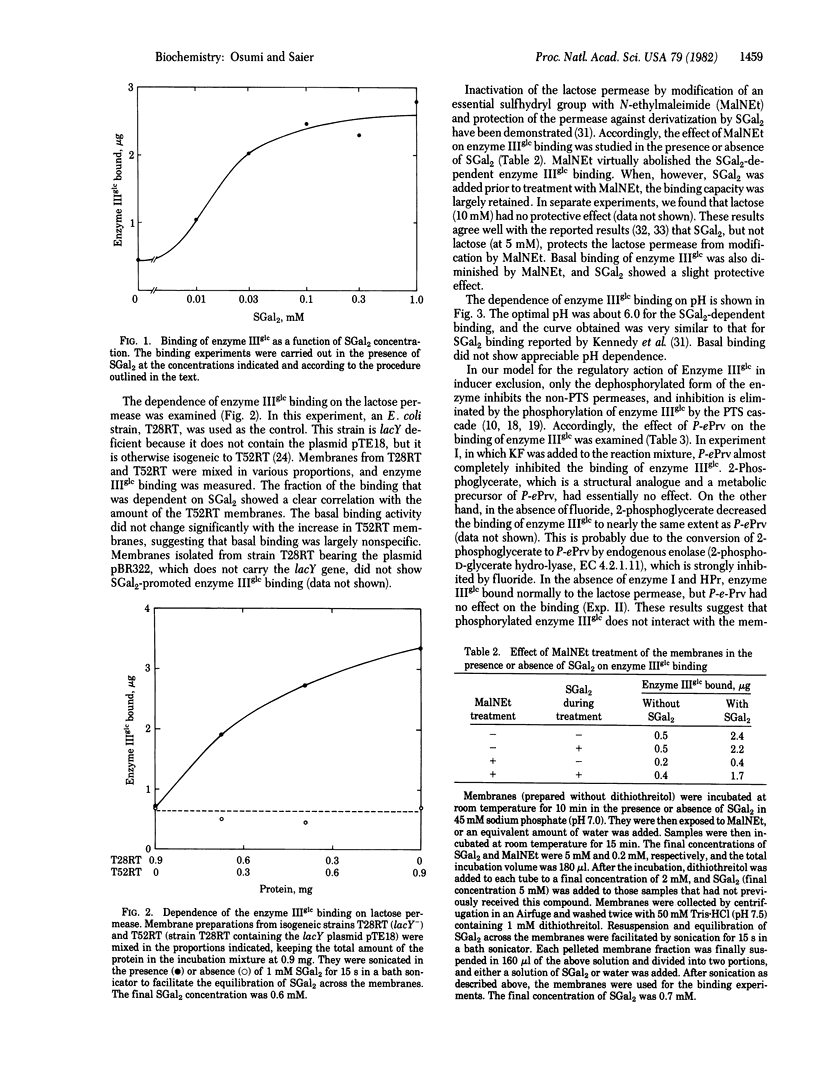
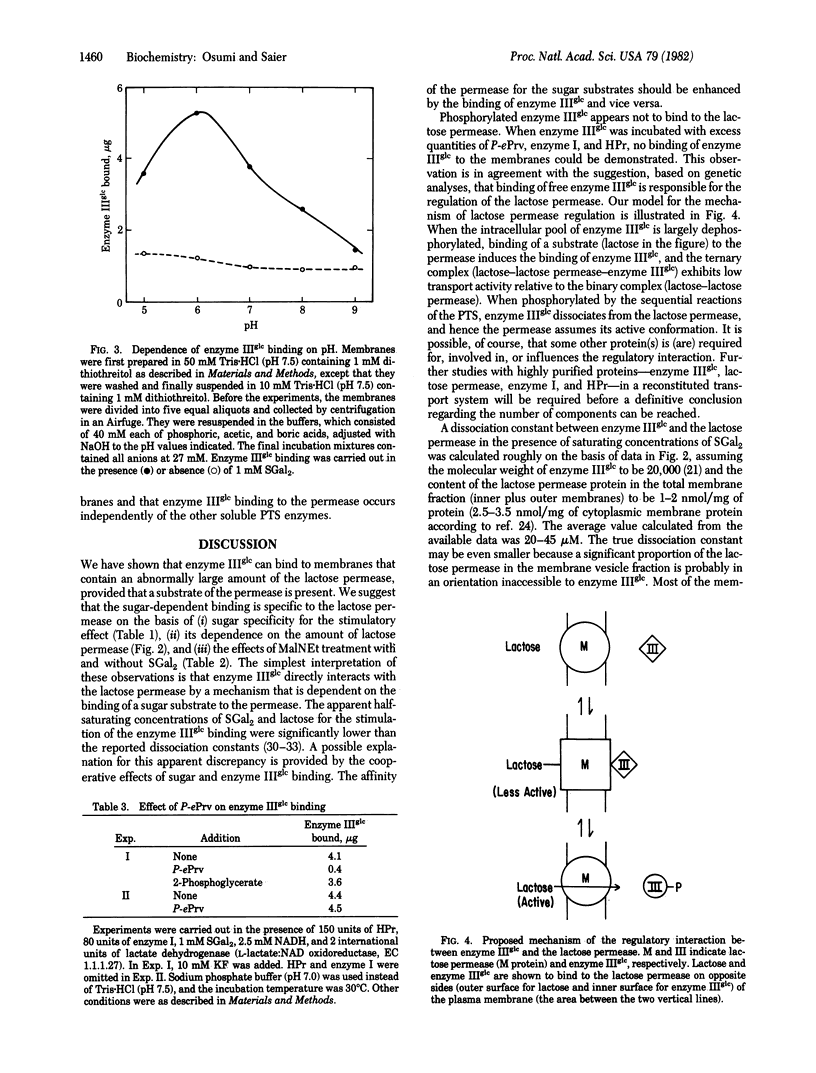
Abstract
Interaction between the glucose-specific enzyme III (enzyme IIIglc) of the phosphoenolpyruvate:sugar phosphotransferase system and the lactose permease was studied with membrane fragments from an Escherichia coli strain that overproduces the lactose permease. Substrates of the permease markedly and specifically stimulated binding of enzyme IIIglc to the membranes. The sugar-stimulated binding of enzyme IIIglc was concluded to the specific to the lactose permease because it (i) was dependent on the amount of the permease, (ii) was promoted only by sugar substrates of the permease, and (iii) was completely eliminated by treatment of the membranes with N-ethylmaleimide in the absence (but not the presence) of thio-beta-D-digalactoside. The pH dependence of binding was similar to that reported for the binding of thio-beta-D-digalactoside to the permease. Phosphoenolpyruvate prevented the binding of enzyme IIIglc to the lactose permease in the presence (but not the absence) of the other phosphate transfer components of the phosphotransferase system. These results support the hypothesis that enzyme IIIglc, in its dephosphorylated form, modulates the activity of the lactose permease by a direct protein-protein interaction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter J. R., Fox C. F., Kennedy E. P. Interaction of sugars with the membrane protein component of the lactose transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Jun;60(2):725–732. doi: 10.1073/pnas.60.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro L., Feucht B. U., Morse M. L., Saier M. H., Jr Regulation of carbohydrate permeases and adenylate cyclase in Escherichia coli. Studies with mutant strains in which enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system is thermolabile. J Biol Chem. 1976 Sep 25;251(18):5522–5527. [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. FACTORS INFLUENCING THE ENZYMIC ACTIVITIES OF BACTERIA. Bacteriol Rev. 1943 Sep;7(3):139–173. doi: 10.1128/br.7.3.139-173.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Hinkle P. C. Oxidative phosphorylation and proton translocation in membrane vesicles prepared from Escherichia coli. Biochem Biophys Res Commun. 