Abstract
Plastid genomes show an impressive array of sizes and compactnesses, but the forces responsible for this variation are unknown. It has been argued that species with small effective genetic population sizes are less efficient at purging excess DNA from their genomes than those with large effective population sizes. If true, one may expect the primary mode of plastid inheritance to influence plastid DNA (ptDNA) architecture. All else being equal, biparentally inherited ptDNAs should have a two-fold greater effective population size than those that are uniparentally inherited, and thus should also be more compact. Here, we explore the relationship between plastid inheritance pattern and ptDNA architecture, and consider the role of phylogeny in shaping our observations. Contrary to our expectations, we found no significant difference in plastid genome size or compactness between ptDNAs that are biparentally inherited relative to those that are uniparentally inherited. However, we also found that there was significant phylogenetic signal for the trait of mode of plastid inheritance. We also found that paternally inherited ptDNAs are significantly smaller (n = 19, p = 0.000001) than those that are maternally, uniparentally (when isogamous), or biparentally inherited. Potential explanations for this observation are discussed.
Introduction
Plastids originate from an ancient endosymbiosis of a cyanobacterium by a eukaryotic host [1]. They first arose in the ancestor of the Archaeplastida (i.e., Plantae), and were then passed on laterally to diverse lineages through eukaryote-eukaryote endosymbioses [2], [3]. The genomes within contemporary plastids show a remarkable, and puzzling, diversity of sizes (5 to >1000 kilobases; kb) and compactnesses (<5 to >85% noncoding DNA) [4]. However, the evolutionary forces that gave rise to this variation are poorly understood.
The mutational hazard hypothesis argues that large, bloated genomes, with lots of intergenic and intronic DNA, pose a greater mutational burden to their hosts than genomes that are compact [5]. This is because any expansion in DNA content increases the potential for deleterious mutations, where the higher the mutation rate the greater the burden of having excess DNA. It follows, therefore, that species with large effective genetic population sizes (Ne), where natural selection is efficient, are better at perceiving and eliminating “burdensome” excess DNA than those with a small Ne [5]. Many studies have explored the relationship between Ne and genome compactness [6]–[8], but few have employed plastid DNA (ptDNA).
Effective genetic population size is a difficult parameter to measure, and one that is likely influenced by the mode of inheritance. Plastid genomes, unlike most nuclear chromosomes, are typically uniparentally inherited [9]. For sexually reproducing species with male and female gametes, maternal plastid inheritance is the norm. Studies, however, have identified diverse species with paternal or biparental modes of plastid inheritance [10]–[13]. Other things being equal, the Ne of uniparentally inherited plastid genomes should be half that of biparentally inherited ones. Further, the influence of differential migration (e.g. seeds are heavier and less numerous than pollen) and an individual’s size at reproduction (e.g. smaller individuals produce greater amounts of pollen vs. seeds) mean that maternal vs. paternal modes of organellar inheritance can also lead to overall differences in the Ne of ptDNAs [14].
In this study, we use newly available data on plastid genome sequence and inheritance pattern to investigate how differing modes of inheritance impact ptDNA architecture. Based on the mutational hazard hypothesis, we predict that biparentally inherited ptDNAs, given their potential for having a higher Ne , will be more compact than those that are uniparentally inherited. We also expect to see differences in genomic architecture between paternally vs. maternally vs. uniparentally (when isogamous) inherited ptDNAs.
Methods
By searching the literature, we found 81 species for which both plastid inheritance statistics and complete ptDNA sequence data are available, including 69 land plants, 6 green algae, 2 red algae, 2 apicomplexans, and 2 stramenopile (Table 1). The mode of plastid inheritance is thought to vary continuously rather than discretely between taxa; however, determining an appropriate scale for ranking the degree of biparental inheritance was difficult because of large differences in sample sizes between species. Instead, we categorized the primary pattern of plastid inheritance using the following: inheritance determined from genetic analysis of mutant plastids; ptDNA restriction analysis and/or analysis of ptDNA sequence data of progeny with known parentage; epifluorescence microscopy employing DNA fluorochromes to detect plastids in viable, mature sperm cells; and ultrastructural observations using transmission electron microscopy (TEM). Further, we noted cases where interspecific, intergeneric, or widely divergent strain cross was used to assess plastid mode of inheritance because at least one previous study has shown that taxonomically divergent crosses can cause the breakdown of the typical pattern of cytoplasmic maternal inheritance [15]. In a few cases the primary mode of inheritance was undetermined for the species with a complete plastid sequence in our dataset, so we screened the literature for plastid inheritance studies from other members of the same genera or higher-level taxonomic group; if the mode of inheritance was identical within the group, then we assumed all members from that group had the same mode of plastid inheritance (e.g. maternal inheritance for the genus Cuscuta or paternal inheritance for the order Pinales).
Table 1. Organisms, coarse taxonomic group, plastid genome size, coding proportion of ptDNA, primary mode of plastid inheritance, and references to support the mode of inheritance.
