Abstract
OBJECTIVE:
The optimal strategy for fluid management during gastrointestinal surgery remains unclear. Minimizing the variation in arterial pulse pressure, which is induced by mechanical ventilation, is a potential strategy to improve postoperative outcomes. We tested this hypothesis in a prospective, randomized study with lactated Ringer's solution and 6% hydroxyethyl starch solution.
METHOD:
A total of 60 patients who were undergoing gastrointestinal surgery were randomized into a restrictive lactated Ringer's group (n = 20), a goal-directed lactated Ringer's group (n = 20) and a goal-directed hydroxyethyl starch group (n = 20). The goal-directed fluid treatment was guided by pulse pressure variation, which was recorded during surgery using a simple manual method with a Datex Ohmeda S/5 Monitor and minimized to 11% or less by volume loading with either lactated Ringer's solution or 6% hydroxyethyl starch solution (130/0.4). The postoperative flatus time, the length of hospital stay and the incidence of complications were recorded as endpoints.
RESULTS:
The goal-directed lactated Ringer's group received the greatest amount of total operative fluid compared with the two other groups. The flatus time and the length of hospital stay in the goal-directed hydroxyethyl starch group were shorter than those in the goal-directed lactated Ringer's group and the restrictive lactated Ringer's group. No significant differences were found in the postoperative complications among the three groups.
CONCLUSION:
Monitoring and minimizing pulse pressure variation by 6% hydroxyethyl starch solution (130/0.4) loading during gastrointestinal surgery improves postoperative outcomes and decreases the discharge time of patients who are graded American Society of Anesthesiologists physical status I/II.
Keywords: Pulse Pressure Variation, Goal-Directed Fluid Therapy, Restrictive Fluid Therapy, Gastrointestinal Surgery
INTRODUCTION
Fluid therapy is a routine practice during surgery; however, the type, amount and timing of fluids administered to patients who undergo major abdominal surgery are under debate among anesthesiologists. The perioperative fluid balance has been highlighted as a major contributory factor in postoperative morbidity and mortality. Fluid therapy strategies have been developed and implemented in clinical practice over several decades. The data suggest that aggressive or “liberal” intraoperative fluid resuscitation is harmful during open abdominal operations, whereas a restrictive fluid protocol has better outcomes, including fewer postoperative complications and a shorter discharge time (1)-(3). However, a restrictive fluid regimen has several limitations (4). Overly restricted or inadequate fluid administration may lead to insufficient intravascular volume, tissue hypoperfusion, cellular oxygenation impairment and potential organ dysfunction (5), which will increase the complication rate, hospital stay and mortality.
Recently, goal-directed fluid therapy has been the focus of several studies on fluid therapy strategies. These studies demonstrated a reduction in the postoperative complication rate and the length of hospital stay with a goal-directed strategy compared with a conventional strategy (6). However, few studies have examined the comparative outcome performances of restrictive and goal-directed fluid therapy in major intra-abdominal surgery; therefore, we conducted a prospective, randomized, controlled study to compare the length of postoperative hospital stay and the recovery of bowel function in patients who were undergoing gastrointestinal surgery with either an intraoperative restrictive or pulse pressure variation [PPV]-directed fluid therapy protocol.
MATERIALS AND METHODS
Subjects
After receiving approval from the institutional review board of Huashan hospital and obtaining written informed consent from the study participants, 60 patients who were undergoing elective gastrointestinal surgeries with an anticipated blood loss of less than 500 ml were included in the study. The inclusion criteria were patients with gastric or colonic cancer who were 18-64 years of age. Patients with a body mass index (BMI)>30, significant arrhythmias, cardiopulmonary dysfunction, extensive peripheral arterial occlusive disease, significant renal or liver diseases, pregnancy or lactation and coagulopathy were excluded.
Anesthesia and monitoring
The surgery was preceded by an 8-hour fasting period. Upon the arrival of the patients in the operating room, the left side of the cubital vein was catheterized in all of the patients. In addition to routing monitoring, including noninvasive arterial blood pressure, electrocardiography and pulse oximetry, invasive hemodynamic monitoring (Datex Ohmeda S/5, Helsinki, Finland) was initiated in all of the patients under local anesthesia. An A-line was established via a 20-G catheter that was inserted in the radial artery for invasive arterial pressure measurement. A double-lumen central venous catheter (CV-17702, Arrow International, Inc., Reading, PA) was inserted in the right internal jugular vein to monitor the central venous pressure (CVP). All of the monitoring transducers were positioned and zeroed at the midaxillary level. The data registration was excluded when a fast flush test indicated an unacceptable pressure recording. The heart rate, the mean arterial pressure (MAP) and the CVP were continuously monitored during the operation.
