Abstract
Enterohemorrhagic Escherichia coli (EHEC) is dependent on acid resistance for gastric passage and low oral infectious dose, and the locus of enterocyte effacement (LEE) for intestinal colonization. Mutation of rpoN, encoding sigma factor N (σN), dramatically alters the growth-phase dependent regulation of both acid resistance and the LEE. This study reports on the determinants of σN-directed acid resistance and LEE expression, and the underlying mechanism attributable to this phenotype. Glutamate-dependent acid resistance (GDAR) in TW14359ΔrpoN correlated with increased expression of the gadX-gadW regulatory circuit during exponential growth, whereas upregulation of arginine-dependent acid resistance (ADAR) genes adiA and adiC in TW14359ΔrpoN did not confer acid resistance by the ADAR mechanism. LEE regulatory (ler), structural (espA and cesT) and effector (tir) genes were downregulated in TW14359ΔrpoN, and mutation of rpoS encoding sigma factor 38 (σS) in TW14359ΔrpoN restored acid resistance and LEE genes to WT levels. Stability, but not the absolute level, of σS was increased in TW14359ΔrpoN; however, increased stability was not solely attributable to the GDAR and LEE expression phenotype. Complementation of TW14359ΔrpoN with a σN allele that binds RNA polymerase (RNAP) but not DNA, did not restore WT levels of σS stability, gadE, ler or GDAR, indicating a dependence on transcription from a σN promoter(s) and not RNAP competition for the phenotype. Among a library of σN enhancer binding protein mutants, only TW14359ΔntrC, inactivated for nitrogen regulatory protein NtrC, phenocopied TW14359ΔrpoN for σS stability, GDAR and ler expression. The results of this study suggest that during exponential growth, NtrC-σN regulate GDAR and LEE expression through downregulation of σS at the post-translational level; likely by altering σS stability or activity. The regulatory interplay between NtrC, other EBPs, and σN–σS, represents a mechanism by which EHEC can coordinate GDAR, LEE expression and other cellular functions, with nitrogen availability and physiologic stimuli.
Introduction
Enterohemorrhagic Escherichia coli (EHEC) is an enteric pathogen commonly implicated in food-borne outbreaks of hemorrhagic colitis, and in the life-threatening illness hemolytic uremic syndrome [1]–[3]. To cause disease in humans, EHEC must overcome two formidable innate barriers to infection: the acidity of the stomach, and competition for intestinal colonization sites. For the former, EHEC (and other E. coli) has evolved multiple discrete acid resistance mechanisms [4], which allow for survival in highly acidic environments such as the stomach, and which determine a low oral infectious dose [5], [6]. For competitive gut colonization, EHEC utilize a type III secretion system (T3SS) encoded on the locus of enterocyte effacement (LEE) pathogenicity island [7]–[10]. This T3SS translocates EHEC effector proteins into host intestinal cells that mediate intimate attachment to the gut and subvert host cellular processes [11].
The expression of acid resistance and the LEE is influenced by various environmental and intracellular signals, including nutrient availability, stress, and growth phase [12]–[21]. During exponential growth acid resistance is largely repressed, but is activated as cultures transition into stationary phase [13]; for the LEE, the inverse is true [18]. This pattern of expression may reflect the importance of colonization and replication when resources are abundant, and that of stress durability when they are scarce. Many auxiliary regulators communicate these changes in growth conditions to regulatory components of both acid resistance and the LEE [12], [22]–[28]. Alternative sigma factor 38 (σS) is a global regulator that plays an important role in coordinating acid resistance and LEE expression with growth phase. σS is a protein of low abundance during exponential growth, but accumulates during transition into stationary phase [29]. The acid resistance phenotype of stationary phase cultures is largely attributed to σS and expectedly, strains mutated for rpoS (encoding σS) are sensitive to acid [13], [14], whereas LEE expression is both decreased and increased in response to rpoS mutation, depending on growth conditions [28], [30]–[32]. Not surprisingly, rpoS mutants are impaired in their ability to survive passage in both murine and bovine models of infection [33]. σS is tightly regulated at multiple levels of control [34], and the factors that dictate rpoS/σS expression indirectly influence acid resistance, the LEE, and EHEC pathogenesis.
Recently, another alternative sigma factor, sigma N (σN), has been shown to control structural and regulatory genes of both acid resistance and the LEE in EHEC serotype O157:H7 [35]. When bound to RNA polymerase (RNAP), the RNAP-σN holoenzyme (EσN) directs transcription from an estimated twenty-one promoters in E. coli which specify the transcription of over sixty genes involved in nitrogen and carbon metabolism, and stress resistance [36]–[39]. EHEC strains null for rpoN (encoding σN) express elevated levels of acid resistance genes belonging to the glutamate-dependent acid resistance (GDAR) system, and reduced levels of expression for genes encoded on all five operons of the LEE [35]. This altered expression of GDAR and LEE genes is restricted to exponential phase cultures. Furthermore, GDAR upregulation in rpoN mutants is correlated with increased survival in acidic environments, and is dependent on an intact rpoS gene, suggesting that GDAR is controlled by an as yet uncharacterized σN–σS regulatory pathway in E. coli [35].
There is precedent for such a pathway in Borrelia burgdorferi, in which a σN–σS regulatory pathway controls the expression of membrane lipoproteins essential for transmission and pathogenesis [40]–[42]. In the B. burgdorferi model, σN has been shown to directly activate rpoS transcription, which is contrary to E. coli in which rpoS inactivation abrogates the GDAR phenotype of an rpoN null mutant, suggesting that σN downregulates rpoS/σS by some unknown mechanism. There is evidence that this negative regulation is at the post-transcriptional level, as rpoN mutation does not alter rpoS mRNA levels [35]. In addition, a recent study reported increased levels and stability of σS in an rpoN mutant of the nonpathogenic E. coli strain K-12 MG1655 [43]. This study further explores the regulatory interplay of σN and σS, and uncovers mechanistic details about σN–σS directed control of acid resistance and the LEE, and other genetic factors which contribute to the expression of this regulatory pathway.
Results
σN–σS Directed Regulation of Glutamate-dependent Acid Resistance and the Locus of Enterocyte Effacement
Independent regulatory pathways control glutamate-dependent acid resistance (GDAR) genes in response to discrete environmental stimuli through transcriptional modulation of the central regulator gadE. These include pathways that stimulate gadE during exponential growth in minimal, acidified media (EvgAB-YdeO) [16], [44], or during stationary phase growth in rich media (σS-GadX-GadW) [12], or rich media containing glucose (TrmE) [15]. The growth conditions under which rpoN-dependent acid resistance is expressed do not conform precisely to any of these stimulating environments. And yet, mutation of rpoS in an rpoN null background suppresses GDAR, suggesting that in the WT background σN negatively regulates GDAR through a σS-dependent pathway; namely, σS-GadX-GadW. To explore this further, transcript levels of GDAR regulatory genes from these activating circuits were measured in WT and mutant backgrounds of TW14359 during exponential growth.
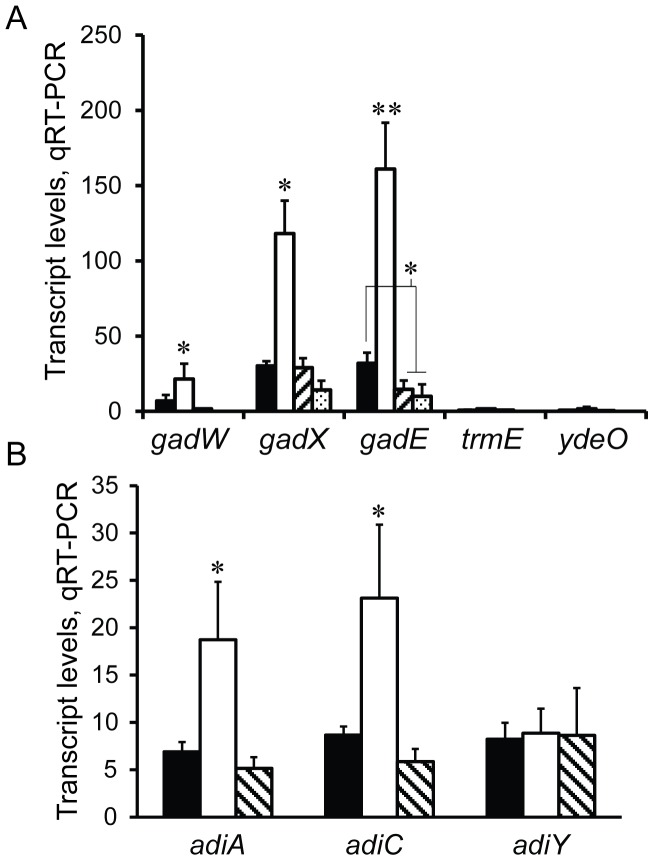
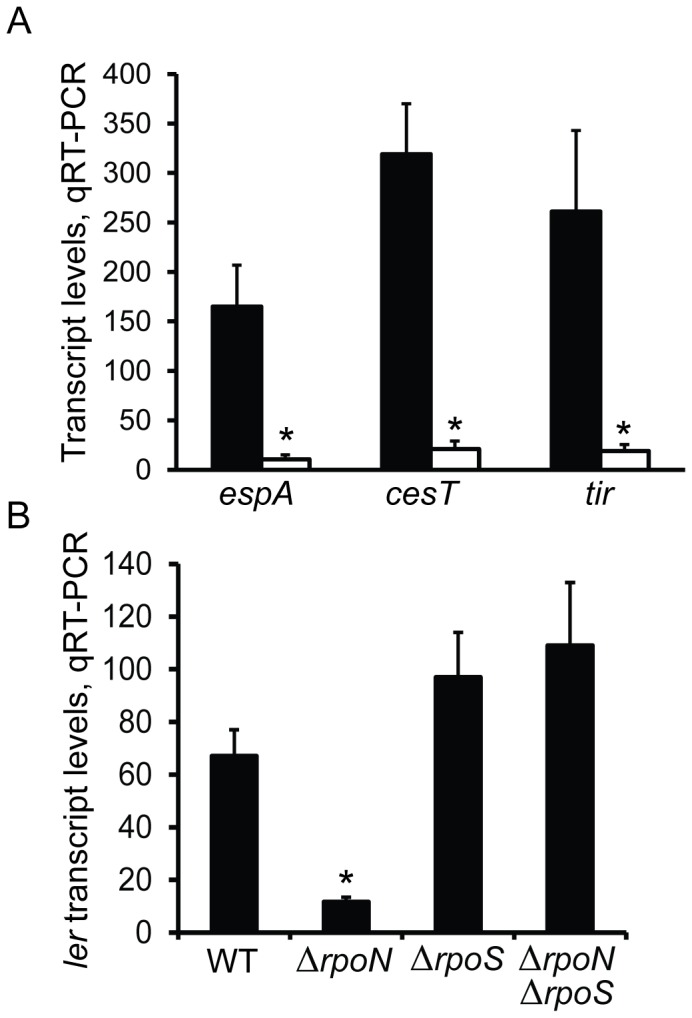
As anticipated, gadE transcript levels were significantly higher in TW14359ΔrpoN compared to TW14359 (p = 0.001), as well as TW14359ΔrpoS (p = 0.007), and TW14359ΔrpoNΔrpoS (p = 0.005) (Fig. 1A). Adding to this, both gadX and gadW transcripts were upregulated in TW14359ΔrpoN (p<0.05), but not in TW14359ΔrpoS for gadX, or TW14359ΔrpoNΔrpoS for either gadX or gadW. Transcript levels for trmE and ydeO, key regulators of alternative pathways for gadE activation, were in low abundance, and did not differ significantly between strains (Fig. 1A); the presence of amplicons for trmE and ydeO was validated by gel electrophoresis. Thus, a rpoN null mutation leads to increased expression of the GDAR-activating GadX-GadW pathway, agreeing with the rpoS-dependency of the phenotype.