| Organism | Accession # | TaxonomicGroup | Plastid GenomeSize (bp) | Codingproportion | PrimaryInheritance | Reference |
| Cicer arietinum | NC_011163 | Land Plant | 125319 | 0.52 | Biparental | [10], [37] * |
| Ectocarpus siliculosus | NC_013498 | Stramenopile | 139954 | 0.74 | Biparental | [38], [39] * |
| Equisetum arvense | NC_014699 | Land Plant | 133309 | 0.54 | Biparental | [40] |
| Geranium palmatum | NC_014573 | Land Plant | 155794 | 0.37 | Biparental | [10] |
| Ipomoea purpurea | NC_009808 | Land Plant | 162046 | 0.54 | Biparental | [41], [42] |
| Medicago truncatula | NC_003119 | Land Plant | 124033 | 0.53 | Biparental | [43] |
| Oenothera argillicola | NC_010358 | Land Plant | 165055 | 0.49 | Biparental | [10], [44] |
| Oenothera biennis | NC_010361 | Land Plant | 164807 | 0.49 | Biparental | [10], [44] |
| Oenothera elata subsp. hookeri | NC_002693 | Land Plant | 165728 | 0.49 | Biparental | [10], [44] |
| Oenothera glazioviana | NC_010360 | Land Plant | 165225 | 0.49 | Biparental | [10], [44] |
| Oenothera parviflora | NC_010362 | Land Plant | 163365 | 0.49 | Biparental | [10], [44] |
| Pelargonium×hortorum | NC_008454 | Land Plant | 217942 | 0.52 | Biparental | [10], [45] |
| Phaseolus vulgaris | NC_009259 | Land Plant | 150285 | 0.54 | Biparental | [10], [46] * |
| Pisum sativum | NC_014057 | Land Plant | 122169 | 0.53 | Biparental | [10], [47]–[49] |
| Psilotum nudum | NC_003386 | Land Plant | 138829 | 0.65 | Biparental | [50] |
| Selaginella moellendorffii | NC_013086 | Land plant | 143780 | 0.54 | Biparental | [51] |
| Trifolium subterraneum | NC_011828 | Land Plant | 144763 | 0.39 | Biparental | [52] |
| Solanum lycopersicum | NC_007898 | Land Plant | 155461 | 0.58 | Maternal | [53] |
| Arabidopsis thaliana | NC_000932 | Land Plant | 154478 | 0.51 | Maternal | [10], [54] |
| Bryopsis hypnoides | NC_013359 | Green Algae | 153429 | 0.35 | Maternal | [55] |
| Carica papaya | NC_010323 | Land Plant | 160100 | 0.49 | Maternal | [56] * |
| Chara vulgaris | NC_008097 | Green Algae | 184933 | 0.48 | Maternal | [57] |
| Cheilanthes lindheimeri | NC_014592 | Land Plant | 155770 | 0.52 | Maternal | [58] |
| Coffea arabica | NC_008535 | Land Plant | 155189 | 0.51 | Maternal | [10] |
| Cucumis sativus | NC_007144 | Land Plant | 155293 | 0.5 | Maternal | [10], [59] * |
| Cuscuta exaltata | NC_009963 | Land Plant | 125373 | 0.48 | Maternal | [10] |
| Cuscuta gronovii | NC_009765 | Land Plant | 86744 | 0.61 | Maternal | [10] |
| Cuscuta obtusiflora | NC_009949 | Land Plant | 85286 | 0.6 | Maternal | [10] |
| Cuscuta reflexa | NC_009766 | Land Plant | 121521 | 0.49 | Maternal | [10] |
| Cycas taitungensis | NC_009618 | Land Plant | 163403 | 0.55 | Maternal | [60] * |
| Daucus carota | NC_008325 | Land Plant | 155911 | 0.5 | Maternal | [61], [62] |
| Eimeria tenella | NC_004823 | Apicomplexan | 34750 | 0.67 | Maternal | [63] |
| Ephedra equisetina | NC_011954 | Land Plant | 109518 | 0.66 | Maternal | [33] |
| Eucalyptus globulus subsp. globulus | NC_008115 | Land Plant | 160286 | 0.5 | Maternal | [64] * |
| Fragaria vesca subsp. vesca | NC_015206 | Land Plant | 155691 | 0.53 | Maternal | [65] |
| Fucus vesiculosus | NC_016735 | Stramenopile | 124986 | 0.79 | Maternal | [66] |
| Ginkgo biloba | NC_016986 | Land Plant | 156988 | 0.42 | Maternal | [67] |
| Glycine max | NC_007942 | Land Plant | 152218 | 0.51 | Maternal | [10] |
| Gossypium hirsutum | NC_007944 | Land Plant | 160301 | 0.49 | Maternal | [68] |
| Gracilaria tenuistipitata | NC_006137 | Red Algae | 183883 | 0.82 | Maternal | [69] |
| Helianthus annuus | NC_007977 | Land Plant | 151104 | 0.51 | Maternal | [70] |
| Hordeum vulgare subsp. vulgare | NC_008590 | Land Plant | 136462 | 0.44 | Maternal | [71], [72] |
| Lolium perenne | NC_009950 | Land Plant | 135282 | 0.44 | Maternal | [73] |
| Manihot esculenta | NC_010433 | Land Plant | 161453 | 0.45 | Maternal | [10] |
| Nicotiana tabacum | NC_001879 | Land Plant | 155943 | 0.54 | Maternal | [74] |
| Olea europaea | NC_013707 | Land Plant | 155888 | 0.53 | Maternal | [75] |
| Oryza sativa Indica Group | NC_008155 | Land Plant | 134496 | 0.36 | Maternal | [10] |
| Oryza sativa Japonica Group | NC_001320 | Land Plant | 134525 | 0.49 | Maternal | [10] |
| Panicum virgatum | NC_015990 | Land Plant | 139619 | 0.43 | Maternal | [76] * |
| Populus trichocarpa | NC_009143 | Land Plant | 157033 | 0.53 | Maternal | [77] * |
| Porphyra purpurea | NC_000925 | Red Algae | 191028 | 0.81 | Maternal | [78] * |
| Ricinus communis | NC_016736 | Land Plant | 163161 | 0.49 | Maternal | [10] |
| Silene vulgaris | NC_016727 | Land Plant | 151583 | 0.53 | Maternal | [79] |
| Solanum tuberosum | NC_008096 | Land Plant | 155296 | 0.53 | Maternal | [10] |
| Sorghum bicolor | NC_008602 | Land Plant | 140754 | 0.42 | Maternal | [10] |
| Toxoplasma gondii | NC_001799 | Apicomplexan | 34996 | 0.6 | Maternal | [80] |
| Triticum aestivum | NC_002762 | Land Plant | 134545 | 0.45 | Maternal | [10] |
| Vitis vinifera | NC_007957 | Land Plant | 160928 | 0.49 | Maternal | [10] |
| Volvox carteri | GU084820 | Green Algae | 461064 | 0.2 | Maternal | [81] * |
| Zea mays | NC_001666 | Land Plant | 140384 | 0.48 | Maternal | [82] * |
| Cathaya argyrophylla | NC_014589 | Land Plant | 107122 | 0.57 | Paternal | [33], [83]–[87] * |
| Cedrus deodara | NC_014575 | Land Plant | 119299 | 0.53 | Paternal | [33], [83]–[87] * |
| Cephalotaxus wilsoniana | NC_016063 | Land Plant | 136196 | 0.58 | Paternal | [33], [83]–[87] * |
| Cryptomeria japonica | NC_010548 | Land Plant | 131810 | 0.56 | Paternal | [88] |
| Keteleeria davidiana | NC_011930 | Land Plant | 117720 | 0.54 | Paternal | [33], [83]–[87] * |
| Larix decidua | NC_016058 | Land Plant | 122474 | 0.5 | Paternal | [89] |
| Picea morrisonicola | NC_016069 | Land Plant | 124168 | 0.48 | Paternal | [90] * |
| Picea sitchensis | NC_011152 | Land Plant | 120176 | 0.37 | Paternal | [90] * |
| Pinus contorta | NC_011153 | Land Plant | 120438 | 0.49 | Paternal | [33], [83]–[87] * |
| Pinus gerardiana | NC_011154 | Land Plant | 117618 | 0.51 | Paternal | [33], [83]–[87] * |
| Pinus koraiensis | NC_004677 | Land Plant | 117190 | 0.54 | Paternal | [33], [83]–[87] * |
| Pinus krempfii | NC_011155 | Land Plant | 116989 | 0.51 | Paternal | [33], [83]–[87] * |
| Pinus lambertiana | NC_011156 | Land Plant | 117239 | 0.52 | Paternal | [33], [83]–[87] * |
| Pinus monophylla | NC_011158 | Land Plant | 116479 | 0.52 | Paternal | [33], [83]–[87] * |
| Pinus nelsonii | NC_011159 | Land Plant | 116834 | 0.52 | Paternal | [33], [83]–[87] * |
| Pinus thunbergii | NC_001631 | Land Plant | 119707 | 0.62 | Paternal | [33], [83]–[87] * |
| Pseudotsuga sinensis var. wilsoniana | NC_016064 | Land Plant | 122513 | 0.56 | Paternal | [33], [83]–[87] * |
| Taiwania cryptomerioides | NC_016065 | Land Plant | 132588 | 0.62 | Paternal | [33], [83]–[87] * |
| Chlamydomonas reinhardtii | NC_005353 | Green Algae | 203828 | 0.39 | Uniparental | [91] * |
| Nephroselmis olivacea | NC_000927 | Green Algae | 200799 | 0.63 | Uniparental | [92] |
| Zygnema circumcarinatum | NC_008117 | Green Algae | 165372 | 0.51 | Uniparental | [93] |
Evidence for plastid inheritance in one or more studies listed was obtained from an interspecific or widely divergent strain cross.
