Abstract
Signals that determine fast- and slow-twitch phenotypes of skeletal muscle fibers are thought to stem from depolarization, with concomitant contraction and activation of calcium-dependent pathways. We examined the roles of contraction and activation of calcineurin (CN) in regulation of slow and fast myosin heavy chain (MHC) protein expression during muscle fiber formation in vitro. Myotubes formed from embryonic day 21 rat myoblasts contracted spontaneously, and ∼10% expressed slow MHC after 12 d in culture, as seen by immunofluorescent staining. Transfection with a constitutively active form of calcineurin (CN*) increased slow MHC by 2.5-fold as determined by Western blot. This effect was attenuated 35% by treatment with tetrodotoxin and 90% by administration of the selective inhibitor of CN, cyclosporin A. Conversely, cyclosporin A alone increased fast MHC by twofold. Cotransfection with VIVIT, a peptide that selectively inhibits calcineurin-induced activation of the nuclear factor of activated T-cells, blocked the effect of CN* on slow MHC by 70% but had no effect on fast MHC. The results suggest that contractile activity-dependent expression of slow MHC is mediated largely through the CN–nuclear factor of activated T-cells pathway, whereas suppression of fast MHC expression may be independent of nuclear factor of activated T-cells.
INTRODUCTION
Skeletal muscle fibers exhibit a range of phenotypes that are characterized by morphological, biochemical, and functional properties. The phenotypes form a continuum ranging from white, glycolytic, fast-twitch fibers to red, oxidative, slow-twitch fibers. The differences are due to variations in gene expression of numerous proteins and their isoforms, including those of metabolic pathways, excitation–contraction coupling, and the contractile apparatus. Hence the phenotype expressed by an individual fiber has important consequences with respect to the energy demands and functional parameters of that fiber.
Although the plasticity of muscle phenotype has been characterized in adult models, little is known about the signals involved in the initial determination of fiber type. Development of vertebrate skeletal muscle is biphasic. Primary (or embryonic) fibers form in the limb before innervation. They express both fast and slow myosin heavy chain (MHC) isoforms, a key determinant of fiber type (Butler-Browne and Whalen, 1984; Condon et al., 1990a). These fibers serve as a scaffold for the formation of the secondary (or fetal) population of fibers, which compose the bulk of muscle.
During development and maturation, secondary fibers in the rat hindlimb first express the embryonic MHC isoform, followed by neonatal and then adult fast or slow isoforms (Whalen et al., 1987; Condon et al., 1990a). The signals that govern secondary fiber development and phenotypic expression are just beginning to be elucidated. In vivo work has shown that formation of the secondary fibers in rats requires contractile activity in that it is blocked by administration of tetrodotoxin (TTX) (Harris, 1981).
In adult muscle, the contractile activity pattern has been shown to have an important role in regulation of muscle phenotype as demonstrated by cross-innervation (Buller et al., 1960) and chronic motoneuron stimulation experiments (for review, see Pette and Vrbova, 1999). Tonic, low-frequency neural stimulation induces a slow fiber phenotype, whereas phasic, high-frequency bursts induce a fast fiber phenotype; however, the route by which the information encoded in the neural firing/contractile pattern is translated into changes in gene expression is not known. One potential decoding pathway involves calcineurin (CN), a serine/threonine protein phosphatase (PP2B). When activated by Ca2+/calmodulin, calcineurin dephosphorylates a number of proteins, including nuclear factor of activated T-cells (NFAT), a transcription factor (for reviews, see Rao et al., 1997; Klee et al., 1998). Activation of this pathway is dependent on the [Ca2+]i pattern (Timmerman et al., 1996; Dolmetsch et al., 1997); thus the ability of this pathway to decipher information contained in the stimulation pattern makes it an ideal candidate for a signaling decoder in muscle tissue. Indeed, a role for the calcineurin pathway in the regulation of fiber type has been described recently in cell lines and postnatal systems (Chin et al., 1998; Bigard et al., 2000; Delling et al., 2000; Naya et al., 2000). It is not known whether this pathway plays a role in the establishment of fiber type during development.
The goal of this study was to dissect potential signaling pathways involved in fiber type determination during development as monitored by expression of MHC protein isoforms. We examined the roles of contraction and the calcineurin pathway during muscle fiber formation in vitro. We found that the calcineurin pathway was important for determining MHC phenotype, and that its effects were influenced by depolarization with subsequent contraction and were mediated via both NFAT and non-NFAT routes.
MATERIALS AND METHODS
Cell Culture
Myoblast cultures were obtained as detailed previously (Dutton et al., 1995; Daniels et al., 2000). Briefly, muscles were stripped from the hindlimbs of 21-d-old fetal rats, trypsinized to dissociate, plated for 1 h to remove fibroblasts, and then replated on 0.5% gelatin-coated dishes in DMEM supplemented with 10% horse serum (Life Technologies, Gaithersburg, MD) and 10% fetal calf serum (Intergen, Purchase, NY) (growth medium). Approximately 48 h later, cells were incubated with 0.05% Dispase II (Roche Molecular Biochemicals, Indianapolis, IN) to selectively detach myoblasts and replated for 30 min to remove more fibroblasts. Finally, 0.5 × 106 cells were plated in 35-mm dishes on either gelatin- and carbon-coated 13-mm-round coverslips (Clay Adams Gold Seal, Becton-Dickinson Labware, Oxnard, CA) (for immunostaining) or on gelatin-coated dishes only (for protein). Forty-eight hours after cell plating, all cultures were refed with 90% DMEM, 10% horse serum to discourage fibroblast growth. Twenty-four hours later, the cultures were again refed with 95% DMEM, 5% horse serum. All media also contained penicillin/streptomycin (100U/100 mg/ml) and fungizone (2.5 μg/ml). Freshly isolated cells were used for each experiment. Cells were maintained at 37°C, 100% humidity with 8% CO2. Some cultures were treated with 1.5 μM TTX to inhibit myotube contraction, starting 2 d after plating and continuing until the time of harvest, 12–13 d after final myoblast plating. At the time of harvest, very few myoblasts were detected by desmin immunostaining, which indicated a fusion index of >95% (our unpublished results).