Figure 1. Transcript levels for acid resistance genes.
Gene transcript levels as determined by qRT-PCR are plotted for genes of the GDAR system (panel A) and genes of the ADAR system (panel B). Mean transcript levels are normalized to the 16S rRNA gene rrsH. Transcript levels are plotted against WT TW14359 (filled), TW14359ΔrpoN (empty), TW14359ΔrpoN ΔrpoS (hatched), and TW14359ΔrpoS (stippled, gadX and gadE only) for panel A. Asterisks denote significant differences by Tukey’s HSD following a significant F-test (n≥3, p<0.05 [*]; p<0.01 [**]). Error bars indicate standard error of the mean.
In addition to GDAR, σS regulates at least two more acid resistance systems in E. coli: the arginine-dependent acid resistance (ADAR) system [45], and the oxidative-dependent acid resistance (ODAR) system [33]. Both GDAR and ADAR systems protect the cell from acid by a proton scavenging mechanism that is facilitated by the conversion of glutamate to γ-aminobutyric acid (GDAR) or arginine to agmatine (ADAR), and catalyzed by amino acid decarboxylases. ODAR on the other hand does not require glutamate or arginine, and is repressed by glucose [4]. Except for rpoS, the regulatory and structural determinants of ODAR are not well understood, and thus were not investigated in this study. For ADAR, the structural genes adiA (arginine decarboxylase) and adiC (arginine-agmatine exchanger) were slightly but significantly upregulated in TW14359ΔrpoN relative to TW14359 and TW14359ΔrpoNΔrpoS (p<0.05) (Fig. 1B). However, adiY, encoding a putative regulator of adiA and adiC [46], was not altered in expression in either of the mutant backgrounds. Despite the increase in adiA and adiC expression in TW14359ΔrpoN, there was no corresponding increase in acid resistance by the ADAR mechanism (Table 1), and exclusion of either glutamate or arginine from acidified EG media resulted in no growth for any strains (data not shown). Therefore the only known requirements for rpoN-dependent acid resistance are rpoS, gadE, and glutamate.
Table 1. Acid resistance by the GDAR and ADAR mechanisms.
| Percent survival (SD)a | |||
| Growth condition | Strain/genotype | GDAR | ADAR |
| DMEM | TW14359 | <0.01b | <0.01 |
| TW14359ΔrpoN | 24.2 (0.24) | <0.01 | |
| TW14359ΔfhlA | 21.2 (0.31) | NDc | |
| TW14359ΔglnG | 15.7 (1.88) | ND | |
| TW14359ΔrpoNΔrpoS | <0.01 | <0.01 | |
| TW14359ΔrpoN pRAM-1 | 0.141 (0.11) | 0.125 (0.79) | |
| TW14359ΔrpoN pRAM-2 | 10.61 (1.22) | ND | |
| DMEM +3, 4-DCId | TW14359 | <0.01 | ND |
| TW14359ΔrpoN | 29.1 (9.3) | ND | |
Percent survival by the glutamate-dependent (GDAR) and arginine-dependent (ADAR) acid resistance system; standard deviation (SD).
Less than 10 CFU/ml remains following 1 h exposure to acidified GDAR or ADAR test environment.
Not determined (ND).
DMEM growth media with addition of 5 µM 3,4-dichloroisocoumarin (3,4-DCI).
σS has also been shown to upregulate and downregulate transcription of LEE genes in EHEC. For upregulation, σS is hypothesized to enhance expression of the central regulator of the LEE, ler (encoded on operon LEE1), in a manner dependent on the non-coding RNA DsrA [28]. It has also been reported that both the LEE3 and LEE5 operons possess σS-responsive promoters [30]. For downregulation, σS is proposed to stimulate an unknown repressor of PchA, which is a positive regulator of ler [31], [32], [47]. The mutation of rpoN leads to the downregulation of LEE genes during exponential growth [35]. Since σN controls GDAR through a σS-dependent pathway, it was predicted that σN-directed regulation of the LEE may be similarly dependent on rpoS. As expected, transcript levels for LEE genes encoding the T3SS translocon component espA (encoded on LEE4), the effector chaperone cesT (on LEE5), and the translocated intimin receptor tir (on LEE5) were downregulated during exponential growth of TW14359ΔrpoN relative to TW14359 (p<0.05) (Fig. 2A). In addition, transcript levels of ler (on LEE1) were reduced in TW14359ΔrpoN compared to TW14359 (p = 0.015) and TW14359ΔrpoS (p = 0.011) (Fig. 2B). Importantly, mutation of rpoS in TW14359ΔrpoN restored ler expression to levels consistent with TW14359ΔrpoS; ler expression was increased in rpoS null backgrounds relative to WT, but not significantly increased. These results indicate that σN positively regulates the LEE during exponential growth in an rpoS-dependent manner, and is consistent with the role of σS as a negative regulator of LEE expression via the PchA-Ler pathway [31], [32], [47].
Figure 2. Transcript levels for LEE genes.
(Panel A): gene transcript levels as determined by qRT-PCR are plotted for representative LEE genes in WT TW14359 (filled) and TW14359ΔrpoN (empty). (Panel B): ler transcript levels by qRT-PCR are plotted against TW14359 and various mutant derivative strains of TW14359. Mean transcript levels are normalized to the 16S rRNA gene rrsH. For panel A, an asterisk denotes a significant difference between TW14359 and TW14359ΔrpoN for each gene by Welch’s t-test (n≥3, p<0.05). For panel B, the asterisk denotes a significant difference between TW14359ΔrpoN and the remaining strains by Tukey’s HSD following a significant F-test (n≥3, p<0.05). Error bars indicate standard error of the mean.
Effect of rpoN Mutation on rpoS mRNA and σS Stability in EHEC
There is evidence that the mutation of rpoN in EHEC does not alter rpoS mRNA levels, but instead leads to post-transcriptional alternations in rpoS/σS [35]. The mutation of rpoN in E. coli strain K-12 MG1655 was recently shown to lead to increased σS levels and stability [43]. However, there are substantial differences at the genomic level between K-12 and EHEC O157:H7 strains [48]. As an important example, the TW14359 genome (and the genomes of many other EHEC strains), does not contain two of the thirteen σN enhancer-binding proteins found in K-12 and most other E. coli. This study thus aimed to validate the effect of rpoN mutation on σS levels and stability in the EHEC background and under the growth conditions that promote σN-dependent control of GDAR and the LEE.
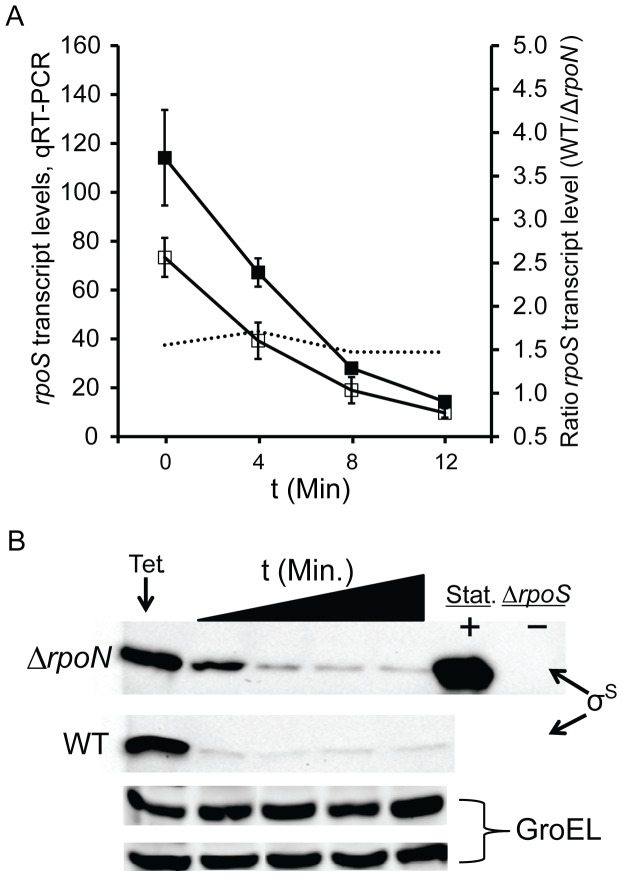
As anticipated, no difference was observed in the stability of rpoS mRNA between TW14359 and TW14359ΔrpoN (Fig. 3A). After 12 min of rifampin addition, rpoS transcript was barely detectable in both backgrounds and the mean half-life for rpoS transcript was estimated at 2.43 min (TW14359) and 2.51 min (TW14359ΔrpoN), which agrees with previous estimates [49], [50]. Before addition of rifampin, however, levels of rpoS transcript were higher (1.5-fold) in TW14359ΔrpoN compared to TW14359, but not significantly higher. In agreement with experiments using strain MG1655, σS was more stable in TW14359ΔrpoN compared to TW14359, however absolute levels were not observed to be higher in TW14359ΔrpoN (Fig. 3B) as described for MG1655 [43]. In TW14359, σS was barely detectable after 4 min of tetracycline addition, but was detected for up to 12 min in TW14359ΔrpoN. The mean half-life for σS was estimated at 2.4 min for TW14359 and 5.5 min for TW14359ΔrpoN, increasing by 2.3-fold in the rpoN null background. The half-life for σS has been estimated at 1.4–6.5 min in exponential cultures of E. coli [29], [51], [52], and 10.5–30 min in stationary phase cultures [29], [51]. These results reveal that in TW14359ΔrpoN, rpoS-dependency and control of GDAR and the LEE is correlated with an increase in exponential phase stability, but not absolute levels, of σS.