Noncoding ptDNA content was calculated as follows: genome length minus the collective length of all annotated protein-, rRNA-, and tRNA-coding regions, not including the portions of these regions that are also annotated as introns. Intronic and non-standard open reading frames were treated as noncoding DNA. This method is contingent on the authors of the GenBank records having properly annotated their entry.
We performed a linear regression between plastid genome length (independent variable) and the amount of noncoding ptDNA (dependent variable). Both variables were log-transformed to meet the assumptions of homoscedasticity and normality. To test the effect of plastid inheritance pattern on noncoding ptDNA content and plastid genome size, we performed two non-parametric analyses. The factor “plastid inheritance” contained four levels: biparental vs. maternal vs. paternal vs. uniparental isogamous. The first analysis tested how all four levels affected the dependent variables (using separate Kruskal-Wallis tests for each variable). For the second analysis, we pooled the last three levels into ‘uniparental’ and used Wilcoxon rank sign tests. We applied non-parametric tests because our data were not normally distributed and because of the uneven sample sizes between levels of the factor “mode of plastid inheritance.” When more than two levels were used, we looked for significant differences between the various levels by performing post-hoc multiple comparisons using the Kruskal-Wallis test (function ‘kruskalmc’ in the R package ‘pgirmess’). Statistical analyses were performed with R v.2.14.2 (R Core Development Team 2012).
Phylogenetic Independent Contrasts and Phylogenetic Signal in Our Dataset
Because our dataset was comprised of several groups of very closely related species (Table 1), we considered if the effects of phylogenetic non-independence (and by proxy pseudoreplication) [16], [17] were influencing the conclusions from our initial analyses. First we checked the tree topology of our dataset using a taxonomic tree generated from the NCBI Taxonomy Database [18], [19], and a maximum-likelihood phylogeny (10000 bootstraps using the PhyML plugin for Geneious Pro v. 5.4.4 [20]) based on the deduced amino acid sequences of the plastid-encoded rbcL gene (see Table 1 for GenBank accession numbers). Both trees had identical topologies except that the rbcL tree contained no apicomplexans because their ptDNAs do not contain rbcL. Because most tests of phylogenetic independence require a tree to be rooted, we forcibly rooted our rbcL tree in the red algal species Gracilaria tenuistipitata var. liui.
Phylogenetic independent contrasts (PICs) for the continuous variables of ptDNA size and noncoding content were performed using the ‘crunch’ function within the ‘caper’ package [20] of R v.2.14.2 (R Core Development Team 2012). To investigate the association between plastid genome size and noncoding ptDNA content, we fit a linear model of the standardized contrasts against each other. We were unable to obtain a large number of contrasts for our dataset that incorporated all nodes of the phylogeny (taxonomic or gene tree) for the categorical variable of primary mode of inheritance. This is because the tips of our phylogeny did not possess sufficient variation in the categorical trait, and with categorical variables only the tips are used in assessing the role of phylogenetic non-independence [21], [22]. Instead, we performed an analysis of phylogenetic signal strength (D) [23] for the binary trait of biparental vs. uniparental plastid inheritance to see if these traits were “clumped” or randomly distributed [22], [23] in the phylogeny. D values that are negative or close to 0 are more phylogenetically conserved (or clumped), which can indicate non-independent evolutionary events, whereas D values closer to 1 are overdispersed and therefore can be a sign of randomness in the trait’s distribution within a phylogeny.
Results and Discussion
As Plastid Genome Size Increases so does the Amount of Noncoding ptDNA
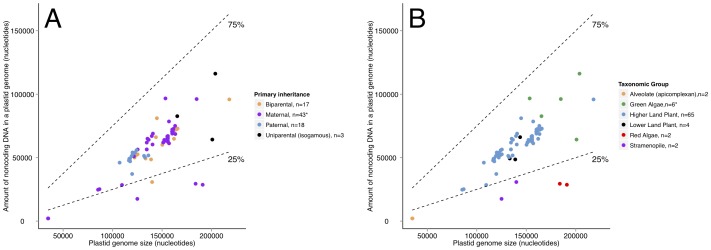
Consistent with previous observations [5], [24], the amount of noncoding ptDNA in nucleotides co-varied positively with plastid genome size for our dataset (n = 81), adjusted R2 = 0.78, p≤0.000001 (Fig. 1 A and B). Logged transformation of both variables enabled our linear model to meet the more crucial assumption for linear regression – homoscedasticity, but transformation did not improve normality. There was one significant high-leverage outlier (Volvox carteri) and two moderate statistical outliers (the apicomplexans Toxoplasma gondii and Eimeria tenella). Removal of these statistical outliers from our dataset (n = 78) did not alter the significance of the linear relationship, adjusted R2 = 0.76, p≤0.000001. When we fit a linear model to our standardized phylogenetic independent contrasts there was still a positive significant relationship (p = 0.00078) between plastid genome size and amount noncoding ptDNA, but the strength of the relationship decreased, adjusted R2 = 0.136. The assumptions of homoscedasticity and normality were violated in fitting this linear model, and neither log transformation of the variables nor the removal of the high-leverage outlier Volvox carteri helped us meet these assumptions. Overall, we contend that if more taxa were added to our dataset, this pattern would remain consistent with the past observations that plastid genome size scales positively with the amount of noncoding ptDNA [24].