Transfection
Muscle cultures were transfected with a constitutively active form of calcineurin (CN*) that was created by making a deletion mutant of the calcineurin catalytic subunit, which lacked the functional calmodulin-binding and autoinhibitory domains (O'Keefe et al., 1992). The calcineurin, linked to a CMV promoter in a pCI-NEO vector (Chin et al., 1998), was a gift from R. S. Williams (Southwestern Medical Center, Dallas, TX). Some cultures were transfected with a VIVIT-GFP (VIVIT) plasmid. This was constructed by inserting the sequence MAGPHPVIVITGPHEE into the pEGFP.N1 vector and was a gift from Dr. A. Rao (Harvard Medical School, Boston, MA). The sequence encodes a peptide that has been shown to be a highly selective inhibitor of the transcription factor NFAT (Aramburu et al., 1999). Myoblasts were transfected with CN* and/or VIVIT, or with vector(s) alone 5–6 h after final cell plating, using 2–4 μg of DNA per 35-mm dish, with a DNA:transfectant ratio of 1 μg DNA:3 μl reagent (FuGENE 6, Roche Molecular Biochemicals). We typically obtain transfection efficiencies above 25% with this protocol (our unpublished results). Four days after transfection, some cultures were administered 250 nM cyclosporin A (CSA; Sigma, St. Louis, MO), a specific blocker of calcineurin, or vehicle (ethanol). Cultures were refed with CSA- or vehicle-containing media every 3 d until harvest.
Immunostaining
Cells were rinsed with Dulbecco's PBS (DPBS), fixed in −20°C methanol for 5–7 min, washed in DPBS, incubated in DPBS supplemented with 1% BSA and 0.05% saponin (permeabilization solution), and blocked for 30 min with 10% goat serum and 1% BSA in DPBS. Cultures were then incubated overnight with primary antibodies against slow (type I) MHC (NOQ7.5.4D-IgG, diluted in the permeabilization solution 1:4000; Sigma) and fast (type II) or neonatal MHC (N3.36-IgM, diluted 1:4; Developmental Studies Hybridoma Bank, Iowa City, IA [please see ACKNOWLEDGMENTS]), followed by the secondary antibodies Cy3 anti-mouse IgG1, Fcγ fragment (1:400), and FITC anti-mouse IgM, Mu chain specific (1:300; both from Jackson ImmunoResearch Laboratories, West Grove, PA). Samples were mounted with Vectashield (Vector Laboratories, Burlingame, CA) to prevent fading. Some cultures were also immunostained for embryonic MHC with F1.652-IgG (used as the supernatant, DSHB) followed by the Cy3 anti-mouse IgG secondary antibody.
Photomicroscopy
Cultures were examined with a Zeiss Axioplan microscope (Carl Zeiss, Thornwood, NY) equipped for phase-contrast and fluorescence microscopy. Standard bandpass filter sets for tetramethylrhodamine and fluorescein isothiocyanate were used to detect Cy3 and FITC, respectively. Images were acquired with a Hamamatsu C4742–95 digital camera (Hamamatsu Photonic Systems, Bridgewater, NJ).
Western Blotting
Cells were washed in DPBS, scraped from the dish, centrifuged to pellet, and stored at −80°C until analysis. Contractile proteins were extracted from the cell samples according to standard procedures (Butler-Browne and Whalen, 1984). Briefly, the cell pellet was resuspended in 150 μl of a high-salt buffer (300 mM NaCl, 100 mM NaH2PO4, 50 mM Na2HPO4, 10 mM Na4P2O7, 10 mM EDTA, 1 mM MgCl2, pH 6.5, with 0.1% β-mercaptoethanol). After extraction on ice for 40 min, samples were centrifuged at 13,000 × g for 30 min to remove debris. Supernatants were diluted 10× in filament formation buffer (1 mM EDTA, 0.1% β-mercaptoethanol), and incubated on ice overnight to precipitate the myofibrillar proteins. The sample was centrifuged at 13,000 × g for 30 min, and the pellet was resuspended in sample buffer (500 mM NaCl, 12.5 mM NaH2PO4, pH 7.0, with 0.1 mg/ml pepstatin, antipain, and leupeptin) and then incubated on ice overnight to dissolve. Protein was determined using a bicinchoninic acid detection technique (Pierce, Rockford, IL).
Myofibrillar protein (8 μg/lane) was separated on 8% SDS-PAGE gels using the Laemmli buffer system (Laemmli, 1970) on a minigel apparatus (Novex, San Diego, CA). Gels were transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA) in 12 mM Tris, 96 mM glycine, and 0.05% SDS. Membranes were blotted for slow (type I) myosin (NOQ7.5.4D, diluted 1:40,000) or fast (type II) myosin (NCL MHCf, diluted 1:100; Vector Laboratories). After detection with the ECL system (Amersham Pharmacia Biotech, Piscataway, NJ), blots were stripped in 62.5 mM Tris-HCl, pH 6.8, 2% SDS, and 100 mM β-mercaptoethanol for 50 min at 50°C, reblocked, and then reblotted for total sarcomeric MHC (MF20, diluted 1:100, DSHB; see ACKNOWLEDGMENTS). Resultant bands were quantitated by densitometry (Fujifilm Image Analyzer LAS-1000, Fuji Medical Systems, Stamford, CT). All samples were run in duplicate. Fast and slow MHC were normalized to the total amount of sarcomeric MHC for each sample to control for variations inherent in cell culture and Western blotting. The specificity of the antibodies used for immunostaining and immunoblotting has been detailed previously by others (Narusawa et al., 1987; Ecob-Prince et al., 1989; Cho et al., 1993; Hughes et al., 1993) and was verified before data collection (our unpublished results). Differences between treatments were assessed with unpaired t tests, with p < 0.05. Data for the CSA, TTX, and CN experiments were obtained from three to six separate cell isolations, and data for the VIVIT studies were derived from six independent isolations.