Figure 3. Stability of rpoS mRNA and σS.
(Panel A): Mean rpoS transcript levels (1st ordinate) and ratio of rpoS transcript (2nd ordinate) plotted against time following addition of rifampin at t = 0 min for WT TW14359 (filled) and TW14359ΔrpoN (empty); ratio is indicated by the dotted line. Error bars denote standard error of the mean (n≥3). (Panel B): Representative western immunoblot for σS as a function to time following addition of tetracycline at t = 0 min for TW14359 (WT) and TW14359ΔrpoN (ΔrpoN); blots are in increments of 4 min. Stationary phase (Stat.) protein extracts were used as a positive control for σS, and TW14359ΔrpoS (ΔrpoS) as a negative control. Equal loading was controlled for by westerns for GroEL (top row is ΔrpoN, bottom row is WT).
Role for Core RNA Polymerase and σN–dependent Transcription in the σS Stability, GDAR and LEE Expression Phenotype of TW14359ΔrpoN
The ability of E. coli sigma factors to successfully compete for core RNA polymerase (RNAP) differs substantially. For example, the RNAP binding affinity of σN is second only to the primary sigma factor, σ70, whereas σS binding affinity lies at the bottom of this rank order [53], [54]. In addition, the relative cellular abundance of each sigma factor influences gene expression through competition for RNAP [55]. During exponential growth, σN levels have been estimated at 10–16% those of σ70, whereas σS is barely detectable [56]–[58]. Together, this suggests that σS is at a substantial disadvantage for competitive RNAP binding during exponential growth. However, in an rpoN null background, the absence of competing σN may allow for an increase in σS RNAP binding sufficient enough to protect σS from ClpXP degradation, leading to increased transcription from σS promoters. This hypothesis might explain the σS stability, GDAR and LEE expression phenotype of TW14359ΔrpoN. To examine this possibility, a mutant version of the rpoN gene (rpoN R456A) was constructed, the product of which can efficiently form EσN holoenzyme but cannot bind DNA to direct transcription from σN promoters [91], [92]. If the increased stability of σS in TW14359ΔrpoN is solely the result of increased RNAP binding by σS, the expression of rpoN R456A in TW14359ΔrpoN should reproduce WT levels of σS stability. This was not determined to be the case however, as the stability of σS in TW14359ΔrpoNpRAM-2 did not differ from that of TW14359ΔrpoN, and both were increased in comparison to TW14359 and TW14359ΔrpoNpRAM-1 (Fig. 4A). The effect of rpoN R456A expression on the GDAR and LEE expression phenotype of TW14359ΔrpoN was also examined. Transcript levels for the GDAR regulator gadE, and the LEE regulator ler in TW14359ΔrpoN and TW14359ΔrpoNpRAM-2 did not differ, and were significantly higher or lower than TW14359 and TW14359ΔrpoNpRAM-1, respectively (p<0.05) (Fig. 4B). Interestingly, survival by GDAR for TW14359ΔrpoNpRAM-2 was partially reduced compared to TW14359ΔrpoN, but remained substantially higher than TW14359 and TW14359ΔrpoNpRAM-1 (Table 1).
Figure 4. Effect of rpoN R456A expression in TW14359ΔrpoN on σS stability, gadE and ler transcription.
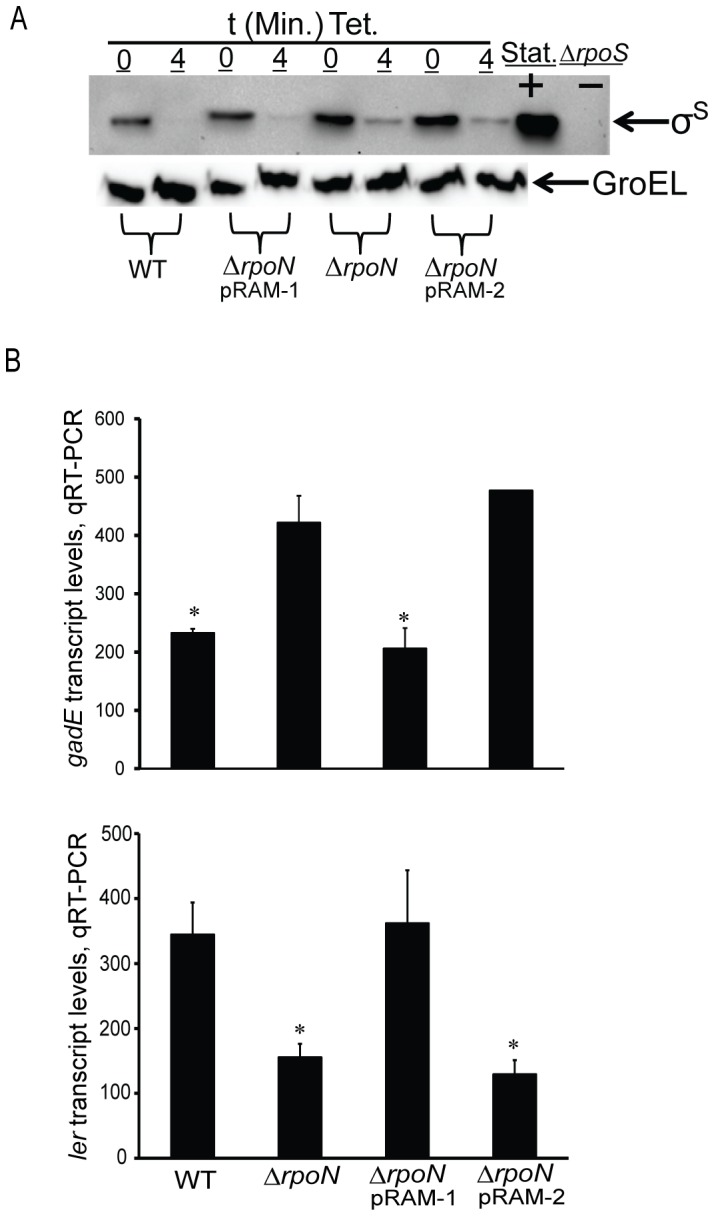
(Panel A): Representative western immunoblots for σS in TW14359 (WT), TW14359ΔrpoN complemented with rpoN + (TW14359ΔrpoNpRAM-1), TW14359ΔrpoN (ΔrpoN), TW14359ΔrpoN complemented with rpoN R456A (TW14359ΔrpoNpRAM-2) before (t = 0 min) and 4 min after addition of tetracycline (Tet.). Stationary phase (Stat.) protein extracts were used as a positive control for σS, and TW14359ΔrpoS (ΔrpoS) as a negative control. Equal gel loading was controlled for by westerns for GroEL. (Panel B): Mean gadE and ler transcript levels by qRT-PCR are plotted against TW14359 (WT) and derivative strains from Panel A. Transcript levels are normalized to the 16S rRNA gene rrsH. Asterisks denote significant differences between WT and TW14359ΔrpoNpRAM-1 when compared to TW14359ΔrpoN and TW14359ΔrpoNpRAM-2 by Tukey’s HSD following a significant F-test (n≥3, p<0.05). Error bars indicate standard error of the mean.
Sensitivity of σN-dependent GDAR and LEE Expression to Protease Inhibition
The low abundance of σS during exponential growth is due to rapid proteolytic turnover by the serine protease complex ClpXP [29], [51]. In strains mutated for clpP (the protease of ClpXP), σS is completely stable in exponential phase [51], however in exponential phase cultures of TW14359ΔrpoN, σS is still largely unstable (Fig. 3B), suggesting that there remains a sufficient amount of σS proteolysis. To reproduce the level of increased σS stability characteristic of TW14359ΔrpoN in the WT background, subinhibitory concentrations of the serine protease inhibitor 3, 4-dichloroisocoumarin (3, 4-DCI) [97] were titrated into growing exponential cultures and σS stability was measured.