Figure 1. Amount noncoding ptDNA regressed on plastid genome size with mode of inheritance indicated and amount noncoding ptDNA regressed on plastid genome size with major taxonomic group indicated.
Dashed lines on both figures indicate the 25% and 75% bounds for percent of noncoding DNA in a plastid genome. Analysis was carried out with all taxa (n = 82), and with logged variables. *We present the raw data here with Volvox carteri not pictured for ease of visual display (n = 81).
Plastid Genome Size and Compactness do not Vary Significantly between Taxa with Biparental vs. Uniparental Plastid Inheritance Patterns
her plastid genome size nor the amount of noncoding ptDNA varied significantly with respect to the primary mode of plastid inheritance when only two types of inheritance pattern were considered (uniparental vs. biparental) (plastid genome size: Wilcoxon signed rank test 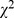 = 2, df = 1, p-value = 0.12; noncoding ptDNA: Wilcoxon signed rank test
= 2, df = 1, p-value = 0.12; noncoding ptDNA: Wilcoxon signed rank test 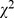 = 2, df = 1, p-value = 0.23). Our analysis of phylogenetic signal strength revealed that the binary trait of mode of plastid inheritance was clumped, D = −0.0052, and the probability that this trait was distributed at random in the phylogeny is effectively zero. This is likely due to the pseudoreplication produced from including multiple species of the same genus (e.g. Oenothera, Pinus, Cuscuta, Picea). Reducing our dataset, by randomly including only one taxon from each of the pseudoreplicated genera produced no significant difference between biparental and uniparental taxa (Wilcoxon signed rank test, df = 1, p-value range = 0.32–0.54). We expected uniparentally-inherited plastids, because of their potential for a reduced Ne, to have more bloated ptDNAs than those with biparentally inherited ones, especially when looking within lineages. Our results suggest that forces other than, or in addition to, inheritance pattern are influencing Ne
(ptDNA) and ultimately shaping plastid genome architecture.
= 2, df = 1, p-value = 0.23). Our analysis of phylogenetic signal strength revealed that the binary trait of mode of plastid inheritance was clumped, D = −0.0052, and the probability that this trait was distributed at random in the phylogeny is effectively zero. This is likely due to the pseudoreplication produced from including multiple species of the same genus (e.g. Oenothera, Pinus, Cuscuta, Picea). Reducing our dataset, by randomly including only one taxon from each of the pseudoreplicated genera produced no significant difference between biparental and uniparental taxa (Wilcoxon signed rank test, df = 1, p-value range = 0.32–0.54). We expected uniparentally-inherited plastids, because of their potential for a reduced Ne, to have more bloated ptDNAs than those with biparentally inherited ones, especially when looking within lineages. Our results suggest that forces other than, or in addition to, inheritance pattern are influencing Ne
(ptDNA) and ultimately shaping plastid genome architecture.
Population bottlenecks can severely reduce the effective population size of a species [25]. Our dataset includes many crop and model species (e.g., Triticum aestivum and Arabidopsis thaliana), including some that show biparental plastid inheritance (e.g., Pisum sativum and Medicago truncatula). In the process of being bred for “desirable traits” or under laboratory conditions, it is likely that these species experienced multiple and frequent bottlenecks, which may have greatly reduced Ne (ptDNA) and canceled out the slight increases in Ne (ptDNA) due to biparental modes of plastid inheritance. Similarly, several of the taxa showing biparental plastid inheritance are the products of hybridizations – events that can alter genome architecture and size [26]. Indeed, the hybrid Pelargonium×hortorum (the garden geranium) has a very large ptDNA genome (217 kb), and one that is thought to have been shaped by one or many hybridization events [27]. In contrast, Geranium palmatum, a close relative of Pelargonium×hortorum but not a hybrid, has a relatively small ptDNA genome (156 kb).
It has also been argued that biparental organelle inheritance as compared to uniparental inheritance is more likely to cause the rapid spread of deleterious cytoplasmic elements (such as a mutant organelle genome with a replication advantage over the wild-type genome) through a sexual population [28]. Although our study was not designed test this particular hypothesis, our observation that ptDNA architecture did not vary significantly with respect to the primary mode of plastid inheritance does not support the view that biparental organelle inheritance promotes the spread of selfish cytoplasmic elements.
Reduced ptDNA Size for Species with Paternally Inherited Plastomes: Lineage Specific Gene Loss or Male-biased Mutation?
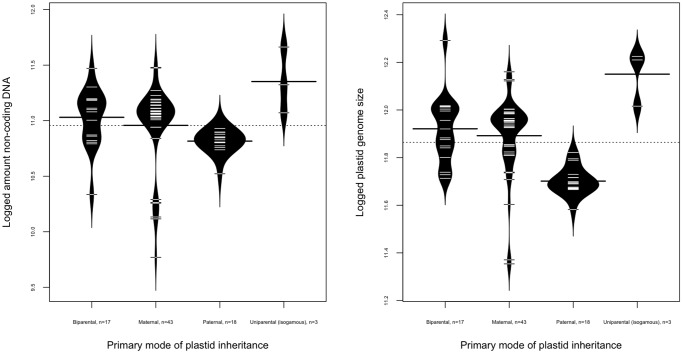
Both plastid genome size and compactness differed significantly with respect to plastid inheritance pattern when four different modes of inheritance were considered: biparental, uniparental isogamous, maternal, and paternal (Fig. 2) (plastid genome size: Kruskal-Wallis 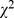 = 30.3, df = 3, p-value = 0.0000012; noncoding ptDNA: Kruskal-Wallis
= 30.3, df = 3, p-value = 0.0000012; noncoding ptDNA: Kruskal-Wallis 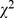 = 19.2, df = 3, p-value = 0.00025). Post-hoc tests revealed that paternally inherited plastid genomes are significantly smaller (plastid genome size) and more compact (amount of noncoding ptDNA) than plastid genomes inherited biparentally, maternally or through uniparental isogamous (critical probability level for post-hoc tests set at p = 0.001).