RESULTS
Expression of Slow MHC Protein in Myotube Cultures
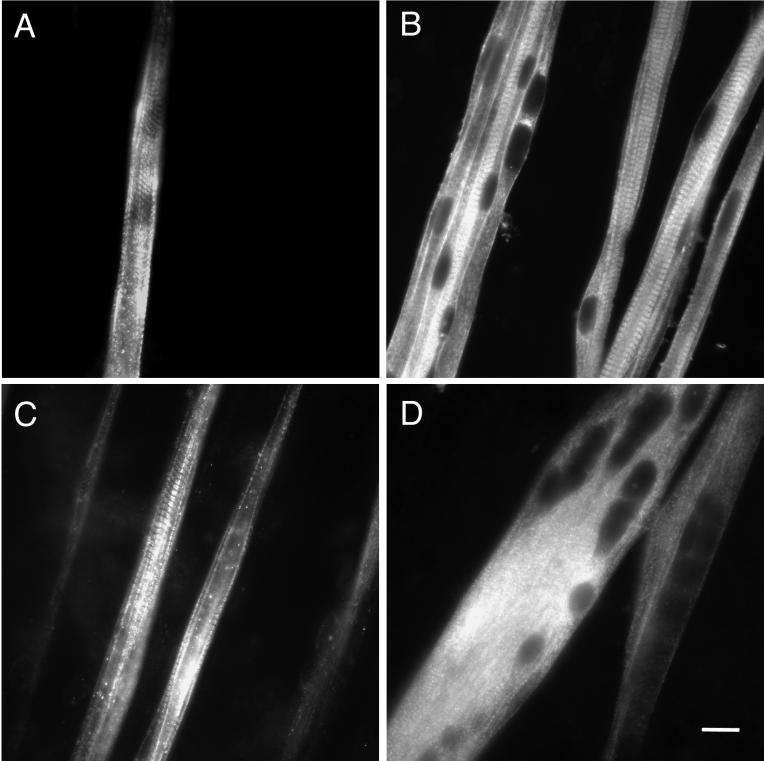
The initial goal of this study was to determine whether myotubes derived from myoblasts isolated from the secondary population of developing rat muscle could express slow MHC protein in culture. After plating, myoblasts fused within a few days into multinucleated myotubes that spontaneously contracted. After 7 d of culture, all myotubes immunostained for embryonic MHC. A subpopulation also stained with a monoclonal antibody that recognizes both neonatal and fast (type II) MHC (our unpublished results). At this time only diffuse staining for slow MHC (type I), which was barely distinguishable from background, was seen (our unpublished results). When the cultures were maintained for a total of 12–13 d after plating, slow MHC was detected by immunofluorescent staining. Distinct immunoreactivity was observed in a fraction of the myotubes (≤10%). Typically, the staining pattern was punctate, with some cross-striations indicating assembly of the slow MHC into sarcomeres. Staining intensity varied over the length of the myotubes (Figure 1A). At this time point, all myotubes were positive for embryonic MHC (Figure 1B), and many were also positive for neonatal/fast MHC (Figure 1C). Most myotubes exhibited well organized cross-striations and peripheral nuclei, indicating that they were well along in the course of muscle fiber development. In summary, we were able to detect slow MHC at the protein level in myotubes originating from the secondary population of muscle. These findings indicate that intrinsic signals necessary for turning on the slow program of MHC expression were present in this in vitro system.
Figure 1.
Protein expression of MHC isoforms in primary cultures of rat myotubes. Myoblasts were isolated from embryonic day 21 rats and cultured for 12–13 d, during which time they fused into multinucleated myotubes. They were fixed and immunostained as described in MATERIALS AND METHODS. (A) Typical view of myotubes stained for slow MHC, showing nonuniform staining and the presence of cross-striations. (B) Myotubes stained for embryonic MHC. Myotubes exhibited well organized cross-striations and often had peripheral nuclei. (C) Myotubes stained for fast/neonatal MHC illustrating the onset of sarcomeric organization and variations in staining intensity. (D) Culture treated with 1.5 μM TTX and stained for embryonic MHC. Myotubes were broader, had a paucity of cross-striations, and more central nuclei. Bar, 20 μm.
The Effects of Depolarization with Subsequent Contraction on Slow MHC Expression
The myotubes exhibited spontaneous contractions by 3 d of culture, continuing until the time of harvest. To determine whether spontaneous depolarization or contractile activity (for simplicity, henceforth termed “contraction”) influenced expression of slow MHC, cultures were treated with TTX from 2 d after plating until the time of harvest (12–13 d). This sodium channel blocker virtually abolished visible myotube contractions and eliminated distinct staining of slow MHC; however, TTX had no effect on slow MHC expression as detected by immunoblot (Figure 2A). TTX also had no effect on embryonic MHC as detected by Western blotting or immunostaining (our unpublished results). Immunostaining for embryonic and fast/neonatal MHC persisted, but cross-striations were rarely seen and myotubes tended to be broader, with more central nuclei (Figure 1D).
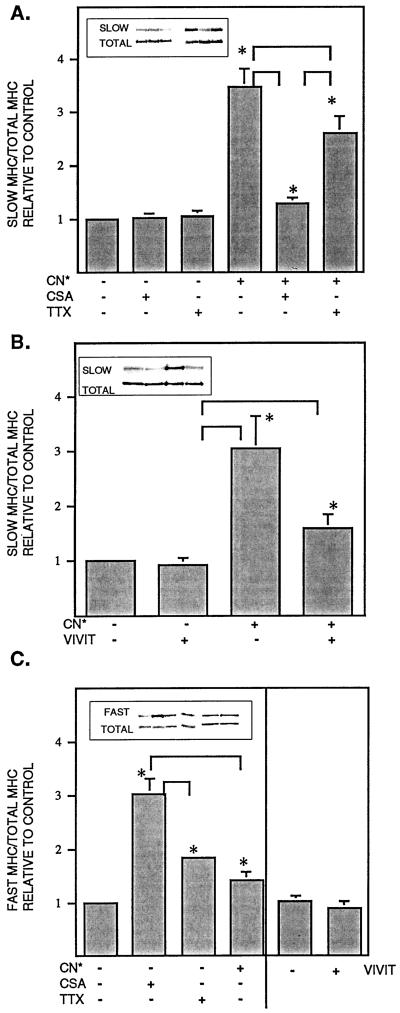
Figure 2.