The induction of anesthesia was conducted with intravenous (IV) midazolam (0.04 mg/kg), propofol (1.5-2 mg/kg) and fentanyl (3 µg/kg). Succinylcholine (2 mg/kg) IV was used to facilitate tracheal intubation. A volume-controlled mode with zero end-expiratory pressure was used to ventilate the lungs in all of the patients (Drager Julian, Philips Healthcare, Tilburg, The Netherlands). The tidal volume was maintained at 8-10 ml/kg, and the respiratory rate was maintained at 10 beats/minute to maintain the end-tidal carbon dioxide partial pressure at 35-40 mmHg. The ventilator settings were unchanged during the study. Patients whose peak airway pressures exceeded 40 mmHg were excluded from the study. Anesthesia was maintained with a 2.5-3% concentration of sevoflurane in O2, and fentanyl and vecuronium were administered intermittently for intraoperative analgesia and muscle relaxation. Immediately after induction, all of the patients received 2.0 g of cefazolin intravenously as an antibiotic prophylaxis. The body temperature was maintained over 36°C with a fluid warmer throughout surgery. All of the surgeries in this study were performed by the same surgical team.
Pulse pressure variation measurement
The PPV was measured using the simple tools in the Datex Ohmeda S/5 Monitor as previously described (7,8). The PPV (%) was calculated using the following formula:$${\rm PPV }\left( \% \right)\equals {\rm 2}00 \times \left( {{\rm PP}_{{\rm max}} \minus {\rm PP}_{{\rm min}} } \right)/\left( {{\rm PP}_{{\rm max}} \plus {\rm PP}_{{\rm min}} } \right)$$where PPmax and PPmin are the maximal and minimal pulse pressure values, respectively, within one respiratory cycle. The PPV (%) was calculated in triplicate over three consecutive respiratory cycles. The mean value of the three determinations was used for the analysis. As a reference for fluid administration in the goal-directed therapy groups, the PPV values were measured every 15 minutes during the operation.
Grouping and fluid therapy protocol
Baseline measurements were instituted after induction and hemodynamic stabilization. Next, the patients were randomly assigned to one of three groups according to the intraoperative fluid protocol using a random number generator in sealed envelopes.
The restrictive Ringer's lactate (R-RL) group (n = 20) received a fixed infusion of 4 ml/kg per hour of lactated Ringer's solution exclusively throughout the operation. The PPV was not measured in the R-RL group. If the urine output was continuously <0.5 ml/kg/h over two hours or the CVP was less than 4 mmHg, 250-ml boluses of lactated Ringer's solution were administered until these targets were restored.
The goal-directed Ringer's lactate (GD-RL) group (n = 20) received a fixed infusion of 4 ml/kg per hour of lactated Ringer's solution throughout the operation. In addition, this group received 250 ml of lactated Ringer's solution as a bolus in 15 minutes if the PPV was >11%.
The goal-directed colloid (GD-C) group (n = 20) received a fixed infusion of 4 ml/kg per hour of lactated Ringer's solution throughout the operation. In addition, this group received 250 ml of 6% hydroxyethyl starch (HES, 130/0.4) as a bolus in 15 minutes if the PPV was >11%.
Intraoperative 4 ml/kg/h lactated Ringer's solution was infused continuously at a constant rate via an infusion pump (TOP-3300®, TOP Corporation, Japan). The mean arterial pressure was maintained within ±20% of the baseline value during the operation. Blood loss was replaced with HES at a 1:1 ratio, and the blood transfusion was started when clinically indicated and supported by laboratory evidence of a hematocrit less than 28%.