= 19.2, df = 3, p-value = 0.00025). Post-hoc tests revealed that paternally inherited plastid genomes are significantly smaller (plastid genome size) and more compact (amount of noncoding ptDNA) than plastid genomes inherited biparentally, maternally or through uniparental isogamous (critical probability level for post-hoc tests set at p = 0.001).
Figure 2. Beanplot in left panel depicts the difference in the amount of logged noncoding DNA content between four modes of plastid inheritance.
Beanplot in right panel depicts the difference in the logged total plastome size between the four modes of plastid inheritance. The dashed line in the middle of each of the plots is the overall average of the continuous variable on the y-axis. The thick black line in the middle of each level for the factor of primary inheritance is the median for the continuous variable. The black curved beanpod surrounding the observations “beans” is the theoretical probability density distribution of these observations (n = 78, outliers removed in figure, not analysis).
Are Paternally Inherited ptDNAs Truly Smaller than those Following Other Patterns of Inheritance?
In our dataset, all of the taxa with paternally inherited plastid genomes belong to pinophytes (i.e., conifers). The ptDNAs of pinophytes tend to have fewer NADH dehydrogenase-encoding ndh genes (because of gene loss or gene transfer to the nuclear genome) than those from most other land plant lineages [29], [30], which largely explains their smaller sizes. Gnetophytes, which are close relatives of pinophytes, also have small plastid genomes with a reduced number of ndh genes [31]. However, unlike pinophytes, gnetophytes are believed to have maternally inherited plastids (at least for some Ephedra species) [32], [33], supporting the notion that the small ptDNAs within these two groups are probably the product of gene loss and not plastid inheritance pattern.
That said, male-biased mutation pressure [34]–[36] may also help to explain why pinophytes have smaller plastid genomes. It is well-established that male-biased mutation occurs in the biparentally inherited nuclear genomes of various animal taxa because male germ-lines cells go through many rounds of cell division, which means they are subjected to increased mutation rates compared to female germ-line cells. Female germ-line cells do not typically undergo cell division throughout the lifespan, and so are effectively buffered from the potentially deleterious effects of mutation. However, plants (unlike animals) were long hypothesized not to have a separation between germ-line and somatic cells, yet both nuclear- and plastid-encoded genes that are transferred paternally still undergo greater amounts of mutation compared to those that are maternally transmitted [34]–[36]. It is possible that paternally inherited plastid genomes have higher mutation rates because of male-biased mutation, and thus are potentially subject to more intense selection pressure for genome compaction [5].
Concluding Remarks
Considering all of the data available at present, we have shown that the ptDNA genomic traits of size and compactness do not vary significantly with respect to mode of plastid inheritance, i.e. biparental vs. uniparental modes of inheritance. These observations are not in line with our expectations formulated under the mutational hazard hypothesis. We expected species with uniparentally inherited plastids to be larger and more bloated than biparentally inherited ones – they were not. However, we did find that paternally inherited ptDNAs were more compact and smaller than maternally and biparentally inherited plastid genomes. One hypothesis for this observation is that paternally inherited ptDNAs have a higher mutation rate due to male-biased mutation pressure. If true, this may mean that there is a greater “burden” associated with carrying excess DNA in plastid genomes that are paternally inherited relative to those that are maternally or biparentally inherited.
Acknowledgments
We thank two anonymous reviewers for helpful comments, and M. Lacharité for reviewing an initial draft of the paper.
Funding Statement
DRS is supported by postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada and the Izaak Walton Killam Trusts. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Palmer JD (2003) The symbiotic birth and spread of plastids: how many times and whodunit? Journal of Phycology 39: 4–12. [Google Scholar]
- 2. Keeling PJ (2004) Diversity and evolutionary history of plastids and their hosts. Am J Bot 91: 1481–1493 doi:10.3732/ajb.91.10.1481. [DOI] [PubMed] [Google Scholar]
- 3. Archibald JM (2009) The Puzzle of Plastid Evolution. Current Biology 19: R81–R88 doi:10.1016/j.cub.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 4. Green BR (2011) Chloroplast genomes of photosynthetic eukaryotes. The Plant Journal 66: 34–44 doi:10.1111/j.1365-313X.2011.04541.x. [DOI] [PubMed] [Google Scholar]
- 5.Lynch M (2007) The Origins of Genome Architecture. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 6. Lynch M, Conery JS (2003) The origins of genome complexity. Science 302: 1401–1404 doi:10.1126/science.1089370. [DOI] [PubMed] [Google Scholar]
- 7. Moens P, Gregory TR, Witt JDS (2008) Population size and genome size in fishes: a closer look. Génome 51: 309–313 doi:10.1139/G08-003. [DOI] [PubMed] [Google Scholar]
- 8. Whitney KD, Garland T (2010) Did Genetic Drift Drive Increases in Genome Complexity? PLoS Genet 6: e1001080 doi:10.1371/journal.pgen.1001080.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Birky CW Jr (2001) The inheritance of genes in mitcohondria and chloroplast: Laws, Mechanisms, and Models. Annu Rev Genet 35: 125–148 doi:10.1146/annurev.genet.35.102401.090231. [DOI] [PubMed] [Google Scholar]
- 10.Corriveau JL, Coleman AW (1988) Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am J Bot: 1443–1458. [Google Scholar]
- 11.Harris SA, Ingram R (1991) Chloroplast DNA and biosystematics: the effects of intraspecific diversity and plastid transmission. Taxon: 393–412. [Google Scholar]
- 12. Reboud X, Zeyl C (1994) Organelle inheritance in plants. Heredity 72: 132–140 doi:10.1038/hdy.1994.19. [Google Scholar]
- 13. Zimmermann J, Jahn R, Gemeinholzer B (2011) Barcoding diatoms: evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org Divers Evol 11: 173–192 doi:10.1007/s13127-011-0050-6. [Google Scholar]
- 14. Latta RG, Mitton JB (1997) A comparison of population differentiation across four classes of gene marker in limber pine (Pinus flexilis James). Genetics 146: 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen AK, Escobar LK, Gilbert LE, Jansen RK (2007) Paternal, maternal, and biparental inheritance of the chloroplast genome in Passiflora (Passifloraceae): implications for phylogenetic studies. Am J Bot 94: 42–46 doi:10.3732/ajb.94.1.42. [DOI] [PubMed] [Google Scholar]
- 16. Felsenstein J (1985) Phylogenies and the Comparative Methods. Am Nat 125: 1–15. [Google Scholar]
- 17.Garland T Jr, Harvey P, Ives A (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Systematic Biology. [Google Scholar]
- 18. Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2009) GenBank. Nucl Acids Res 37: D26–D31 doi:10.1093/nar/gkn723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sayers EW, Barrett T, Benson DA, Bryant SH, Canese K, et al. (2009) Database resources of the National Center for Biotechnology Information. Nucl Acids Res 37: D5–D15 doi:10.1093/nar/gkn741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, et al.. (2011) Geneious v5.4. Available:http://www.geneious.com/.