Protein levels of slow and fast MHC in myotube cultures. (A) Slow MHC protein after administration of TTX, CSA, and/or transfection with CN*. (B) Slow MHC protein after transfection with CN* and/or VIVIT. (C) Fast MHC protein after administration of TTX, CSA, or transfection with CN* or VIVIT. Myoblasts were cultured for 12–13 d, then myofibrillar proteins were isolated and subjected to Western blotting as described in MATERIALS AND METHODS. Samples were blotted for slow or fast MHC, stripped, and then reblotted for total MHC. Values for slow or fast MHC were taken as a percentage of total MHC and then normalized to controls. Inset, blots from a single experiment shown in the same order as the data bars. Values are the mean ± SE of three to six independent experiments. *Significantly different from control, p < 0.05. Brackets, the two indicated values are significantly different, p < 0.05.
Although the levels of slow MHC that were detected by immunostaining in contracting myotubes were reduced by treatment with TTX (or CSA or VIVIT; see below), no changes in levels were detected by Western blot. This suggests that there was widespread constitutive expression of slow MHC at a level barely detectable by immunofluorescence but detectable via Western blotting of myofibrillar protein preparations. The small population of strongly immunopositive myotube segments in contracting cultures probably contributed only a small fraction of the total pool of slow MHC visualized by Western blotting.
Influence of the Calcineurin Pathway
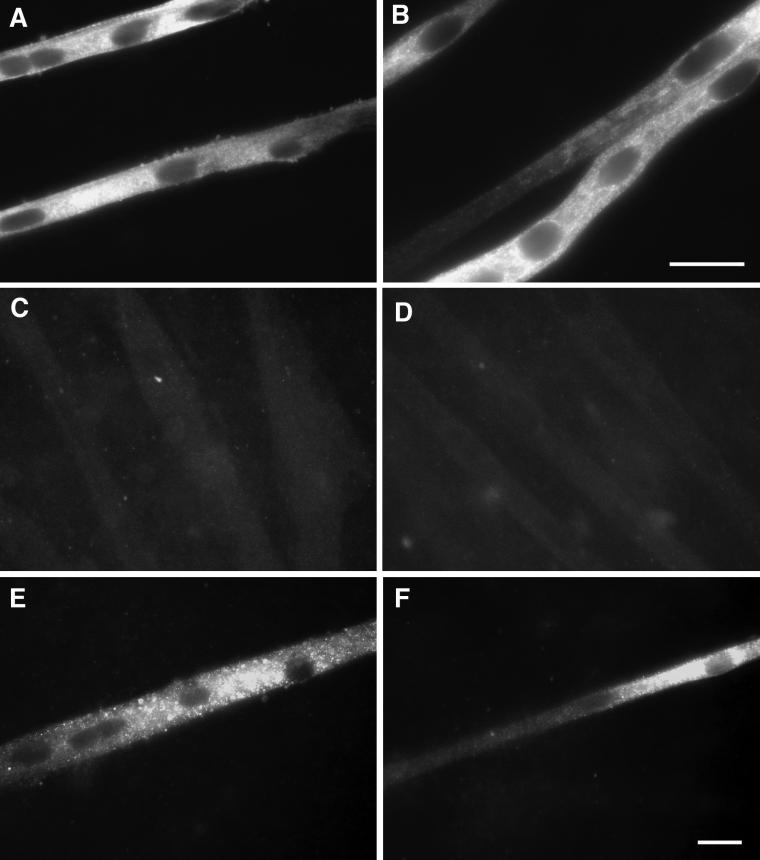
To determine whether calcineurin activation could induce expression of slow MHC during development, myoblasts were transfected with a constitutively active form of calcineurin (CN*) and cultured for 12–13 d. As shown in Figure 2A, CN* transfection resulted in a 2.5-fold increase in slow MHC as detected by Western blotting. Immunostaining of these cultures revealed a large population of myotubes that were very intensely labeled for slow MHC (Figure 3, A and B). This slow MHC was distributed throughout the myotubes in a punctate pattern. The nuclei were centrally located, and cross-striated patterns were noted only occasionally, suggesting that development was retarded. Administration of the selective calcineurin inhibitor CSA to CN*-transfected cultures eliminated 90% of the CN* effect as monitored by Western blotting (Figure 2A). Slow MHC was undetectable via immunostaining in all CSA-treated cultures (Figure 3, C and D). In control cultures transfected with the vector alone, CSA had no effect on slow MHC protein as detected by Western blot.
Figure 3.
Protein expression of slow MHC in myotube cultures. Cells were cultured as described in Figure 1 and immunostained with an antibody against slow MHC. (A and B) Culture transfected with CN*, showing widespread, intense punctate staining, central nuclei, and a scarcity of cross-striations. (C) Culture administered CSA. No staining above background was detected. (D) Culture transfected with CN* and administered CSA. No staining above background was detected. (E) Culture transfected with CN* and administered tetrodotoxin. Staining was decreased compared with transfection with CN* only. (F) Culture transfected with CN* and VIVIT. Staining was absent, or the intensity was greatly decreased compared with transfection with CN* only, except in limited regions of a few myotubes. Bars, 20 μm (bar in B refers only to that panel).