Postoperative management
Anesthesia was discontinued when the operation was completed. A total of 80 mg of parecoxib and 5 mg of morphine was injected intravenously for analgesia 30 minutes before the end of the surgery. Patients were extubated in the operating room when they fulfilled the standard clinical criteria (adequate protective reflexes, adequate oxygenation, and stable hemodynamics). Once the patients were sent to the ward, follow-up was conducted by an independent researcher (Zhiyong He) who was unaware of the randomization of the patient until the patient was discharged from the hospital. The same surgical team was in charge of the postoperative care of the patients, including fluid infusion (a baseline crystalloid infusion of 1.5-2 ml/kg/h to maintain normovolemia with 2.0 grams of cefazolin daily for three days) and postoperative analgesia (a daily intravenous infusion of 100 mg of flurbiprofen axetil for three days following surgery and an intramuscular injection of 10 mg of morphine as a rescue analgesic). To treat postoperative nausea and vomiting, 10 mg of metoclopramide was administered intravenously. The discharge criteria adopted the standard protocol that was predefined by the Department of General Surgery at our hospital.
The endpoints of the study
In this study, the primary endpoint was the postoperative length of hospital stay, and the secondary endpoints were the time to bowel flatus and postoperative complications. Additionally, the preoperative and postoperative biochemical and hemodynamic variables; the type and volume of the intraoperative fluid infusions; the estimation of blood loss; the urine output; and the medications were recorded.
Statistical analysis
To calculate the sample size, we used the retrospective data that were available for the same surgical population at our hospital. A one-day reduction in the postoperative length of hospital stay was considered to be clinically relevant, which required approximately 20 patients with a type I error of 0.05 and a power of at least 90%.
The patient characteristics and the perioperative data were analyzed using a one-way analysis of variance (ANOVA), and the differences between the individual treatment groups were determined using the Student–Newman–Keuls test. The differences between the treatment groups according to the incidence of adverse events were determined using the two-tailed Fisher's exact test. The statistical analysis was performed using the SPSS 15.0 statistical software (SPSS, Inc., Chicago, IL). The statistical significance was accepted at p<0.05.
RESULTS
A total of 60 patients with gastrointestinal cancer who were undergoing laparotomy were enrolled. No patients were excluded or dropped out of the study. There were no significant differences between the groups regarding the demographic characteristics and preoperative diseases (Table 1).
Table 1.
Patient demographic data and clinical characteristics.
| GD-RL(n = 20) | GD-C(n = 20) | R-RL(n = 20) | |
| Age (yrs) | 56.7±6.9 | 52.8±11.8 | 53.3±13.0 |
| Sex (M/F) | 14/6 | 14/6 | 14/6 |
| Height (cm) | 167.1±6.6 | 167.3±9.4 | 165.0±8.0 |
| Weight (kg) | 60.1±10.7 | 62.9±7.4 | 62.3±9.6 |
| ASA class (I/II) | 11/9 | 10/10 | 11/9 |
| Comorbidity | |||
| Diabetes | 3 (15%) | 2 (10%) | 3 (15%) |
| Hypertension | 5 (25%) | 4 (25%) | 3 (15%) |
| CAD | 2 (10%) | 1 (5%) | 1 (5%) |
| COPD | 4 (20%) | 5 (20%) | 3 (15%) |
| Asthma | 1 (5%) | 0 (0) | 0 (0) |
ASA: American Society of Anesthesiologists; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease.
The data are presented as the means±SD or the number (%).
Notes: All of the patients with comorbidities had been optimized before anesthesia and surgery.
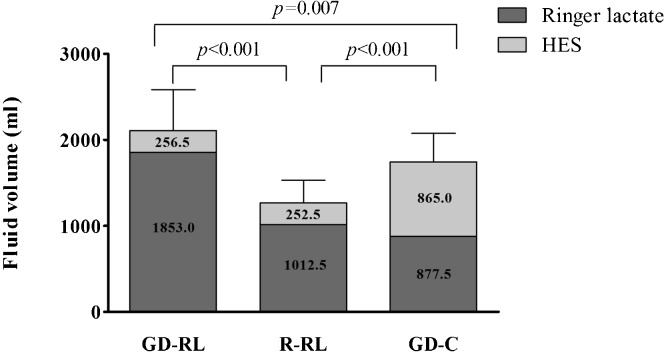
The perioperative data are shown in Table 2. No differences were observed between the types of surgery, operation duration, intraoperative blood loss, and analgesic consumption (fentanyl). However, the urine output in the GD-RL group (485.0±93.3 ml) was greater than that in the other two groups (GD-C: 295.0±48.4 ml, p<0.001; R-RL: 277.5±63.8 ml, p<0.001). The patients in the GD-C group required less vasopressors (phenylephrine or ephedrine) than the patients in the GD-RL group (p = 0.001) or the R-RL group (p<0.001). The amount of the intraoperative fluid infusion in the GD-RL group (2109.50±474.25 ml) was significantly higher than those in the other groups (GD-C: 1742.50±333.01 ml, p = 0.007; R-RL: 1260.00±269.44 ml, p<0.001) (Figure 1). Specifically, the GD-RL group (1853.0±381.3 ml) received more crystalloid solution than the GD-C group (877.5±130.0 ml, p<0.001) or the R-RL group (1012.5±238.4 ml, p<0.001), whereas the GD-C group (865.0±297.4 ml) received more colloid solution than the GD-RL group (256.5±139.9 ml, p<0.001) and the R-RL group (252.5±44.4 ml, p<0.001) (Figure 1). No patient required a blood transfusion according to the hematocrit measurements during the operation.