- 21. Purvis A, Rambaut A (1995) Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. Comput Appl Biosci 11: 247–251. [DOI] [PubMed] [Google Scholar]
- 22.Orme D (2011) The caper package: comparative analysis of phylogenetics and evolution in R. [Google Scholar]
- 23. Fritz S, Purvis A (2010) Selectivity in Mammalian Extinction Risk and Threat Types: a New Measure of Phylogenetic Signal Strength in Binary Traits. Conservation Biology 24: 1042–1051 doi:10.1111/j.1523–1739.2010.01455.x. [DOI] [PubMed] [Google Scholar]
- 24. Lynch M, Koskella B, Schaack S (2006) Mutation Pressure and the Evolution of Organelle Genomic Architecture. Science 311: 1727–1730 doi:10.1126/science.1118884. [DOI] [PubMed] [Google Scholar]
- 25. Frankham R, Lees K, Montgomery ME, England PR, Lowe EH, et al. (1999) Do population size bottlenecks reduce evolutionary potential? Animal Conservation 2: 255–260. [Google Scholar]
- 26. Baack EJ, Whitney KD, Rieseberg LH (2005) Hybridization and genome size evolution: timing and magnitude of nuclear DNA content increases in Helianthus homoploid hybrid species. New Phytol 167: 623–630 doi:10.1111/j.1469–8137.2005.01433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chumley TW (2006) The Complete Chloroplast Genome Sequence of Pelargonium×hortorum: Organization and Evolution of the Largest and Most Highly Rearranged Chloroplast Genome of Land Plants. Molecular Biology and Evolution 23: 2175–2190 doi:10.1093/molbev/msl089. [DOI] [PubMed] [Google Scholar]
- 28. Cosmides LM, Tooby J (1981) Cytoplasmic inheritance and intragenomic conflict. J Theor Biol 89: 83–129. [DOI] [PubMed] [Google Scholar]
- 29. Wakasugi T, Tsudzuki J, Ito S, Nakashima K, Tsudzuki T, et al. (1994) Loss of all ndh genes as determined by sequencing the entire chloroplast genome of the black pine Pinus thunbergii. Proc Natl Acad Sci USA 91: 9794–9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braukmann TWA, Kuzmina M, Stefanović S (2009) Loss of all plastid ndh genes in Gnetales and conifers: extent and evolutionary significance for the seed plant phylogeny. Curr Genet 55: 323–337 doi:10.1007/s00294-009-0249-7. [DOI] [PubMed] [Google Scholar]
- 31. Zhong B, Yonezawa T, Zhong Y, Hasegawa M (2010) The Position of Gnetales among Seed Plants: Overcoming Pitfalls of Chloroplast Phylogenomics. Molecular Biology and Evolution 27: 2855–2863 doi:10.1093/molbev/msq170. [DOI] [PubMed] [Google Scholar]
- 32. Carmichael JS, Friedman WE (1995) Double Fertilization in Gnetum gnemon: The Relationship between the Cell Cycle and Sexual Reproduction. Plant Cell 7: 1975–1988 doi:10.1105/tpc.7.12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogensen HL (1996) The hows and whys of cytoplasmic inheritance in seed plants. Am J Bot: 383–404. [Google Scholar]
- 34. Whittle C-A, Johnston MO (2002) Male-driven evolution of mitochondrial and chloroplastidial DNA sequences in plants. Molecular Biology and Evolution 19: 938–949. [DOI] [PubMed] [Google Scholar]
- 35. Whittle C-A, Johnston MO (2003) Male-biased transmission of deleterious mutations to the progeny in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 4055–4059 doi:10.1073/pnas.0730639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whittle C-A, Malik MR, Krochko JE (2007) Gender-specific selection on codon usage in plant genomes. BMC Genomics 8: 169 doi:10.1186/1471-2164-8-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumari M, Clarke HJ, Francs-Small des CC, Small I, Khan TN, et al. (2011) Albinism does not correlate with biparental inheritance of plastid DNA in interspecific hybrids in Cicer species. Plant Science 180: 628–633 doi:10.1016/j.plantsci.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 38. Peters AF, Scornet D, Müller DG, Kloareg B, Cock JM (2004) Inheritance of organelles in artificial hybrids of the isogamous multicellular chromist alga Ectocarpus siliculosus(Phaeophyceae). European Journal of Phycology 39: 235–242 doi:10.1080/09670260410001683241. [Google Scholar]
- 39. Motomura T, Nagasato C, Kimura K (2010) Cytoplasmic inheritance of organelles in brown algae. J Plant Res 123: 185–192 doi:10.1007/s10265-010-0313-x. [DOI] [PubMed] [Google Scholar]
- 40. Renzaglia K, Dengate S, Schmitt S, Duckett J (2002) Novel features of Equisetum arvense spermatozoids: insights into pteridophyte evolution. New Phytol 154: 159–174. [Google Scholar]
- 41.Zanmin H, Shiyi H, Jinzhong Z (1996) Paternal inheritance of plastid DNA in genus Pharbitis. Acta Botanica Sinica 38. [Google Scholar]
- 42.Hu Z-M, Hu S-Y (1995) Study on organelle DNA within the generative cell and sperm cells in Pharbitis. Acta Botanica Sinica: 1–6. [Google Scholar]
- 43. Matsushima R, Hu Y, Toyoda K, Sodmergen, Sakamoto W (2008) The Model Plant Medicago truncatula Exhibits Biparental Plastid Inheritance. Plant and Cell Physiology 49: 81–91 doi:10.1093/pcp/pcm170. [DOI] [PubMed] [Google Scholar]
- 44. Corriveau JL, Coleman AW (1990) Plastid inheritance in Oenothera: paternal input may influence transmission patterns. Curr Genet 17: 327–330. [Google Scholar]
- 45. Baur E (1909) Das Wesen und die Erblichkeit sver- haltnisse der “Varietates albomarginatae hort” von Pelargonium zonale. Z Indukt Abstammungs-Ver- erbungsl 1: 330–351. [Google Scholar]