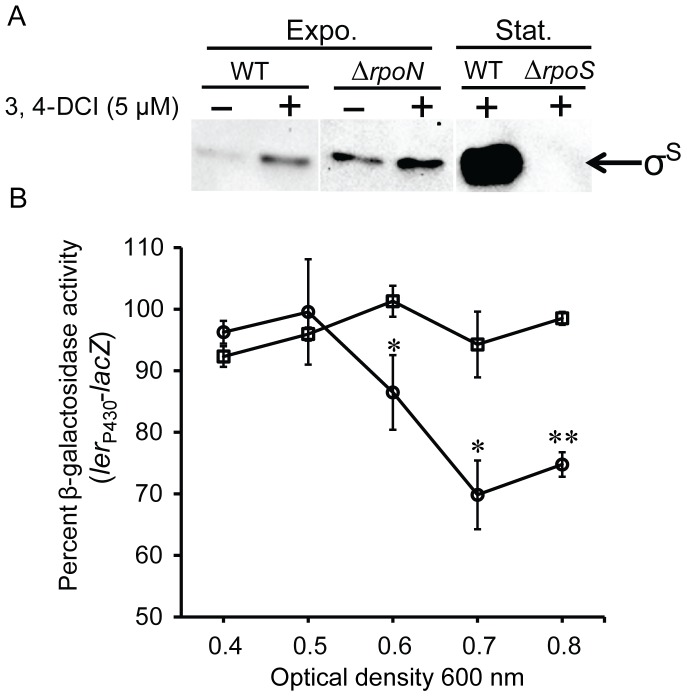
The addition of 5 µM 3, 4-DCI (or 1/12X MIC) increased σS stability levels in TW14359 similar to σS stability levels observed in TW14359ΔrpoN without the addition of 3, 4-DCI (Fig. 5A). Addition of 3, 4-DCI further increased σS levels in TW14359ΔrpoN as well, revealing that σS stability is sensitive to serine protease inhibition in both backgrounds. It was predicted that if the GDAR and LEE expression phenotype of TW14359ΔrpoN was simply a result of decreased σS proteolysis, then experimentally increasing σS stability with 3,4-DCI should reconstitute a similar phenotype in TW14359. For GDAR this was not shown to be true, as 3, 4-DCI had no impact on survival of TW14359 in acid, and only marginally increased percent survival in TW14359ΔrpoN (Table 1). Thus increased stability of σS alone cannot account for GDAR in TW14359ΔrpoN. The expression of LEE genes is known to be positively influenced by ClpP through its proteolytic effect on σS [31], [32]. Consistent with this, 3, 4-DCI addition reduced expression from ler P430-lacZ in TW14359 as indicated by a decrease in percent β-galactosidase activity relative to untreated controls (Fig. 5B). Since addition of 3,4-DCI further increased σS stability in TW14359ΔrpoN, it was expected that this increase would correspond with a further decrease in ler expression. On the contrary, ler P430-lacZ expression did not differ in 3,4-DCI-treated TW14359ΔrpoN cultures compared to untreated controls, and β-galactosidase activity was unchanged throughout growth compared to significantly reduced activity in TW14359 (p<0.05) (Fig. 5B). These results reveal that although σS stability is sensitive to protease inhibition using 3, 4-DCI in TW14359ΔrpoN, GDAR and ler expression is not and indicates that the underlying mechanism responsible for these phenotypes are at least partially distinct. The addition of 1/2X MIC of 3, 4-DCI did not significantly alter the outcome for GDAR or ler expression in either strain (data not shown).
Figure 5. Effect of the serine protease inhibitor 3,4-DCI on σS stability and ler expression.
(Panel A): Representative western immunoblot for σS stability in TW14359 (WT) and TW14359ΔrpoN (ΔrpoN) during exponential phase (Expo.) 4 min after the addition of tetracycline, and with or without 3,4-DCI, as well as in WT and TW14359ΔrpoS (ΔrpoS) during stationary phase (Stat.) with 3,4-DCI. Equal gel loading was controlled for by westerns for GroEL. (Panel B): Expression from ler P430-lacZ as measured by mean percent β-galactosidase activity following addition of 3,4-DCI and relative to untreated controls during exponential growth for TW14359 (circles) and TW14359ΔrpoN (squares). Asterisks denote significant differences between TW14359 and TW14359ΔrpoN at each OD600 by Welch’s t-test (n≥3, p<0.05 [*]; p<0.01 [**]).
Identification of the Enhancer-binding Protein Required for σN-directed Regulation of GDAR and the LEE
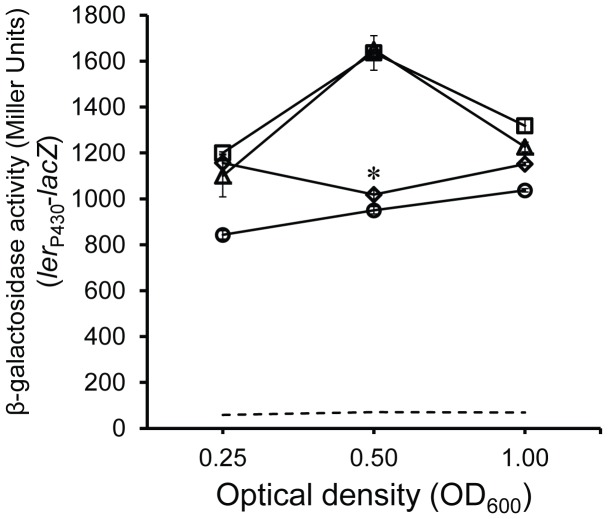
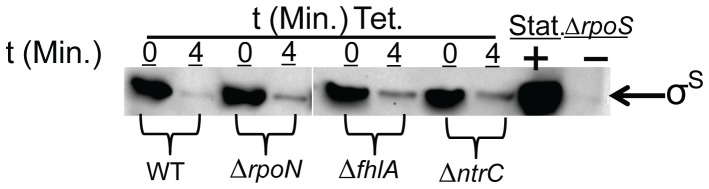
σN is a unique sigma factor in its requirement for enhancer-binding proteins (EBP) to initiate transcription [59]. If σS stability, GDAR and LEE expression in TW14359ΔrpoN is dependent on σN-directed transcription, at least one of these EBPs is required for this control. To examine this, a library of EBP isogenic deletion mutants in TW14359 was constructed and screened for GDAR during exponential growth. Of the eleven mutants, only TW14359ΔglnG and TW14359ΔfhlA expressed GDAR comparable to levels observed for TW14359ΔrpoN (Table 1). fhlA encodes a regulator of formate metabolism [60], and ntrC (also glnG) encodes NtrC, a major regulator of nitrogen assimilation [61], [62]. The impact of fhlA or ntrC mutation on LEE expression was then determined by transforming pRJM-1 containing ler P430-lacZ into both EBP isogenic backgrounds, TW14359ΔrpoN and TW14359, and β-galactosidase activity was measured during exponential growth. Expression from ler P430-lacZ increased in TW14359 to mid-exponential phase (OD600 = 0.5), then tapered off as cells entered late exponential phase (OD600 = 1.0) (Fig. 6). For TW14359ΔrpoN, ler P430-lacZ expression only slightly increased during growth, and was significantly reduced to 56% of WT levels at OD600 = 0.5, concordant with qRT-PCR data (p = 0.008) (Figs. 2 and 6). Mutation of fhlA had no apparent effect on ler P430-lacZ expression, yet ntrC mutation reduced ler P430-lacZ expression to 50% of WT at OD600 = 0.5 (p = 0.006) to levels comparable with TW14359ΔrpoN (Fig. 6). Thus the mutation of ntrC faithfully reproduces the GDAR and LEE expression phenotype of TW14359ΔrpoN. Interestingly, σS stability was increased in both EBP mutant backgrounds to the level of stability observed in TW14359ΔrpoN (Fig. 7). These results reveal that mutation of fhlA and ntrC similarly influence σS stability, yet only ntrC mutation phenocopies GDAR and LEE expression observed in TW14359ΔrpoN. A strain deleted for both rpoN and ntrC was constructed to validate the dependence on rpoN for NtrC-directed GDAR and LEE expression, but the mutant was too growth-impaired in DMEM to be phenotypically informative.
Figure 6. Expression from ler P430-lacZ in σN enhancer binding protein mutants.
Mean expression from ler P430-lacZ represented as β-galactosidase activity during exponential growth for TW14359 (triangles), TW14359ΔrpoN (circles), TW14359ΔfhlA (squares), TW14359ΔntrC (diamonds) and empty vector pRS551 (hatched line). The asterisk denotes a significant difference for TW14359ΔrpoN and TW14359ΔntrC when compared to the remaining strains by Tukey’s HSD following a significant F-test (n≥3, p<0.05).
Figure 7. Stability of σS in σN enhancer binding protein mutants.
Representative western immunoblots for σS in TW14359 (WT), TW14359ΔrpoN (ΔrpoN), TW14359ΔfhlA (ΔfhlA), and TW14359ΔntrC (ΔntrC) before (t = 0 min) and 4 min after addition of tetracycline (Tet.). Stationary phase (Stat.) protein extracts were used as a positive control for σS, and TW14359ΔrpoS (ΔrpoS) as a negative control. Equal loading was controlled for by westerns for GroEL.
Discussion
The importance of σN in E. coli metabolism, particularly nitrogen metabolism, is undisputed. Strains mutated for rpoN are growth-impaired under nitrogen-limiting conditions due to an inability to activate nitrogen regulatory response promoters. Mutation of rpoN also clearly affects many genes in E. coli that are not directly tied to metabolism, but which are perhaps cued to the metabolic status of the cell through σN, such as those involved in the regulation of motility [63], [64], NO detoxification [65], and biofilm formation [66]. In the present study, the phenotype of acid resistance and LEE expression previously described for rpoN mutants in EHEC [35], represents a case in which σN-dependent regulation is indirectly communicated through the downregulation of another sigma factor, σS. The antagonistic interplay of σN and σS in the control of these discrete systems resembles that described on a genomic scale by Dong et al. [43], in which it was estimated that as many as 60% of σN regulated genes are counter-regulated by σS.
For control of acid resistance, σN negatively regulates the σS-directed GadX-GadW pathway of glutamate-dependent acid resistance (GDAR) activation. This agrees with the dependence on rpoS and gadE for acid resistance formerly described for rpoN mutants [35], and with research showing that rpoS expression in a ΔgadXW background cannot induce the GDAR central regulator gadE [67]. In this regulatory circuit, σS drives the transcription of gadX, the product of which then activates gadE transcription. GadX also downregulates GadW, which is a negative regulator of σS [12]. As observed for GDAR, σN is clearly dependent on rpoS for upregulation of the LEE, conforming to the role of σS as a negative regulator of LEE expression [31], [32]. This σN–σS regulatory pathway is predicted to converge on the LEE central regulator, ler. The fact that ler expression was not observed to be significantly decreased in previous microarray studies of rpoN mutated EHEC [35] but is in the current study, may be explained by the increased sensitivity of qRT-PCR.