If the effects of contraction are mediated exclusively through calcineurin, we would anticipate that the consequences of CN* transfection would not be affected by TTX administration; however, addition of TTX to cells transfected with CN* resulted in a 34% reduction of the CN*-induced increase in slow MHC (Figure 2A). Immunostaining revealed two distinct populations of myotubes in the CN*/TTX cultures. In one group the myotubes were broad and stained for fast/neonatal MHC in the pattern described above for TTX-treated cultures (Figure 1D). A second population stained intensely for slow MHC, although less so than in the absence of TTX (Figure 3E). The morphology of these myotubes resembled that of CN*-transfected myotubes in the absence of TTX. This suggests that the effect of contraction on slow MHC expression was partially independent of calcineurin. Moreover, activation of calcineurin may modify the effects of blocking contraction. TTX, CN*, or the combination had no effect on levels of embryonic MHC (our unpublished results).
Alterations in Fast MHC
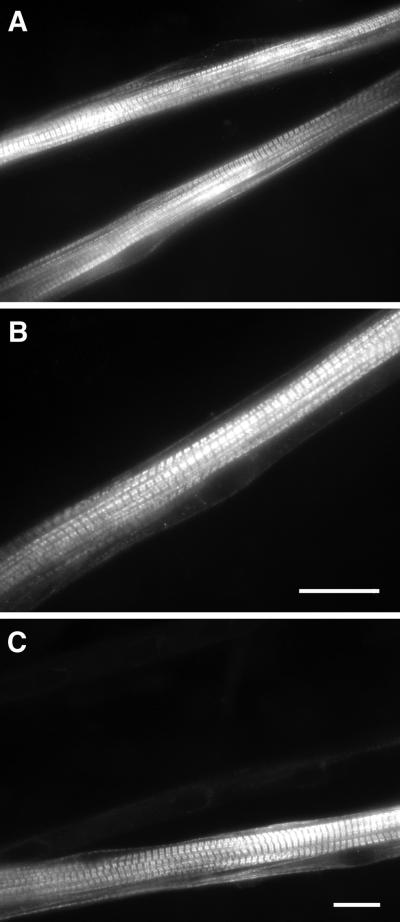
There is reciprocal regulation of slow-specific and fast-specific genes in muscle; hence inhibition of signal transduction pathways that lead to increased expression of slow MHC might be expected to increase expression of fast MHC and vice versa. We examined the levels of fast (type II) MHC in response to treatment with CN*, CSA, and TTX. Administration of CSA to control cultures resulted in a twofold increase in fast MHC expression as detected by Western blotting (Figure 2C). Immunostaining with an antibody that recognizes fast and neonatal MHC showed brightly stained, well developed myotubes with extensively aligned sarcomeres and peripheral nuclei (Figure 4, A and B).
Figure 4.
Protein expression of fast/neonatal MHC in myotube cultures. Cells were cultured as described in Figure 1 and immunostained with an antibody that crosses to both fast and neonatal MHC. (A and B) Culture treated with CSA showing intensely stained myotubes with well aligned sarcomeres and peripheral nuclei. (C) Culture transfected with calcineurin (CN*) demonstrating a typical pattern of organized cross-striations and peripheral nuclei. Note: The field in C is the same field as that of Figure 3A. Myotubes that were positive for fast/neonatal MHC were negative for slow MHC and vice versa. Bars, 20 μm (bar in B refers only to that panel).
Fast MHC protein levels were increased 40% (p < 0.05) in the CN*-transfected cultures (Figure 2C). Double immunostaining of the CN*-transfected cultures showed that myotubes stained strongly for either slow or fast/neonatal MHC, but not for both (Figures 3A and 4C). This suggests that there were two or more populations of myotubes that may have responded differently to the presence of CN*, resulting in a modest net increase in fast MHC levels as detected in immunoblots. Fast MHC protein levels increased 85% in control cultures treated with TTX (Figure 2C) (p < 0.05). As described above, the morphology of TTX-treated myotubes was relatively undifferentiated.
Calcineurin Activation: Role of NFAT
To determine whether the effects of CN* were mediated via activation of the transcription factor NFAT, myoblasts were cotransfected with VIVIT. This peptide selectively inhibits NFAT activation but does not disrupt other CN-dependent pathways (Aramburu et al., 1999). As shown in Figure 2B, transfection with CN* and the control vector resulted in a twofold increase in slow MHC, which was similar to the increase seen with CN* alone. Cotransfection of CN* with VIVIT blocked this increase by 70% as assessed by Western blotting (p < 0.05). Immunostaining revealed a marked decrease in the number of slow MHC-positive myotubes, as well as a decrease in staining intensity (Figure 3F). In marked contrast to the CSA-induced increase on fast MHC protein levels in control cultures, VIVIT had no effect (Figure 2C), indicating that the NFAT pathway was not involved in suppressing fast MHC expression.
DISCUSSION
Expression of Slow MHC
The first goal of this study was to determine whether myotubes isolated from a secondary (fetal) population of myoblasts from rat hindlimb could express the slow MHC isoform, an important determinant of the slow muscle phenotype. We detected slow MHC by both immunostaining and Western blot analysis after 12–13 d of culture, but not before. These results agree with Cho et al. (1993), who found that myoblast clones isolated from human tissue at various stages of embryonic and fetal development could express slow MHC after differentiation. In contrast, others were unable to detect slow MHC in secondary populations of myoblasts isolated from rats (Pin and Merrifield, 1993). This discrepancy may be attributable to differences in the length of time in culture; Pin and Merrifield (1993) cultured their cells for a maximum of 6 d. Vivarelli et al. (1988) detected slow MHC in ∼5% of their myotubes derived from secondary myoblasts maintained for 20 d. In agreement with this, we detected slow MHC via immunostaining in ≤10% of the myotubes. In vivo, only a small fraction of secondary fibers express slow MHC in the prenatal rat hindlimb (Condon et al., 1990a), and very few of the muscles ultimately express the slow phenotype in the adult (Ariano et al., 1973). Thus the ability to detect slow MHC in culture may depend additionally on the prospective phenotype of the muscles that are harvested.