Table 2.
Perioperative data.
| GD-RL | GD-C | R-RL | |
| Type of surgery | |||
| Gastrectomy | 15 | 12 | 13 |
| Colectomy | 5 | 8 | 7 |
| Duration of surgery (min) | 190.3±40.2 | 183.0±13.8 | 182.5±18.0 |
| Blood loss (ml) | 256.5±139.9 | 265.0±46.2 | 252.5±44.4 |
| Urine output (ml) | 485.0±93.3† | 295.0±48.4 | 277.5±63.8 |
| Medications | |||
| Phenylephrine (µg) | 120 (120-160) | 80 (40-80) † | 190 (180-200) |
| Ephedrine (mg) | 12.5 (10-18.75) | 5 (0-5) † | 15 (15-20) |
| Fentanyl (µg) | 625.0±67.9 | 655.0±48.4 | 660.0±80.5 |
| PPV value (%) | |||
| Baseline | 13.6±2.4 | 13.7±2.1 | – |
| End of surgery | 7.1±1.2*) | 7.0±1.0*) | – |
| CVP value (mmHg) | |||
| Baseline | 6.2±1.1 | 6.3±1.2 | 6.2±1.0 |
| End of surgery | 8.7±0.9 | 8.6±1.0 | 7.8±1.5 |
| Albumin (g/l) | |||
| Preoperative | 39.5±2.5 | 40.0±2.8 | 40.1±3.5 |
| First postoperative day | 33.5±2.1*) | 34.1±2.0*) | 38.7±2.9† |
| Creatine (µmol/l) | |||
| Preoperative | 71.1±8.0 | 66.8±10.4 | 68.9±10.6 |
| First postoperative day | 66.7±8.1 | 61.4±8.9 | 63.1±10.2 |
| Hematocrit (%) | |||
| Preoperative | 41.9±3.0 | 43.3±3.8 | 42.5±2.8 |
| First postoperative day | 35.9±2.0*) | 38.8±2.3*) | 41.3±2.6† |
HES: 6% hydroxyethyl starch (130/0.4); CVP: central venous pressure.
The data are presented as the means±SD or the medians (25-75 interquartile range).
, p<0.05, compared with the preoperative data; †, p<0.05, compared with the GD-RL group and the GD-C group.
Figure 1.
Intraoperative fluid infusion volume.
The baseline hematocrit, biochemical parameters (albumin and creatine levels) and hemodynamic variables (CVP and PPV) were comparable among the three groups (Table 2). Significant differences were found between the hematocrit, albumin and PPV values at the end of surgery and the baseline values in the GD-RL and GD-C groups (Table 2).