The GDAR and LEE expression phenotype of TW14359ΔrpoN correlates with an increase in σS stability similar to that described for K-12 [43], however no increase in σS levels was observed as was for K-12. This disparity in results could reflect genetic differences between K-12 and TW14359, or differences in experimental growth conditions. For the latter, the M9 glucose media used by Dong et al. [43] should be strongly growth restrictive for rpoN mutants, which are auxotrophic for glutamine in minimal media containing glucose [61]. As the production of σS is sensitive to reduced growth [29], increased σS levels during growth of rpoN mutants in M9 glucose may be attributed to metabolic stress, and not specific to σN. The growth of rpoN mutants is impaired in DMEM (Fig. S1), but not prohibitively, as it contains glutamine.
This study further scrutinized the genetic basis for and significance of increased σS stability in the GDAR and LEE expression phenotype of rpoN. The expression of a transcriptionally silent allele of σN (rpoN R456A) in TW14359ΔrpoN did not reconstitute WT levels of σS stability, gadE or ler expression, suggesting that competition for core RNAP is unlikely to be the primary underlying mechanism for this phenotype, and that transcription from a σN promoter(s) is a requirement. The RNAP competition hypothesis implies that the simple removal of a competing sigma factor may allow for increased competition of the remaining sigma factors for RNAP core. However, due to the low intrinsic affinity of σS for RNAP [53], all else being equal, it is more likely that σ70, or other sigma factors present during exponential phase (ex. σF) will out-compete σS for extant core. Naturally, this competition dynamic changes in stationary phase cultures, as small molecules and proteins modulate the ability of specific sigma subunits to interact with RNAP.
Addition of the serine protease inhibitor 3,4-DCI was shown to result in increased σS stability in TW14359, and further increased σS stability in TW14359ΔrpoN. This cumulative increase in σS stability in TW14359ΔrpoN could reflect the sum of effects of 3,4-DCI and rpoN mutation on a common pathway (i.e. ClpP), or independent pathways. There is no direct evidence however, that 3, 4-DCI is increasing σS stability by inhibiting ClpP. Regardless of which is true, increasing σS stability alone by interfering with proteolysis did not alter GDAR and LEE expression in TW14359ΔrpoN, suggesting that the mechanistic basis of these phenotypes is distinct. Mutation of rpoN could lead to increased σS activity at promoters, or modulate its affinity for RNAP. For the former, both FliZ and 6S RNA have been reported to reduce σS activity at selective promoters [68], [69]. Interestingly, transcript levels of fliZ were markedly upregulated in rpoN null K-12 [43], but not in EHEC [35]. For the latter, various proteins and small molecules are known to facilitate EσS holoenzyme formation, including Crl [70], Rsd [71], and ppGpp [72]. Currently, the involvement of any of these regulators in σN–σS control of GDAR and the LEE is unknown.
This study revealed that a strain mutated for ntrC, encoding nitrogen regulatory protein NtrC, is phenotypically similar to an rpoN mutant in regards to σS stability, GDAR and LEE expression. NtrC is a canonical σN EBP, activating transcription from at least 16 promoters in E. coli by binding as a hexameric ring to an upstream activator sequence (UAS) distal to the σN−24/−12 binding site [62], [73], [74]. The transcription of ntrC dramatically increases when E. coli is grown in media that does not contain ammonia (i.e. DMEM), and plays an integral role in controlling nitrogen utilization pathways. This finding suggests that the product(s) of an NtrC/σN driven promoter directly or indirectly downregulates σS, which in-turn affects GDAR and LEE expression. Currently however, there is no experimental evidence to support a role for any of the known NtrC/σN regulated genes in this. Alternatively, NtrC could activate σN promoters independent of DNA binding, which may relax the site selectivity of NtrC/σN dependent transcription initiation. Examples of this have been described for Rrp2 of B. burgdorferi, and FlgR of Campylobacter jejuni, that activate σN promoters in the absence of known UAS sites for these EBPs by some unknown mechanism [75]–[77]. There is also a precedent for NtrC regulating transcription independent of σN. NtrC binds to the core promoters of glnA P1 and glnA P3, repressing glnLG/glnALG (glutamine synthetase operon) transcription by interfering with σ70-dependent initiation [61]. Other E. coli promoters that are directly downregulated by NtrC have not however been described.
This study further identified FhlA as a putative EBP involved in the control of σS and GDAR, but not the LEE. FhlA activates transcription from multiple operons involved in formate metabolism, including structural components of the formate hydrogen lysase hydrogenase-3 (Hyd-3) complex. Interestingly, the Hyd-3 complex has been reported to confer acid resistance by a unique mechanism that involves the consumption of protons during the conversion of formic acid to CO2 and H2 [78]. However, the fact that fhlA mutation leads to acid resistance is inconsistent with its role as a positive regulator of the Hyd-3 acid resistance mechanism. Adding to this, Hyd-3 has only been shown to be protective under anaerobic growth conditions [78], together suggesting that the acid resistance conferred by fhlA mutation is independent of this mechanism. Alternatively, mutation of fhlA may lead to the accumulation of formic acid during growth on glucose (DMEM contains 4 g/l glucose) leading to acid-adaptation. Volatile fatty acid (VFAs, including acetic, formic and butyric acid) production during growth on glucose has been attributed to inorganic acid resistance in Salmonella and E. coli [79], [80]. The broader significance of this finding is that multiple σN EBPs regulate GDAR and the LEE by discrete pathways, some of which may be independent of rpoS. In further support of this hypothesis, the EBP QseF has been independently shown to be important for attaching and effacing lesion formation, and for the control of T3SS effectors in response to autoinducer 3 (AI-3) and norepinephrine/epinephrine [81]–[83]. The mutation of qseF did not however affect GDAR in this study (data not shown).
Given the essential roles of NtrC and σN in nitrogen metabolism, the results of this study infer that these proteins coordinate the expression of GDAR and the LEE with nitrogen (i.e. NH3) availability through σS. This proposed regulatory pathway shares many similarities with that described for rfaH expression and O-antigen production in Salmonella enterica. Specifically, σN has been observed to activate rfaH transcription in an rpoS-dependent manner [84]. However, the mutation of rpoN was epistatic for rfaH control by σS, indicating a regulatory relationship in which σS is positively controlling σN; there is no evidence that σS influences rpoN/σN expression or activity in E. coli [35], [43]. Remarkably however, rfaH transcription was further determined to be stimulated under nitrogen-limiting conditions [85], which suggests the potential for involvement of NtrC in σN–σS dependent control of O-antigen production in S. enterica.
This study concludes that σN exerts its regulatory influence on GDAR and the LEE through negative post-translational control of σS. Thus the inactivation of rpoN relaxes the requirement for stationary phase-induced mechanisms of σS accumulation during exponential growth. Furthermore, the results suggest that σN–σS dependent GDAR and LEE expression is at least partially controlled by NtrC, an EBP that activates transcription from σN promoters specifying genes for nitrogen utilization. The regulatory interplay of NtrC and other EBPs with σN and σS is likely to play a significant role in coordinating transcription with the various nutritional and physiological stimuli EHEC is exposed to during transmission, and in the course of infection.
Materials and Methods
Bacterial Strains and Culture Conditions
The strains and plasmids used in this study are listed in Table 2. Strains were stocked at −80°C in glycerol (15% v/v final) diluted in Lysogeny Broth (LB) and were maintained in LB or on LB with 1.5% agar (LBA). Unless otherwise noted, overnight (18–20 h) cultures grown in MOPS (50 mM)-buffered Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, cat. #D2902, St. Louis, MO) [86] containing 4 g/l glucose and 4 mM glutamine (pH 7.4) were used to inoculate fresh DMEM to a final OD600 = 0.05 and cultured at 37°C on a rotary shaker (200 RPM) using a 1∶10 ratio of media-to-flask volume as described [35]. The growth of strains in DMEM was monitored by taking OD600 readings at 1 h intervals over 12 h (Fig. S1). Antibiotics (Sigma-Aldrich) were added to cultures when required. The rpoS + status of strains was confirmed by catalase activity and glycogen storage following previous protocols [87], [88].
Table 2. Strains and plasmids used in this study.
| Strain/plasmid | Relevant characteristics | Source/reference |
| Strain name: | ||
| DH5α | Vector propagation, recA1 endA1 | |
| XL10-Gold® | Competent cells | Agilent, Santa Clara, CA |
| TW14359 | WT 2006 outbreak, western U.S. | [98] |
| EcRPF-6 | TW14359ΔrpoN | This study |
| EcRPF-9 | TW14359ΔrpoNΔrpoS | This study |
| EcRPF-7 | TW14359ΔrpoS | This study |
| EcRAM-26 | TW14359ΔglnG | This study |
| EcRAM-25 | TW14359ΔfhlA | This study |
| EcRAM-28 | TW14359ΔqseF | This study |
| EcRAM-27 | TW14359ΔpspF | This study |
| EcRAM-29 | TW14359ΔygeV | This study |
| EcRAM-4 | TW14359norR::kan KanR | This study |
| EcRAM-7 | TW14359rtcR::kan KanR | This study |
| EcRAM-3 | TW14359hyfR::kan KanR | This study |
| EcRAM-11 | TW14359zraR::kan KanR | This study |
| EcRAM-8 | TW14359tyrR::kan KanR | This study |
| EcRAM-5 | TW14359prpR::kan KanR | This study |
| Plasmid name: | ||
| pACYC177 | Low copy cloning vector, AmpR KanR P15A | [99] |
| pRAM1 | rpoN::pACYC177, AmpR KanS | This study |
| pRAM2 | rpoN R456A::pACYC177 AmpR KanS | This study |
| pRS551 | lac fusion vector, AmpR KanR lacZ + ColE1 | [95] |
| pRJM-1 | pRS551 containing ler P430-lacZfusion | This study |
Directed Gene Deletion and Site-specific Mutation
Gene deletion mutants were constructed using the λ Red recombinase-assisted approach [89], [90] and as described [35]. Primers used for the deletion of σN EBPs, as well as rpoN and rpoS are provided in Table S1. For site-specific mutation, a 1,518 bp ClaI/HindIII-digested PCR fragment containing the rpoN gene from strain TW14359 nucleotide positions 4,144,833–4,146,311 was generated using primers rpoN-45/ClaI and rpoN+1455/HindIII (Table S1). This fragment was ligated into ClaI/HindIII-digested pACYC177 to produce pRAM-1 (Table 2). Point mutations C1366G and G1367C were introduced into the rpoN gene present on the pRAM-1 template plasmid by PCR using mutagenic primers rpoNR456A-F and rpoNR456A-R (Table S1) and Pfu Ultra™ high fidelity DNA polymerase (Agilent, Santa Clara, CA) to produce pRAM-2 (Table 2). The resultant σN allele has a R456A mutation (rpoN R456A) in the DNA binding domain which interferes with the ability of the protein to bind DNA, but does not affect its capacity for RNAP association and holoenzyme formation [91], [92]. pRAM-1, in addition to pRAM-2 purified from E. coli XL10-Gold® (Agilent) transformants, were transformed into strain TW14359ΔrpoN as described [35]. Genetic constructs were validated by PCR, and restriction mapping, or by DNA sequencing and qRT-PCR.