- 46. Schmit V, Jardin P, Baudoin J, Debouck D (1993) Use of chloroplast DNA polymorphisms for the phylogenetic study of seven Phaseolus taxa including P. vulgaris and P. coccineus. TAG Theoretical and Applied Genetics 87: 506–516. [DOI] [PubMed] [Google Scholar]
- 47. Corriveau JL, Polans NO, Coleman AW (1989) Cultivar variability for the presence of plastid DNA in pollen of Pisum sativum L.: implications for plastid transmission. Curr Genet 16: 47–51. [DOI] [PubMed] [Google Scholar]
- 48. Bogdanova VS, Galieva ER, Kosterin OE (2008) Genetic analysis of nuclear-cytoplasmic incompatibility in pea associated with cytoplasm of an accession of wild subspecies Pisum sativum subsp. elatius (Bieb.) Schmahl. Theoret Appl Genetics 118: 801–809 doi:10.1007/s00122-008-0940-y. [DOI] [PubMed] [Google Scholar]
- 49.Bogdanova VS, Galieva ER, Yadrikhinskiy AK, Kosterin OE (2012) Inheritance and genetic mapping of two nuclear genes involved in nuclear–cytoplasmic incompatibility in peas (Pisum sativum L.). Theoret Appl Genetics. doi:10.1007/s00122-012-1804-z. [DOI] [PubMed]
- 50. Renzaglia KS, Johnson TH, Gates HD, Whittier DP (2001) Architecture of the sperm cell of Psilotum. Am J Bot 88: 1151–1163. [PubMed] [Google Scholar]
- 51. Renzaglia KS, Bernhard DL, Garbary DJ (1999) Developmental Ultrastructure of the Male Gamete of Selaginella. Int J Plant Sci 160: 14–28 doi:10.1086/314103. [Google Scholar]
- 52. Zhang Q, Liu Y (2003) Sodmergen (2003) Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant and Cell Physiology 44: 941–951. [DOI] [PubMed] [Google Scholar]
- 53. Ruf S, Hermann M, Berger IJ, Carrer H, Bock R (2001) Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat Biotechnol 19: 870–875 doi:10.1038/nbt0901-870. [DOI] [PubMed] [Google Scholar]
- 54. Azhagiri AK, Maliga P (2007) Exceptional paternal inheritance of plastids in Arabidopsis suggests that low-frequency leakage of plastids via pollen may be universal in plants. The Plant Journal 52: 817–823 doi:10.1111/j.1365-313X.2007.03278.x. [DOI] [PubMed] [Google Scholar]
- 55. Kuroiwa T, Hori T (1986) Preferential digestion of male chloroplast nuclei and mitochondrial nuclei during gametogenesis of Bryopsis maxima Okamura. Protoplasma 133: 85–87 doi:10.1007/BF01293191. [Google Scholar]
- 56. Droogenbroeck B, Maertens I, Haegeman A, Kyndt T, O’Brien C, et al. (2005) Maternal inheritance of cytoplasmic organelles in intergeneric hybrids of Carica papaya L. and Vasconcellea spp. (Caricaceae Dumort., Brassicales). Euphytica 143: 161–168 doi:10.1007/s10681-005-3156-0. [Google Scholar]
- 57. Sun GH, Uyeda TQP, Kuroiwa T (1988) Destruction of organelle nuclei during spermatogenesis in Chara corallina examined by staining with DAPI and anti-DNA antibody. Protoplasma 144: 185–188. [Google Scholar]
- 58.Gastony GJ, Yatskievych G (1992) Maternal inheritance of the chloroplast and mitochondrial genomes in cheilanthoid ferns. Am J Bot: 716–722. [Google Scholar]
- 59. Havey M, McCreight J, Rhodes B, Taurick G (1998) Differential transmission of the Cucumis organellar genomes. TAG Theoretical and Applied Genetics 97: 122–128. [Google Scholar]
- 60. Zhong Z-R, Li N, Qian D, Jin J-H, Chen T (2011) Maternal inheritance of plastids and mitochondria in Cycas L. (Cycadaceae). Mol Genet Genomics 286: 411–416 doi:10.1007/s00438-011-0653-9. [DOI] [PubMed] [Google Scholar]
- 61. Boblenz K, Nothnagel T, Metzlaff M (1990) Paternal inheritance of plastids in the genus Daucus. Molecular and General Genetics 220: 489–491. [Google Scholar]
- 62. Vivek BS, Ngo QA, Simon PW (1999) Evidence for maternal inheritance of the chloroplast genome in cultivated carrot (Daucus carota L. ssp. sativus ). Theoret Appl Genetics 98: 669–672 doi:10.1007/s001220051119. [Google Scholar]
- 63. Ferguson DJP, Campbell SA, Henriquez FL, Phan L, Mui E, et al. (2007) Enzymes of type II fatty acid synthesis and apicoplast differentiation and division in Eimeria tenella. Int J Parasitol 37: 33–51 doi:10.1016/j.ijpara.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McKinnon GE, Vaillancourt RE, Tilyard PA, Potts BM (2001) Maternal inheritance of the chloroplast genome in Eucalyptus globulus and interspecific hybrids. Genome 44: 831–835 doi:10.1139/gen-44-5-831. [PubMed] [Google Scholar]
- 65. Reinhard A, Reavey P, Lin J, Zhang H (2009) Chloroplast DNA inheritance, ancestry, and sequencing in Fragaria. Acta Horticulturae 859: 221–228. [Google Scholar]
- 66. Brawley SH, Wetherbee R, Quatrano RS (1976) Fine-structural studies of the gametes and embryo of Fucus vesiculosus L. (Phaeophyta). II. The cytoplasm of the egg and young zygote. J Cell Sci 20: 255–271. [DOI] [PubMed] [Google Scholar]
- 67. Lee C (1955) Fertilization in Ginkgo biloba. Botanical Gazette 117: 79–100. [Google Scholar]
- 68. Kumar S, Dhingra A, Daniell H (2004) Stable transformation of the cotton plastid genome and maternal inheritance of transgenes. Plant Mol Biol 56: 203–216 doi:10.1007/s11103-004-2907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bouzon Z, Miguens F (2000) Male gametogenesis in the red algae Gracilaria and Gracilariopsis (Rhodophyta, Gracilariales). Cryptogamie Algologie 21. [Google Scholar]