1974 May 7;58(1):178–184. doi: 10.1016/0006-291x(74)90908-5. [DOI] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Armstrong J. B. Dierect measurement of the binding of labeled sugars to the lactose permease M protein. J Biol Chem. 1974 Jan 10;249(1):33–37. [PubMed] [Google Scholar]
- Kundig W. Molecular interactions in the bacterial phosphoenolpyruvate-phosphotransferase system (PTS). J Supramol Struct. 1974;2(5-6):695–814. doi: 10.1002/jss.400020514. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. A., Jacobson G. R., Saier M. H., Jr Plasmid-directed synthesis of enzymes required for D-mannitol transport and utilization in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7336–7340. doi: 10.1073/pnas.78.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Hofstadter L. J. Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):883–892. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Regulation of carbohydrate transport activities in Salmonella typhimurium: use of the phosphoglycerate transport system to energize solute uptake. J Bacteriol. 1980 Feb;141(2):611–617. doi: 10.1128/jb.141.2.611-617.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J Biol Chem. 1976 Nov 10;251(21):6598–6605. [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R., Lin P. Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Kinetic characterization. J Biol Chem. 1980 Sep 25;255(18):8579–8584. [PubMed] [Google Scholar]
- Saier M. H., Jr, Straud H., Massman L. S., Judice J. J., Newman M. J., Feucht B. U. Permease-specific mutations in Salmonella typhimurium and Escherichia coli that release the glycerol, maltose, melibiose, and lactose transport systems from regulation by the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1978 Mar;133(3):1358–1367. doi: 10.1128/jb.133.3.1358-1367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Roseman S. Inducer exclusion and repression of enzyme synthesis in mutants of Salmonella typhimurium defective in enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1972 Feb 10;247(3):972–975. [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]