Tests for Acid Resistance
Acid resistance by the glutamate- and arginine-dependent systems was measured as described [35] with slight adaptations. For the glutamate-dependent acid resistance mechanism, mid-exponential (OD600 = 0.5) DMEM cultures were inoculated to 106 CFU/ml final cell density into E minimal glucose (EG) media with or without 5.7 mM L-glutamate at pH 7 (control) or acidified with HCl (pH 2). To test for arginine-dependent acid resistance, exponential phase DMEM cultures were inoculated into EG media as above but with or without 0.6 mM L-arginine at pH 7 and pH 2.5. EG media acid resistance test environments were incubated at 37°C (200 RPM) for 1 h before sampling. For cell counts (CFU/ml) and percent survival determinations, samples were serially-diluted in PBS (pH 7), plated to LBA and incubated overnight at 37°C.
Quantitative Real-time PCR (qRT-PCR)
Primers for qRT-PCR are provided in Table S1. RNA purification, cDNA synthesis, qRT-PCR cycling conditions and data analysis followed previously described protocols [35], [93].
Protein Extraction, SDS-PAGE and Western Immunoblots
To extract total cellular protein, 10 ml culture samples were centrifuged at 10,000×g for 2 min and the cell pellet was washed twice with sterile water with centrifugation as above. Washed cell pellets were resuspended in 0.7 ml 0.5 M triethyl ammonium bicarbonate buffer (TEAB) (Sigma-Aldrich) and sonicated with a Sonic Dismembrator 120 (Fisher, Waltham, MA) at 50% amplitude for 30 sec intervals totaling 5 min, followed by incubation at 95°C in 4X Laemmli Buffer for 5 min. Total cell protein was collected from lysed cells by centrifugation at 10,000×g for 5 min, and supernatant was removed by aspiration. For western immunoblots, 10–30 µg extracted protein was resolved using 10% SDS-PAGE at 13 V/cm for 80 min before transfer at 15 V for 20 min to polyvinylidene fluoride (PVDF) membranes using a Trans-Blot semi-dry transfer cell (Bio-Rad, Hercules, CA). For detection of σS, PVDF membranes were blocked in Tris-buffered saline (1X Tris, pH 7.4) with 0.1% (v/v) Tween-20 (TBST) containing 5% skim milk for 2 h at room temperature before incubation with anti-σS mAbs (Neoclone, Madison, WI) diluted 1∶5000 in TBST containing 2% skim milk overnight on a Veri Mix platform rocker (Fisher) at 4°C. Membranes were then incubated for 1 h at room temperature with HRP-conjugated goat anti-mouse pAbs (Bio-Rad) diluted 1∶10,000 in TBST with 2% skim milk. Protein was detected using an enhanced chemiluminescence (ECL) Plus detection system (Amersham-Pharmacia, Piscataway, NJ) following the manufacturer’s instructions. Protein levels were measured and analyzed using a ChemiDoc XRS and Image Lab Software (Bio-Rad). The amount of protein loaded was measured using a Bradford protein assay standard curve. Equal loading was validated by western blots for GroEL using anti-GroEL mAbs (Bio-Rad) diluted 1∶40,000 in TBST with 2% skim milk. Western blots were repeated a minimum of three times in independent trials.
σS and rpoS mRNA Stability
Cultures were grown to mid-exponential phase (OD600 = 0.5) before the addition of a subinhibitory concentration of the transcription inhibitor rifampin (300 µg/ml final) or the translation inhibitor tetracycline (60 µg/ml final). Sampling was performed immediately before addition of antibiotics, and at 4 min intervals thereafter for 12 min (rpoS mRNA stability) or 16 min (σS protein stability). RNA was purified and validated as described [93]. For rpoS mRNA stability, gene transcript levels were measured using qRT-PCR and primers rpoS+356 and rpoS+466 (Table S1). Protein was extracted, and σS levels measured by western immunoblots. The half-life in minutes for rpoS mRNA and σS was extrapolated from gene transcript or protein levels, respectively, using linear regression analysis and as described [94]. The strength of linearity was estimated by the correlation coefficient (r2), and exceeded 0.85 (85%) for all analyses.
lacZ Transcriptional Fusions and β-galactosidase Assay
A 429-bp BamHI/EcoRI digested PCR fragment generated using primers ler-1/BamHI and ler-430/EcoRI (Table S1) and corresponding to nucleotide positions 4,679,303-4,679,731 in strain TW14359 was cloned into the similarly digested vector pRS551 [95] using T4-DNA ligase (Fisher) to create pRJM-1 (Table 2). This cloned fragment included 429-bp upstream of the translation initiation codon for ler (ECSP_4703) and both ler P1 and P2 promoters transcriptionally fused to lacZ (ler P430-lacZ). pRJM-1 purified from DH5α transformants was used for transformation into various WT and mutant backgrounds. The ler P430-lacZ fusion was confirmed by PCR and sequencing. To measure β-galactosidase activity from ler P430-lacZ, 50 µl culture samples taken at OD600 = 0.25 (early exponential), OD600 = 0.5 (mid-exponential) and OD600 = 1.0 (late exponential) were immediately added to 950 µl Z-buffer (1 M KCl, 1 mM MgSO4, 0.05 M β-mercaptoethanol, 0.06 M Na2HPO4, 0.04 M NaH2PO4⋅H2O, pH 7) with 0.1 ml chloroform and 50 µl 0.1% (v/v) SDS) and mixed vigorously for 30 sec. Samples were then incubated static at 28°C for 5 min before addition of 0.2 ml ortho-nitrophenyl β-D-galactopyranoside (ONPG, 4 mg/ml in 0.1 M phosphate buffer, pH 7) at 28°C for 20 min. Following development of the yellow cleavage product orthonitrophenol, the reaction was terminated by the addition of 0.5 ml Stop Solution (1 M Na2CO3) and samples were mixed and then centrifuged at 21,000×g for 5 min before measuring β-galactosidase activity. β-galactosidase activity was converted to Miller Units as described [96].
Serine Protease Inhibition
Selective inhibition of serine protease activity was performed using subinhibitory concentrations (i.e. 1/12X minimum inhibitory concentration (MIC) or 5 µM) of 3, 4-dichloroisocoumarin (3,4-DCI) (Sigma-Aldrich) [97]. The MIC for 3,4-DCI was at 60 µM for both WT and rpoN null backgrounds. The effect of 3,4-DCI addition to growing cultures on σS stability, GDAR and LEE expression was determined as described above. For σS stability, 3, 4-DCI was added to cultures at mid-exponential phase (OD600 = 0.4) and incubated to OD600 = 0.5 before addition of 60 µg/ml tetracycline. Sampling was performed immediately before tetracycline addition and 4 min after addition. For GDAR and LEE expression, 3,4-DCI was added at OD600 = 0.4 as for σS stability, and then GDAR tested, or β-galactosidase activity measured from ler P430-lacZ as described above. Control cultures did not contain 3, 4-DCI for all experiments.
Supporting Information
Growth of strains in Dulbecco’s Modified Eagle’s Medium (DMEM). Mean (n = 2) optical density 600 nm (OD600) plotted for TW14359 (empty squares), TW14359ΔrpoN (filled squares), TW14359ΔrpoS (circles), TW14359ΔfhlA (plus signs), TW14359ΔntrC (triangles), and TW14359ΔrpoNΔrpoS (diamonds). Individual OD600 measurements for each strain varied by less than 5%. For ler P430-lacZ expression (Fig. 6), sampling was done for all strains except for TW14359ΔrpoS and TW14359ΔrpoNΔrpoS at OD600 = 0.25, OD600 = 0.5, and OD600 = 1.0 approximately corresponding to early-, mid- and late-exponential phase, respectively. For all remaining experiments, sampling was done at OD600 = 0.5.
(TIF)
Primers used in this study.
(PDF)
Acknowledgments
We thank Edward G. Dudley (Pennsylvania State University) for kindly providing vector pRS551 for lacZ reporter fusion construction, and Galeb Abu-Ali (U.S. Food and Drug Administration) for critical review of previous versions of the manuscript.
Funding Statement
This research was supported by intramural start-up funds from the University of South Florida. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, et al. (1999) Food-related illness and death in the United States. Emerg Infect Dis 5(5): 607–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mead P, Bender I, Dembek Z, et al.. (1998) Active surveillance for hemolytic uremic syndrome at selected sites, United States, In Abstracts of the International Conference on Emerging Infectious Diseases (Atlanta), Abstract P-3.12.