The Effects of Contraction
Membrane depolarization and the resulting contraction appear to play a crucial role in secondary muscle fiber development; the formation of these fibers is compromised in rats after treatment of embryos with TTX (Harris, 1981; Harris et al., 1989). In the present study, the cultured myotubes exhibited widespread spontaneous contractions that were almost completely eliminated by the sodium channel blocker TTX. TTX administration had no effect on slow MHC expression as detected by immunoblot; however, as revealed by immunostaining, the TTX-treated myotubes had a more immature morphology, and no myotube segments with cross-striated patterns of slow MHC staining were detected. These findings agree with previous in vitro studies showing that administration of TTX to cultured muscle cells causes centralization of nuclei and disorganization of myofibrils (Tassin et al., 1988; Park-Matsumoto et al., 1992). In chick cultures, TTX inhibits the formation of neonatal MHC, so that only embryonic myosin is expressed (Cerny and Bandman, 1986). DiMario and Stockdale (1997) found that TTX prevented expression of slow MHC in their muscle–spinal cord explant cocultures. Taken together, these results suggest that depolarization or contractile activity plays an important role in in vitro myogenesis. In particular, it may have a role in assembly and maintenance of the sarcomeric lattice, and in the transition between myosin isoforms.
Influence of the Calcineurin Pathway
Calcineurin has been postulated to have a key role in the process by which changes in muscle gene expression are induced by information encoded in the motoneuron firing pattern (Chin et al., 1998; Bigard et al., 2000; Naya et al., 2000). In response to activation by Ca2+/calmodulin, calcineurin dephosphorylates a number of proteins, including the transcription factor, NFAT (for reviews, see Rao et al., 1997; Klee et al., 1998). The role of the calcineurin/NFAT pathway in establishment of fiber type during development has not been investigated. We transfected myoblasts that originated from the fetal/secondary wave of developing fibers with a constitutively active form of calcineurin (CN*). This resulted in a striking increase in slow MHC protein expression that could be blocked by administration of CSA, a specific inhibitor of calcineurin. This is the first report that demonstrates the action of this pathway in a primary cell culture model of development. Our findings are in good agreement with recent reports that activation of calcineurin up-regulates slow-fiber specific gene promoters in C2C12 cells (Chin et al., 1998), induces expression of slow MHC in C2C12 cells and MyoD-converted 10T1/2 cells (Delling et al., 2000), and leads to a doubling in slow MHC expression in the gastrocnemius of transgenic mice (Naya et al., 2000).
Reciprocal Regulation of Fast and Slow MHC
When slow-specific muscle genes are activated, corresponding fast-specific ones are typically repressed (Goldspink, 1998; Pette and Vrbova, 1999). We found that administration of CSA to control cultures resulted in an increase in fast MHC expression, in addition to the repression of slow MHC expression as seen by immunostaining. This suggests that repression of fast MHC expression via endogenous calcineurin occurred, such that CSA treatment derepressed expression. This agrees with in vivo studies demonstrating that in rats chronically administered CSA, some muscles exhibit a partial slow-to-fast MHC transition (Chin et al., 1998; Bigard et al., 2000). Bigard et al. (2000) noted that rats chronically administered CSA for 3 wk only exhibited a transition at the protein level from MHC type I to type IIa. They hypothesized that transition to faster phenotypes (MHC IIx/d, IIb) may involve additional pathways. Further work will be needed to clarify the extent of phenotypic transitions. The shift in MHC isoforms with CSA treatment has been shown to be accompanied by corresponding changes in metabolic markers such as sarcoplasmic reticulum ATPase, lactate dehydrogenase, and creatine kinase (Bigard et al., 2000). Thus the calcineurin-mediated regulation of fiber phenotype in both developmental and adult models probably occurs via both activation of slow-specific targets and repression of fast-specific targets.
Considering the reciprocal effects of CSA on slow and fast MHC, we anticipated that CN* transfection would lead to a decrease in fast MHC protein; however, it resulted in a modest increase in fast MHC. The immunostaining results for MHC isoforms may offer some insight into this apparent contradiction. In CN*-transfected cultures, myotubes that stained for slow MHC did not stain for fast/neonatal MHC and vice versa. It is possible that there were at least two populations of fibers; in one population, calcineurin activated slow MHC and suppressed fast MHC, whereas in a smaller population, calcineurin activated fast MHC, yielding a modest net increase. This would be consistent with the finding that the effect of calcineurin varies in different muscles (Bigard et al., 2000) and the tenet that expression of fiber phenotype is dependent on both extrinsic (contraction, innervation) and intrinsic (lineage) mechanisms (DiMario et al., 1997).
Calcineurin Activation: Role of NFAT and Interaction with Other Contraction-mediated Pathways
A key issue in elucidating the role of calcineurin in fiber type determination is to discern the pathway(s) through which this phosphatase functions. There are numerous substrates for calcineurin, including the transcription factor NFAT (for review, see Rao et al., 1997), of which three isoforms are found in muscle cells (NFATp/1a, c/2a, and x/c3/4x) (Abbott et al., 1998). To assess whether the effects of CN* were mediated via NFAT, we cotransfected a plasmid encoding the VIVIT peptide. This peptide contains a sequence based on the calcineurin docking motif of NFAT that selectively inhibits NFAT activation but does not disrupt other calcineurin-dependent pathways (Aramburu et al., 1999). VIVIT blocked the CN*-induced increase in slow MHC by 70%, suggesting that the ability of calcineurin to increase slow MHC expression is mediated in large part via activation of NFAT. Similarly, Chin et al. (1998) found that disruption of putative NFAT recognition elements in slow-specific promoters diminished the response to calcineurin by ∼75%. Activation of NFAT alone may not be sufficient to induce slow-specific gene activation; binding of other transcription factors such as MEF2 may be required (Chin et al., 1998; Wu et al., 2000). Furthermore, removal of the NFAT site from a 128-bp troponin I slow upstream element that confers slow-fiber–type expression, does not affect its activity (Calvo et al., 1999), suggesting that NFAT is not directly involved in the regulation of this slow gene.