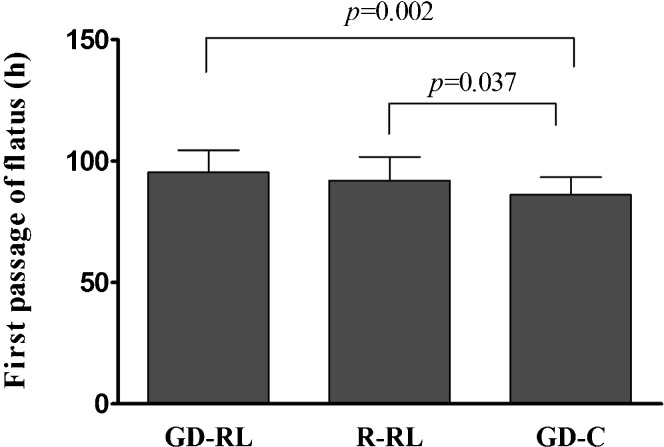
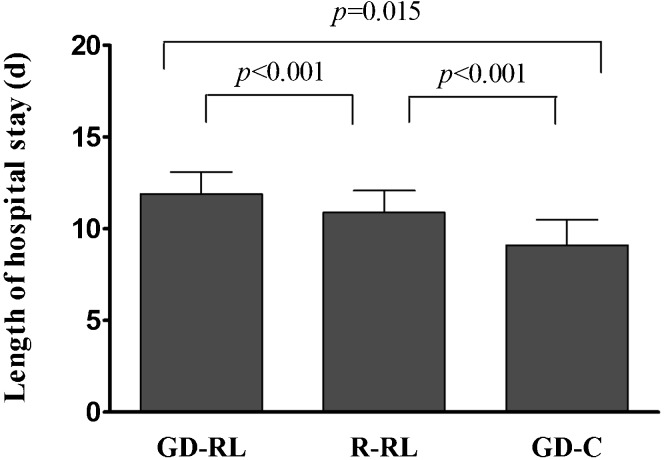
The postoperative analgesic consumption, fluid administration and complications were similar among the three groups (p>0.05, Table 3. No anastomotic leaks or intra-abdominal bleeding occurred, and all of the patients survived during the study period. The time to first passage of flatus was 86.2±7.2 h in the GD-C group versus 92.1±9.7 h (p = 0.03) in the R-RL group and 95.4±9.1 h (p<0.001) in the GD-RL group (Figure 2). The length of postoperative hospital stay was 9.1±1.4 days in the GD-C group versus 10.9±1.2 days (p<0.001) in the R-RL group and 11.9±1.2 days (p<0.001) in the GD-RL group (Figure 3).
Table 3.
Postoperative data (n = 20).
| GD-RL | GD-C | R-RL | |
| Morphine consumption (mg) | 61.7±28.4 | 52.5±30.2 | 59.0±32.0 |
| Fluid infusion volume per day (ml) | 2342.5±242.4 | 2442.5±164.1 | 2412.5±217.6 |
| Complications | |||
| Postoperative vomiting | 5 | 3 | 4 |
| Arrhythmia* | 1 | 0 | 0 |
| Pulmonary infection | 1 | 0 | 0 |
| Bowel obstruction | 0 | 0 | 1 |
| SSI | 1 | 1 | 0 |
| Total complications | 8 | 4 | 5 |
SSI: surgical site infection; Arrhythmia*: paroxysmal ventricular premature.
The data are presented as the means±SD or the number.
Figure 2.
Time to first passage of flatus.
DISCUSSION
Perioperative fluid replacement is a challenging issue in surgical care, especially in a procedure-specific model. Recently, goal-directed fluid therapy has been introduced into clinical practice as part of perioperative management. To our knowledge, this study is the first to directly compare the effects of an intraoperative restrictive protocol with a PPV-directed protocol on the outcomes of patients undergoing gastrointestinal surgery. Our results suggest that PPV-directed therapy with HES solution results in a shorter postoperative hospital stay and faster bowel function recovery than an infusion with either restricted or PPV-directed lactated Ringer's solution. However, the incidence of postoperative complications was similar among the patients who were treated with the three fluid strategies.
The accuracy and early recognition of the intravascular volume status is essential to prevent both hypoperfusion due to volume depletion and fluid overload due to an unnecessary infusion. Therefore, appropriate hemodynamic monitoring is necessary for intraoperative fluid management. A simple, affordable and reliable method to achieve this goal would be appropriate for routine intraoperative application. PPV measurement with a multiparameter monitor that is commonly used in clinical practice has been described and successfully used for intraoperative fluid therapy (9,10). This type of PPV monitoring is not associated with additional costs or complications other than arterial catheterization. PPV monitoring has been recommended to guide volume expansion in surgical patients. Lopes et al. demonstrated that intraoperative PPV-guided fluid therapy during high-risk surgery improved postoperative outcomes and decreased the length of hospital stay (11). Our previous studies demonstrated that the PPV, which was derived from the Datex method, was comparable with the stroke volume variation (SVV) using the FloTracTM/VigileoTM system (7) and better than the CVP measurement (8) for intravascular volume assessment. Therefore, we used PVV = 11%, which was obtained from our previous study (7), as the threshold of hypovolemia to target volume optimization in this study.