- 3. Banatvala N, Griffin PM, Greene KD, Barrett TJ, Bibb WF, et al. (2001) The United States National Prospective Hemolytic Uremic Syndrome Study: microbiologic, serologic, clinical, and epidemiologic findings. J Infect Dis 183(7): 1063–70. [DOI] [PubMed] [Google Scholar]
- 4. Foster JW (2004) Escherichia coli acid resistance: tales of an amateur acidophile. Nat Rev Microbiol 2(11): 898–907. [DOI] [PubMed] [Google Scholar]
- 5. Teunis P, Takumi K, Shinagawa K (2004) Dose response for infection by Escherichia coli O157:H7 from outbreak data. Risk Anal 24(2): 401–7. [DOI] [PubMed] [Google Scholar]
- 6. Chart H (2000) VTEC enteropathogenicity. Symp Ser Soc Appl Microbiol (29): 12S–23S. [DOI] [PubMed] [Google Scholar]
- 7. Perna NT, Mayhew GF, Posfai G, Elliott S, Donnenberg MS, et al. (1998) Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect Immun 66(8): 3810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDaniel TK, Kaper JB (1997) A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol 23(2): 399–407. [DOI] [PubMed] [Google Scholar]
- 9. Hartland EL, Batchelor M, Delahay RM, Hale C, Matthews S, et al. (1999) Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol Microbiol 32(1): 151–8. [DOI] [PubMed] [Google Scholar]
- 10. Elliott SJ, Wainwright LA, McDaniel TK, Jarvis KG, Deng YK, et al. (1998) The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol 28(1): 1–4. [DOI] [PubMed] [Google Scholar]
- 11. Wong AR, Pearson JS, Bright MD, Munera D, Robinson KS, et al. (2011) Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol Microbiol 80(6): 1420–38. [DOI] [PubMed] [Google Scholar]
- 12. Ma Z, Richard H, Foster JW (2003) pH-Dependent modulation of cyclic AMP levels and GadW-dependent repression of RpoS affect synthesis of the GadX regulator and Escherichia coli acid resistance. J Bacteriol 185(23): 6852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW (1999) Control of acid resistance in Escherichia coli . J Bacteriol 181(11): 3525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Small P, Blankenhorn D, Welty D, Zinser E, Slonczewski JL (1994) Acid and base resistance in Escherichia coli and Shigella flexneri: role of rpoS and growth pH. J Bacteriol 176(6): 1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gong S, Ma Z, Foster JW (2004) The Era-like GTPase TrmE conditionally activates gadE and glutamate-dependent acid resistance in Escherichia coli . Mol Microbiol 54(4): 948–61. [DOI] [PubMed] [Google Scholar]
- 16. Ma Z, Masuda N, Foster JW (2004) Characterization of EvgAS-YdeO-GadE branched regulatory circuit governing glutamate-dependent acid resistance in Escherichia coli . J Bacteriol 186(21): 7378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yona-Nadler C, Umanski T, Aizawa S, Friedberg D, Rosenshine I (2003) Integration host factor (IHF) mediates repression of flagella in enteropathogenic and enterohaemorrhagic Escherichia coli . Microbiology 149(Pt 4): 877–84. [DOI] [PubMed] [Google Scholar]
- 18. Bergholz TM, Wick LM, Qi W, Riordan JT, Ouellette LM, et al. (2007) Global transcriptional response of Escherichia coli O157:H7 to growth transitions in glucose minimal medium. BMC Microbiol 7(1): 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abe H, Tatsuno I, Tobe T, Okutani A, Sasakawa C (2002) Bicarbonate ion stimulates the expression of locus of enterocyte effacement-encoded genes in enterohemorrhagic Escherichia coli O157:H7. Infect Immun 70(7): 3500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abe A, Kenny B, Stein M, Finlay BB (1997) Characterization of two virulence proteins secreted by rabbit enteropathogenic Escherichia coli, EspA and EspB, whose maximal expression is sensitive to host body temperature. Infect Immun 65(9): 3547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kenny B, Abe A, Stein M, Finlay BB (1997) Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect Immun 65(7): 2606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krin E, Danchin A, Soutourina O (2010) RcsB plays a central role in H-NS-dependent regulation of motility and acid stress resistance in Escherichia coli . Res Microbiol 161(5): 363–71. [DOI] [PubMed] [Google Scholar]
- 23. Tobe T, Ando H, Ishikawa H, Abe H, Tashiro K, et al. (2005) Dual regulatory pathways integrating the RcsC-RcsD-RcsB signalling system control enterohaemorrhagic Escherichia coli pathogenicity. Mol Microbiol 58(1): 320–33. [DOI] [PubMed] [Google Scholar]
- 24. Shin S, Castanie-Cornet MP, Foster JW, Crawford JA, Brinkley C, et al. (2001) An activator of glutamate decarboxylase genes regulates the expression of enteropathogenic Escherichia coli virulence genes through control of the plasmid-encoded regulator, Per. Mol Microbiol 41(5): 1133–50. [DOI] [PubMed] [Google Scholar]
- 25. Sanchez-SanMartin C, Bustamante VH, Calva E, Puente JL (2001) Transcriptional regulation of the orf19 gene and the tir-cesT-eae operon of enteropathogenic Escherichia coli . J Bacteriol 183(9): 2823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Biase D, Tramonti A, Bossa F, Visca P (1999) The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Mol Microbiol 32(6): 1198–211. [DOI] [PubMed] [Google Scholar]
- 27. Lease RA, Smith D, McDonough K, Belfort M (2004) The small noncoding DsrA RNA is an acid resistance regulator in Escherichia coli . J Bacteriol 186(18): 6179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laaberki MH, Janabi N, Oswald E, Repoila F (2006) Concert of regulators to switch on LEE expression in enterohemorrhagic Escherichia coli O157:H7: interplay between Ler, GrlA, HNS and RpoS. Int J Med Microbiol 296(4–5): 197–210. [DOI] [PubMed] [Google Scholar]
- 29. Lange R, Hengge-Aronis R (1994) The cellular concentration of the sigma S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev 8(13): 1600–12. [DOI] [PubMed] [Google Scholar]
- 30. Sperandio V, Mellies JL, Nguyen W, Shin S, Kaper JB (1999) Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli . Proc Natl Acad Sci U S A 96(26): 15196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iyoda S, Watanabe H (2005) ClpXP protease controls expression of the type III protein secretion system through regulation of RpoS and GrlR levels in enterohemorrhagic Escherichia coli . J Bacteriol 187(12): 4086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomoyasu T, Takaya A, Handa Y, Karata K, Yamamoto T (2005) ClpXP controls the expression of LEE genes in enterohaemorrhagic Escherichia coli . FEMS Microbiol Lett 253(1): 59–66. [DOI] [PubMed] [Google Scholar]
- 33. Price SB, Cheng CM, Kaspar CW, Wright JC, DeGraves FJ, et al. (2000) Role of rpoS in acid resistance and fecal shedding of Escherichia coli O157:H7. Appl Environ Microbiol 66(2): 632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hengge-Aronis R (2002) Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev 66(3): 373–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riordan JT, Tietjen JA, Walsh CW, Gustafson JE, Whittam TS (2010) Inactivation of alternative sigma factor 54 (RpoN) leads to increased acid resistance, and alters locus of enterocyte effacement (LEE) expression in Escherichia coli O157 : H7. Microbiology 156(Pt 3): 719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Powell BS, Court DL, Inada T, Nakamura Y, Michotey V, et al. (1995) Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. Enzyme IIANtr affects growth on organic nitrogen and the conditional lethality of an erats mutant. J Biol Chem 270(9): 4822–39. [DOI] [PubMed] [Google Scholar]
- 37. Bueno R, Pahel G, Magasanik B (1985) Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli . J Bacteriol 164(2): 816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weiner L, Brissette JL, Model P (1991) Stress-induced expression of the Escherichia coli phage shock protein operon is dependent on sigma 54 and modulated by positive and negative feedback mechanisms. Genes Dev 5(10): 1912–23. [DOI] [PubMed] [Google Scholar]
- 39. Reitzer L, Schneider BL (2001) Metabolic context and possible physiological themes of sigma(54)-dependent genes in Escherichia coli . Microbiol Mol Biol Rev 65(3): 422–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boardman BK, He M, Ouyang Z, Xu H, Pang X, et al. (2008) Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi . Infect Immun 76(9): 3844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang XF, Lybecker MC, Pal U, Alani SM, Blevins J, et al. (2005) Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi . J Bacteriol 187(14): 4822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, et al. (2001) Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A 98(22): 12724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dong T, Yu R, Schellhorn H (2011) Antagonistic regulation of motility and transcriptome expression by RpoN and RpoS in Escherichia coli . Mol Microbiol 79(2): 375–86. [DOI] [PubMed] [Google Scholar]
- 44. Masuda N, Church GM (2002) Escherichia coli gene expression responsive to levels of the response regulator EvgA. J Bacteriol 184(22): 6225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin J, Smith MP, Chapin KC, Baik HS, Bennett GN, et al. (1996) Mechanisms of acid resistance in enterohemorrhagic Escherichia coli . Appl Environ Microbiol 62(9): 3094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stim-Herndon KP, Flores TM, Bennett GN (1996) Molecular characterization of adiY, a regulatory gene which affects expression of the biodegradative acid-induced arginine decarboxylase gene (adiA) of Escherichia coli . Microbiology 142 (Pt 5): 1311–20. [DOI] [PubMed] [Google Scholar]
- 47. Iyoda S, Watanabe H (2004) Positive effects of multiple pch genes on expression of the locus of enterocyte effacement genes and adherence of enterohaemorrhagic Escherichia coli O157 : H7 to HEp-2 cells. Microbiology 150(Pt 7): 2357–571. [DOI] [PubMed] [Google Scholar]
- 48. Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, et al. (2001) Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res 8(1): 11–22. [DOI] [PubMed] [Google Scholar]
- 49. Paesold G, Krause M (1999) Analysis of rpoS mRNA in Salmonella dublin: identification of multiple transcripts with growth-phase-dependent variation in transcript stability. J Bacteriol 181(4): 1264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zgurskaya HI, Keyhan M, Matin A (1997) The sigma S level in starving Escherichia coli cells increases solely as a result of its increased stability, despite decreased synthesis. Mol Microbiol 24(3): 643–51. [DOI] [PubMed] [Google Scholar]