Fast MHC expression was unaffected by cotransfection with VIVIT. The novel finding that CSA increased fast MHC but VIVIT had no effect suggests that the ability of calcineurin to repress fast MHC was mediated by a pathway that did not involve NFAT. These results are the first to demonstrate that calcineurin exerts effects on slow and fast targets via distinct pathways. Several substrates for calcineurin, including phosphorylase kinase (Papadopoulos et al., 1989), dystrophin (Walsh et al., 1995), and ryanodine receptors (Cameron et al., 1995), inositol 1,4,5-trisphosphate receptors (Lam et al., 1995), and dihydropyridine receptors (Hosey et al., 1986), are found in muscle. The route by which calcineurin influences fast MHC, as well as whether other markers of the fast phenotype are influenced in a similar manner, remains to be elucidated.
The calcineurin pathway is thought to be activated by a sequence of events proceeding from depolarization to elevated [Ca+2]i to activation of calcineurin (Chin et al., 1998; Hughes, 1998). In this scheme, muscle contraction, although also activated by elevated [Ca+2]i, is not directly involved. Thus we were interested in ascertaining whether the effects of constitutively active calcineurin were independent of depolarization and subsequent contraction. We found that administration of TTX to CN*-transfected cells resulted in a blunting of the CN*-induced increase in slow MHC. This new finding suggests that additional signaling pathways arising from muscle depolarization may mediate fiber type determination. Calcineurin has been shown to function in combination with proteins of the MEF2 and GATA families (Chin et al., 1998; Musaro et al., 1999; Wu et al., 2000). Additional molecules shown to play a role in phenotypic gene expression include Ras (Murgia et al., 2000), MusTRD1 (O'Mahoney et al., 1998), and myogenin (Hughes et al., 1999). Many of these pathways involve calcium, and not surprisingly, treatment of cultured cells with a calcium ionophore has been shown to induce a fast-to-slow fiber type transition (Meissner et al., 2000). Taken together, these findings intimate an explanation for the incomplete fiber-type shifts characteristic of in vivo studies. Transgenic mice that express activated calcineurin (Naya et al., 2000), as well as rats chronically administered CSA (Chin et al., 1998; Bigard et al., 2000), exhibit only modest shifts in fiber type. The calcineurin pathway may act in conjunction with other pathways that are present in neurally intact and spontaneously active models.
In conclusion, it is becoming clear that control of skeletal muscle phenotype during development, as well as in the adult, involves the interplay of numerous signaling pathways. We have shown that the calcineurin pathway plays a role in development in primary cultures of myotubes and that it interacts with other contraction-mediated systems via both NFAT and non-NFAT routes. These findings provide further clues regarding the determinants of phenotypic expression exhibited during skeletal muscle development.
ACKNOWLEDGMENTS
The technical assistance of Brian Lugo, Tehnaz Parakh, and Isaac Bernstein-Hanley is gratefully acknowledged. We thank Dr. Birgit Neuhuber for her assistance with muscle transfection, and Drs. Kathleen McCormick and Evelyn Ralston for critical reading of this article. Monoclonal antibodies against sarcomeric myosin (MF20, developed by D. A. Fischman), embryonic myosin (F1.652, developed by H. M. Blau), and neonatal/fast myosin (N3.36, H. M. Blau) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. We thank Drs. R. S. Williams and A. Rao for the kind gifts of the calcineurin and VIVIT vectors, respectively.
REFERENCES
- Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell. 1998;9:2905–2916. doi: 10.1091/mbc.9.10.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973;21:51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Bigard X, Sanchez H, Zoll J, Mateo P, Rousseau V, Veksler V, Ventura-Clapier R. Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J Biol Chem. 2000;275:19653–19660. doi: 10.1074/jbc.M000430200. [DOI] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurons and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Browne GS, Whalen RG. Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev Biol. 1984;102:324–334. doi: 10.1016/0012-1606(84)90197-0. [DOI] [PubMed] [Google Scholar]
- Calvo S, Venepally P, Cheng J, Buonanno A. Fiber-type-specific transcription of the troponin I slow gene is regulated by multiple elements. Mol Cell Biol. 1999;19:515–525. doi: 10.1128/mcb.19.1.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. 1995;83:N463–N472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Cerny LC, Bandman E. Contractile activity is required for the expression of neonatal myosin heavy chain in embryonic chick pectoral muscle cultures. J Cell Biol. 1986;103:2153–2161. doi: 10.1083/jcb.103.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M, Webster SG, Blau HM. Evidence for myoblast-extrinsic regulation of slow myosin heavy chain expression during muscle fiber formation in embryonic development. J Cell Biol. 1993;121:795–810. doi: 10.1083/jcb.121.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon K, Silberstein L, Blau HA, Thompson WJ. Development of muscle fiber types in the prenatal rat hindlimb. Dev Biol. 1990a;138:256–274. doi: 10.1016/0012-1606(90)90196-p. [DOI] [PubMed] [Google Scholar]