- 70. Wills DM, Hester ML, Liu A, Burke JM (2005) Chloroplast SSR polymorphisms in the Compositae and the mode of organellar inheritance in Helianthus annuus. Theoret Appl Genetics 110: 941–947 doi:10.1007/s00122-004-1914-3. [DOI] [PubMed] [Google Scholar]
- 71. Mogensen H, Rusche ML (1985) Quantitative ultrastructural analysis of barley sperm. Protoplasma 128: 1–13. [Google Scholar]
- 72. Mogensen HL (1988) Exclusion of male mitochondria and plastids during syngamy in barley as a basis for maternal inheritance. Proc Natl Acad Sci USA 85: 2594–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pacini E, Taylor P, Singh M, Knox R (1992) Development of plastids in pollen and tapetum of rye-grass, Lolium perenne L. Annals of Botany. 70: 179–188. [Google Scholar]
- 74. Huang CY, Ayliffe MA, Timmis JN (2003) Direct measurement of the transfer rate of chloroplast DNA into the nucleus. Nature 422: 72–76 doi:10.1038/nature01435. [DOI] [PubMed] [Google Scholar]
- 75. Amane M, Lumaret R, Hany V, Ouazzani N, Debain C, et al. (1999) Chloroplast-DNA variation in cultivated and wild olive (Olea europaea L.). TAG Theoretical and Applied Genetics 99: 133–139. [Google Scholar]
- 76. Caha C, Lee DJ, Martínez-Reyna J, Vogel K (2001) Meiotic stability, chloroplast DNA polymorphisms, and morphological traits of upland× lowland switchgrass reciprocal hybrids. Crop Science 41: 1579–1583. [Google Scholar]
- 77. Mejnartowicz M (1991) Inheritance of chloroplast DNA in Populus. TAG Theoretical and Applied Genetics 82: 477–480. [DOI] [PubMed] [Google Scholar]
- 78. Niwa K, Kobiyama A (2010) Interspecific hybridization in the haploid blade-forming marine crop Porphyra (Bangiales, Rhodophyta): occurrence of allopolyploidy in surviving F1 gametophytic blades. Journal of Phycology 46: 693–702. [Google Scholar]
- 79. Olson MS, McCauley DE (2000) Linkage disequilibrium and phylogenetic congruence between chloroplast and mitochondrial haplotypes in Silene vulgaris. Proceedings of the Royal Society B: Biological Sciences 267: 1801–1808 doi:10.1098/rspb.2000.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ferguson DJP, Henriquez FL, Kirisits MJ, Muench SP, Prigge ST, et al. (2005) Maternal inheritance and stage-specific variation of the apicoplast in Toxoplasma gondii during development in the intermediate and definitive host. Eukaryotic Cell 4: 814–826 doi:10.1128/EC.4.4.814-826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Adams CR, Stamer KA, Miller JK, McNally JG, Kirk MM, et al. (1990) Patterns of organellar and nuclear inheritance among progeny of two geographically isolated strains of Volvox carteri. Curr Genet 18: 141–153. [DOI] [PubMed] [Google Scholar]
- 82. Conde MF, Pring D, Levings C III (1979) Maternal inheritance of organelle DNA’s in Zea mays-Zea perennis reciprocal crosses. Journal of Heredity 70: 2–4. [Google Scholar]
- 83. Chen J, Tauer C, Huang Y (2002) Paternal chloroplast inheritance patterns in pine hybrids detected with trnL-trnF intergenic region polymorphism. Theoret Appl Genetics 104: 1307–1311 doi:10.1007/s00122-002-0893-5. [DOI] [PubMed] [Google Scholar]
- 84. Guo F, Hu S-Y, Yuan Z, Zee S-Y, Han Y (2005) Paternal cytoplasmic transmission in Chinese pine (Pinus tabulaeformis). Protoplasma 225: 5–14 doi:10.1007/s00709-005-0088-4. [DOI] [PubMed] [Google Scholar]
- 85.Neale DB, Wheeler NC, Allard RW (1986) Paternal inheritance of chloroplast DNA in Douglas-fir. http://dxdoiorg/101139/x86-205.
- 86. Neale D, Sederoff R (1989) Paternal inheritance of chloroplast DNA and maternal inheritance of mitochondrial DNA in loblolly pine. TAG Theoretical and Applied Genetics 77: 212–216. [DOI] [PubMed] [Google Scholar]
- 87. Dong J, Wagner D, Yanchuk A, Carlson M, Magnussen S, et al. (1992) Paternal chloroplast DNA inheritance in Pinus consora and Pinus banksiana: independence of parenetal species or cross direction. Journal of Heredity 83: 419–422. [Google Scholar]
- 88. Ohba K, Iwakawa M, Okada Y, Murai M (1971) Paternal transmission of a plastid anomaly in some reciprocal crosses of Sugi, Cryptomeria japonica D. Don. Silvae Genet 20: 101–107. [Google Scholar]
- 89. Szmidt AE, Aldén T, Hällgren JE (1987) Paternal inheritance of chloroplast DNA in Larix. Plant Mol Biol 9: 59–64. [DOI] [PubMed] [Google Scholar]
- 90.Sutton BCS, Flanagan DJ, Gawley JR, Newton CH, Lester DT, et al.. (1991) Inheritance of chloroplast and mitochondrial DNA in Picea and composition of hybrids from introgression zones. Theoret Appl Genetics 82. doi:10.1007/BF00226220. [DOI] [PubMed]
- 91. Lee RW, Jones RF (1973) Induction of Mendelian and non-Mendelian streptomycin resistant mutants during the synchronous cell cycle of Chlamydomonas reinhardtii. Molecular and General Genetics 121: 99–108. [DOI] [PubMed] [Google Scholar]
- 92. Suda S, Watanabe MM, Inouye I (2004) Electron microscopy of sexual reproduction in Nephroselmis olivacea (Prasinophyceae, Chlorophyta). Phycological Res 52: 273–283 doi:10.1111/j.1440-183.2004.00346.x. [Google Scholar]
- 93. Miyamura S (2010) Cytoplasmic inheritance in green algae: patterns, mechanisms and relation to sex type. J Plant Res 123: 171–184 doi:10.1007/s10265-010-0309-6. [DOI] [PubMed] [Google Scholar]