- 51. Schweder T, Lee KH, Lomovskaya O, Matin A (1996) Regulation of Escherichia coli starvation sigma factor (sigma S) by ClpXP protease. J Bacteriol 178(2): 470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Muffler A, Traulsen DD, Lange R, Hengge-Aronis R (1996) Posttranscriptional osmotic regulation of the sigma(s) subunit of RNA polymerase in Escherichia coli . J Bacteriol 178(6): 1607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maeda H, Fujita N, Ishihama A (2000) Competition among seven Escherichia coli sigma subunits: relative binding affinities to the core RNA polymerase. Nucleic Acids Res 28(18): 3497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Colland F, Fujita N, Ishihama A, Kolb A (2002) The interaction between sigmaS, the stationary phase sigma factor, and the core enzyme of Escherichia coli RNA polymerase. Genes Cells 7(3): 233–47. [DOI] [PubMed] [Google Scholar]
- 55. Farewell A, Kvint K, Nystrom T (1998) Negative regulation by RpoS: a case of sigma factor competition. Mol Microbiol 29(4): 1039–51. [DOI] [PubMed] [Google Scholar]
- 56. Ishihama A (1990) Molecular assembly and functional modulation of Escherichia coli RNA polymerase. Adv Biophys 26: 19–31. [DOI] [PubMed] [Google Scholar]
- 57. Jishage M, Iwata A, Ueda S, Ishihama A (1996) Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J Bacteriol 178(18): 5447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jishage M, Ishihama A (1995) Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of sigma 70 and sigma 38. J Bacteriol 177(23): 6832–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shingler V (1996) Signal sensing by sigma 54-dependent regulators: derepression as a control mechanism. Mol Microbiol 19(3): 409–16. [DOI] [PubMed] [Google Scholar]
- 60. Sankar P, Shanmugam KT (1988) Biochemical and genetic analysis of hydrogen metabolism in Escherichia coli: the hydB gene. J Bacteriol 170(12): 5433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reitzer LJ, Magasanik B (1986) Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell 45(6): 785–92. [DOI] [PubMed] [Google Scholar]
- 62. Zimmer DP, Soupene E, Lee HL, Wendisch VF, Khodursky AB, et al. (2000) Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc Natl Acad Sci U S A 97(26): 14674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Claret L, Hughes C (2002) Interaction of the atypical prokaryotic transcription activator FlhD2C2 with early promoters of the flagellar gene hierarchy. J Mol Biol 321(2): 185–99. [DOI] [PubMed] [Google Scholar]
- 64. Zhao K, Liu M, Burgess RR (2010) Promoter and regulon analysis of nitrogen assimilation factor, sigma54, reveal alternative strategy for E. coli MG1655 flagellar biosynthesis. Nucleic Acids Res 38(4): 1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gardner AM, Gessner CR, Gardner PR (2003) Regulation of the nitric oxide reduction operon (norRVW) in Escherichia coli. Role of NorR and sigma54 in the nitric oxide stress response. J Biol Chem 278(12): 10081–6. [DOI] [PubMed] [Google Scholar]
- 66. Belik AS, Tarasova NN, Khmel IA (2008) Regulation of biofilm formation in Escherichia coli K12: effect of mutations in hns, stpA, lon, and rpoN genes. Mol Gen Mikrobiol Virusol (4): 3–5. [PubMed] [Google Scholar]
- 67. Sayed AK, Odom C, Foster JW (2007) The Escherichia coli AraC-family regulators GadX and GadW activate gadE, the central activator of glutamate-dependent acid resistance. Microbiology 153(Pt 8): 2584–92. [DOI] [PubMed] [Google Scholar]
- 68. Pesavento C, Becker G, Sommerfeldt N, Possling A, Tschowri N, et al. (2008) Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. . Genes Dev 22(17): 2434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Trotochaud AE, Wassarman KM (2004) 6S RNA function enhances long-term cell survival. J Bacteriol 186(15): 4978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dong T, Kirchhof MG, Schellhorn HE (2007) RpoS regulation of gene expression during exponential growth of Escherichia coli K12. Mol Genet Genomics 279(3): 267–277. [DOI] [PubMed] [Google Scholar]
- 71. Jishage M, Ishihama A (1999) Transcriptional organization and in vivo role of the Escherichia coli rsd gene, encoding the regulator of RNA polymerase sigma D. J Bacteriol. 181(12): 3768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jishage M, Kvint K, Shingler V, Nystrom T (2002) Regulation of sigma factor competition by the alarmone ppGpp. Genes Dev 16(10): 1260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dixon R, Kahn D (2004) Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2(8): 621–31. [DOI] [PubMed] [Google Scholar]
- 74. Weiss V, Claverie-Martin F, Magasanik B (1992) Phosphorylation of nitrogen regulator I of Escherichia coli induces strong cooperative binding to DNA essential for activation of transcription. Proc Natl Acad Sci U S A 89(11): 5088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burtnick MN, Downey JS, Brett PJ, Boylan JA, Frye JG, et al. (2007) Insights into the complex regulation of rpoS in Borrelia burgdorferi. . Mol Microbiol 65(2): 277–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Blevins JS, Xu H, He M, Norgard MV, Reitzer L, et al. (2009) Rrp2, a {sigma}54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J Bacteriol 191(8): 2902–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Joslin SN, Hendrixson DR (2008) Analysis of the Campylobacter jejuni FlgR response regulator suggests integration of diverse mechanisms to activate an NtrC-like protein. J Bacteriol 190(7): 2422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Noguchi K, Riggins DP, Eldahan KC, Kitko RD, Slonczewski JL (2010) Hydrogenase-3 contributes to anaerobic acid resistance of Escherichia coli. PLoS One, 2010. 5(4): e10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baik HS, Bearson S, Dunbar S, Foster JW (1996) The acid tolerance response of Salmonella typhimurium provides protection against organic acids. Microbiology 142 (Pt 11): 3195–200. [DOI] [PubMed] [Google Scholar]
- 80. Kwon YM, Ricke SC (1998) Induction of acid resistance of Salmonella typhimurium by exposure to short-chain fatty acids. Appl Environ Microbiol 64(9): 3458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Reading NC, Rasko DA, Torres AG, Sperandio V (2010) A transcriptome study of the QseEF two-component system and the QseG membrane protein in enterohaemorrhagic Escherichia coli O157 : H7. Microbiology 156(Pt 4): 1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reading NC, Rasko DA, Torres AG, Sperandio V, et al. (2009) The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. Proc Natl Acad Sci U S A 106(14): 5889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reading NC, Torres AG, Kendall MM, Hughes DT, Yamamoto K, et al. (2007) A novel two-component signaling system that activates transcription of an enterohemorrhagic Escherichia coli effector involved in remodeling of host actin. J Bacteriol 189(6): 2468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bittner M, Saldias S, Altamirano F, Valvano M A, Contreras I (2004) RpoS and RpoN are involved in the growth-dependent regulation of rfaH transcription and O antigen expression in Salmonella enterica serovar Typhi. Microb Pathog 36(1): 19–24. [DOI] [PubMed] [Google Scholar]
- 85. Bittner M, Saldias S, Estevez C, Zaldivar M, Marolda C L, et al. (2002) O-antigen expression in Salmonella enterica serovar Typhi is regulated by nitrogen availability through RpoN-mediated transcriptional control of the rfaH gene. Microbiology 148(Pt 12): 3789–99. [DOI] [PubMed] [Google Scholar]
- 86. Morton HJ (1970) A survey of commercially available tissue culture media. In Vitro 6(2): 89–108. [DOI] [PubMed] [Google Scholar]
- 87. Hengge-Aronis R, Fischer D (1992) Identification and molecular analysis of glgS, a novel growth-phase-regulated and rpoS-dependent gene involved in glycogen synthesis in Escherichia coli. . Mol Microbiol 6(14): 1877–86. [DOI] [PubMed] [Google Scholar]
- 88. Bohannon DE, Connell N, Keener J, Tormo A, Espinosa-Urgel M, et al. (1991) Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of sigma 70. J Bacteriol 173(14): 4482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Murphy KC, Campellone KG (2003) Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. . BMC Mol Biol 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97(12): 6640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Taylor M, Butler R, Chambers S, Casimiro M, Badii F, et al. (1996) The RpoN-box motif of the RNA polymerase sigma factor sigma N plays a role in promoter recognition. Mol Microbiol 22(5): 1045–54. [DOI] [PubMed] [Google Scholar]
- 92. Wang L, Gralla JD (2001) Roles for the C-terminal region of sigma 54 in transcriptional silencing and DNA binding. J Biol Chem 276(12): 8979–86. [DOI] [PubMed] [Google Scholar]
- 93. Neupane M, Abu-Ali GS, Mitra A, Lacher DW, Manning SD, et al. (2011) Shiga toxin 2 overexpression in Escherichia coli O157:H7 strains associated with severe human disease. Microb Pathog 51(6): 466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bernstein JA, Khodursky AB, Lin PH, Lin-Chao S, Cohen SN (2002) Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc Natl Acad Sci U S A 99(15): 9697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simons RW, Houman F, Kleckner N, (1987) Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene, 53(1): p. 85–96. [DOI] [PubMed]
- 96.Miller J (1972) Assay of B-galactosidase In: Experiments in molecular genetics. Cold Spring Harbor, NY. Cold Spring Harbor Laboratory, 352–255.
- 97. Powers JC, Kam CM, Narasimhan L, Oleksyszyn J, Hernandez MA, et al. (1989) Mechanism-based isocoumarin inhibitors for serine proteases: use of active site structure and substrate specificity in inhibitor design. J Cell Biochem 39(1): 33–46. [DOI] [PubMed] [Google Scholar]
- 98. Manning SD, Motiwala AS, Springman AC, Qi W, Lacher DW, et al. (2008) Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc Natl Acad Sci U S A 105(12): 4868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chang AC, SN Cohen (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134(3): 1141–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth of strains in Dulbecco’s Modified Eagle’s Medium (DMEM). Mean (n = 2) optical density 600 nm (OD600) plotted for TW14359 (empty squares), TW14359ΔrpoN (filled squares), TW14359ΔrpoS (circles), TW14359ΔfhlA (plus signs), TW14359ΔntrC (triangles), and TW14359ΔrpoNΔrpoS (diamonds). Individual OD600 measurements for each strain varied by less than 5%. For ler P430-lacZ expression (Fig. 6), sampling was done for all strains except for TW14359ΔrpoS and TW14359ΔrpoNΔrpoS at OD600 = 0.25, OD600 = 0.5, and OD600 = 1.0 approximately corresponding to early-, mid- and late-exponential phase, respectively. For all remaining experiments, sampling was done at OD600 = 0.5.
(TIF)
Primers used in this study.
(PDF)