- Daniels MP, Lowe BT, Shah S, Ma J, Samuelsson SJ, Lugo B, Parakh T, Uhm C-S. A rodent nerve-muscle culture system for studies of neuromuscular junction development: refinements and applications. Microsc Res Tech. 2000;49:26–37. doi: 10.1002/(SICI)1097-0029(20000401)49:1<26::AID-JEMT4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol. 2000;20:6600–6611. doi: 10.1128/mcb.20.17.6600-6611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMario JX, Stockdale FE. Both myoblast lineage and innervation determine fiber type and are required for expression of the slow myosin heavy chain 2 gene. Dev Biol. 1997;188:167–180. doi: 10.1006/dbio.1997.8619. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Dutton EK, Uhm C-S, Samuelsson SJ, Schaffner AE, Fitzgerald SC, Daniels MP. Acetylcholine receptor aggregation at nerve-muscle contacts in mammalian cultures: induction by ventral spinal cord neurons is specific to axons. J Neurosci. 1995;15:7401–7416. doi: 10.1523/JNEUROSCI.15-11-07401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecob-Prince M, Hill M, Brown W. Immunocytochemical demonstration of myosin heavy chain expression in human muscle. J Neurol Sci. 1989;91:71–78. doi: 10.1016/0022-510x(89)90076-2. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Selective gene expression during adaptation of muscle in response to different physiological demands. Comp Biochem Physiol B. 1998;120:5–15. doi: 10.1016/s0305-0491(98)00018-2. [DOI] [PubMed] [Google Scholar]
- Harris AJ. Embryonic growth and innervation of rat skeletal muscles. I. Neural regulation of muscle fiber numbers. Philos Trans R Soc Lond B Biol Sci. 1981;293:257–277. doi: 10.1098/rstb.1981.0076. [DOI] [PubMed] [Google Scholar]
- Harris AJ, Fitzsimons RB, McEwan JC. Neural control of the sequence of expression of myosin heavy chain isoforms in fetal mammalian muscles. Development. 1989;107:751–769. doi: 10.1242/dev.107.4.751. [DOI] [PubMed] [Google Scholar]
- Hosey M, Borsotto M, Lazdunski M. Phosphorylation and dephosphorylation of dihydropyridine-sensitive voltage-dependent Ca2+ channel in skeletal muscle membranes by cAMP- and Ca2+-dependent processes. Proc Natl Acad Sci USA. 1986;83:3733–3737. doi: 10.1073/pnas.83.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SM. Muscle development: electrical control of gene expression. Curr Biol. 1998;8:R892–R894. doi: 10.1016/s0960-9822(07)00554-4. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Chi MM-Y, Lowry OH, Gundersen K. Myogenin induces a shift of enzyme activity from glycolytic to oxidative metabolism in muscles of transgenic mice. J Cell Biol. 1999;145:633–642. doi: 10.1083/jcb.145.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM. Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol. 1993;158:183–199. doi: 10.1006/dbio.1993.1178. [DOI] [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam E, Martin M, Timerman A, Sabers C, Fleischer S, Lukas T, Abraham R, O'Keefe S, O'Neill E, Wiederrecht G. A novel FK506 binding protein can mediate the immunosuppressive effects of FK506 and is associated with the cardiac ryanodine receptor. J Biol Chem. 1995;270:26511–26522. doi: 10.1074/jbc.270.44.26511. [DOI] [PubMed] [Google Scholar]
- Meissner JD, Kubis H-P, Scheibe RJ, Gros G. Reversible Ca2+-induced fast-to-slow transition in primary skeletal muscle culture cells at the mRNA level. J Physiol. 2000;523:19–28. doi: 10.1111/j.1469-7793.2000.t01-1-00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia M, Serrano AL, Calabria E, Pallafacchina G, Lømo T, Schiaffino S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol. 2000;2:142–147. doi: 10.1038/35004013. [DOI] [PubMed] [Google Scholar]
- Musaro A, McCullagh KJA, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- Narusawa M, Fitzsimons RB, Izumo S, Nadal-Ginard B, Rubinstein NA, Kelly AM. Slow myosin in developing rat skeletal muscle. J Cell Biol. 1987;104:447–459. doi: 10.1083/jcb.104.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- O'Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, O'Neill EA. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- O'Mahoney JV, Guven KL, Lin J, Joya JE, Robinson CS, Wade RP, Hardeman EC. Identification of a novel slow-muscle-fiber enhancer binding protein, MusTRD1. Mol Cell Biol. 1998;18:6641–6652. doi: 10.1128/mcb.18.11.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos B, Brown AS, Hall PF. Isolation and characterization of calcineurin from adrenal cell cytoskeleton: identification of substrates for Ca2+-calmodulin dependent phosphatase activity. Mol Cell Endocrinol. 1989;63:23–28. doi: 10.1016/0303-7207(89)90078-6. [DOI] [PubMed] [Google Scholar]
- Park-Matsumoto YC, Askanas V, Engel WK. The influence of muscle contractile activity versus neural factors on morphologic properties of innervated cultured human muscle. J Neurocytol. 1992;21:329–340. doi: 10.1007/BF01191701. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve. 1999;22:666–677. doi: 10.1002/(sici)1097-4598(199906)22:6<666::aid-mus3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Pin CL, Merrifield PA. Embryonic and fetal rat myoblasts express different phenotypes following differentiation in vitro. Dev Genet. 1993;14:356–368. doi: 10.1002/dvg.1020140505. [DOI] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Tassin AM, Pincon-Raymond M, Paulin D, Rieger F. Unusual organization of desmin intermediate filaments in muscular dysgenesis and TTX-treated myotubes. Dev Biol. 1988;129:37–47. doi: 10.1016/0012-1606(88)90159-5. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- Vivarelli E, Brown WE, Whalen RG, Cossu G. The expression of slow myosin during mammalian somitogenesis and limb bud differentiation. J Cell Biol. 1988;107:2191–2197. doi: 10.1083/jcb.107.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M, Busaan J, Fraser E, Fu S, Pato M, Michalak M. Characterization of the recombinant C-terminal domain of dystrophin: phosphorylation by calmodulin-dependent protein kinase II and dephosphorylation by type 2B protein phosphatase. Biochemistry. 1995;34:5561–5568. doi: 10.1021/bi00016a030. [DOI] [PubMed] [Google Scholar]
- Whalen RG, Sell SM, Butler-Browne GS, Schwartz K, Bouveret P, Pinset-Harstrom L. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1987;292:805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- Wu H, Naya FJ, McKinsey TA, Mercen B, Shelton JM, Chin ER, Simard AR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000;19:1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]