [Google Scholar]
- Pralong WF, Spat A, Wollheim CB. Dynamic pacing of cell metabolism by intracellular Ca2+ transients. J Biol Chem. 1994;269:27310–27314. [PubMed] [Google Scholar]
- Pullen TJ, Khan AM, Barton G, Butcher SA, Sun G, Rutter GA. Identification of genes selectively disallowed in the pancreatic islet. Islets. 2010;2:89–95. doi: 10.4161/isl.2.2.11025. [DOI] [PubMed] [Google Scholar]
- Ravier MA, Eto K, Jonkers FC, Nenquin M, Kadowaki T, Henquin JC. The oscillatory behavior of pancreatic islets from mice with mitochondrial glycerol-3-phosphate dehydrogenase knockout. J Biol Chem. 2000;275:1587–1593. doi: 10.1074/jbc.275.3.1587. [DOI] [PubMed] [Google Scholar]
- Rubi B, Ishihara H, Hegardt FG, Wollheim CB, Maechler P. GAD65-mediated glutamate decarboxylation reduces glucose-stimulated insulin secretion in pancreatic beta cells. J Biol Chem. 2001;276:36391–36396. doi: 10.1074/jbc.M104999200. [DOI] [PubMed] [Google Scholar]
- Sener A, Hutton JC, Malaisse WJ. The stimulus-secretion coupling of amino acid-induced insulin release. Synergistic effects of L-glutamine and 2-keto acids upon insulin secretion. Biochim Biophys Acta. 1981a;677:32–38. doi: 10.1016/0304-4165(81)90142-2. [DOI] [PubMed] [Google Scholar]
- Sener A, Malaisse WJ. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980;288:187–189. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- Sener A, Malaisse-Lagae F, Malaisse WJ. Stimulation of pancreatic islet metabolism and insulin release by a nonmetabolizable amino acid. Proc Natl Acad Sci USA. 1981b;78:5460–5464. doi: 10.1073/pnas.78.9.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A, Owen A, Malaisse-Lagae F, Malaisse WJ. The stimulus-secretion coupling of amino acid-induced insulin release. XI. Kinetics of deamination and transamination reactions. Horm Metab Res. 1982;14:405–409. doi: 10.1055/s-2007-1019030. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Peterson PE, Schmidt T, Fang J, Stanley CA. Structures of bovine glutamate dehydrogenase complexes elucidate the mechanism of purine regulation. J Mol Biol. 2001;307:707–720. doi: 10.1006/jmbi.2001.4499. [DOI] [PubMed] [Google Scholar]
- Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, Perlman K, Rich BH, Zammarchi E, Poncz M. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338:1352–1357. doi: 10.1056/NEJM199805073381904. [DOI] [PubMed] [Google Scholar]
- Storto M, et al. Insulin secretion is controlled by mGlu5 metabotropic glutamate receptors. Mol Pharmacol. 2006;69:1234–1241. doi: 10.1124/mol.105.018390. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Huh JW, Kim MJ, Lee WJ, Kim TU, Choi SY, Cho SW. Regulatory effects of 5′-deoxypyridoxal on glutamate dehydrogenase activity and insulin secretion in pancreatic islets. Biochimie. 2003;85:581–586. doi: 10.1016/s0300-9084(03)00092-0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jetton TL, Goshorn S, Lynch CJ, She P. Transamination is required for α-ketoisocaproate but not leucine to stimulate insulin secretion. J Biol Chem. 2010;285:33718–33726. doi: 10.1074/jbc.M110.136846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.