Many studies have investigated the effects of the amount of intraoperative fluid administration on perioperative outcomes. A meta-analysis demonstrated that a restrictive intraoperative (3,12,13) and postoperative (14) fluid protocol in major abdominal surgery reduces the incidence of perioperative complications, such as cardiopulmonary events and disturbances in bowel motility; improves wound and anastomotic healing; and reduces the length of the hospital stay. In addition, several studies examined the effects of intraoperative fluid therapy that is guided by various hemodynamic targets, such as conventional hemodynamic parameter-guided and functional hemodynamic parameter-directed fluid therapy, on perioperative outcomes. Studies demonstrated that patient outcomes improved after functional hemodynamic parameter (PPV or SVV)-directed fluid therapy in major surgery (15)-(18). Therefore, two optimal intraoperative strategies have been proposed: restrictive and goal-directed fluid therapy. However, patients who are treated using a goal-directed protocol receive greater amounts of fluid than patients who are treated with a restrictive protocol. Several studies have questioned the positive effects of goal-directed fluid therapy. Senagore et al. (19) revealed that esophageal Doppler monitoring-guided fluid administration in laparoscopic colectomy predisposed patients to a longer hospital stay and significantly increased complications. Lahner et al. (20) demonstrated that SVV may not be a reliable predictor of fluid responsiveness in the setting of major abdominal surgery. PPV is considered to be more reliable than SVV and can be used to recognize volume contraction earlier than other indicators (21,22); however, amplified PPV does not represent the hypovolemic status, such as anesthesia or inflammation-induced vasodilation (10). Therefore, restrictive fluid infusion with vasopressor administration during abdominal surgery has been advocated to prevent fluid retention after the anesthetic effects have subsided. However, recent randomized trials did not confirm the potential benefits of fluid restriction on recovery after elective surgery (3,13). Intraoperative fluid restriction was associated with frequent episodes of intraoperative hypovolemia (23), which is a major determinant of postoperative organ dysfunction (11) and an independent predictor of postoperative complications, such as an anastomotic leak and postoperative sepsis (5). Whether goal-directed therapy is superior to a restrictive fluid strategy in major abdominal surgery remains uncertain. Therefore, we designed a prospective, randomized study to compare the effects of restrictive and goal-directed fluid therapy on perioperative outcomes in patients who were undergoing open gastrointestinal surgery. The goal-directed therapy group was further divided into two subgroups, a GD-RL group and a GD-C group, to compare the role of fluid type in intraoperative fluid therapy. In this study, a total fluid volume of 1742 ml in the GD-C group was sufficient for organ perfusion in open abdominal surgery over a 3-hour period; however, a greater volume (2109 ml in the GD-RL group) or reduced volume (1260 ml in the R-RL group) was less effective.
Another factor that may influence the outcomes of patients who undergo major abdominal surgery is the type of fluid. In normovolemic healthy volunteers, the blood volume expansion was significantly greater after an infusion of 1 L of colloids (6% HES 130/0.4) than after an infusion of 1 L of crystalloids (saline) over one hour (24). Only 16% of the HES solution and more than 68% of the saline solution escaped into the extravascular fluid compartment after one hour. In the setting of moderate hypovolemia, an infusion of colloids increases blood volume and cardiac output more effectively than the same volume of crystalloids, even when the crystalloid infusion is rapidly administered (over 5-7 min) (25). Therefore, the patients in the GD-C group required less vasoactivators to achieve hemodynamic stability than the patients in the GD-RL and R-RL groups. A greater volume (≈1000 ml) of lactated Ringer's solution was infused into the GD-RL group to achieve PPV≤11% than that (≈600 ml) of the HES solution in the GD-C group. However, in contrast to the crystalloid-to-colloid volume ratio of 4:1 or more, our results (RL: HES = 1.67) are consistent with those of a recent study on goal-directed resuscitation, in which lower ratios that ranged from 1-1.6 were observed (26). Crystalloids enter the extravascular space more rapidly and in greater quantities than colloid; therefore, third-space fluid accumulation and altered capillary permeability may occur (23). Large amounts of intravenous crystalloid solutions can lead to an interstitial volume load, the development of interstitial edema and the impairment of the diffusion of O2 in gut tissues (27), which may prolong the period of ileus and increase postoperative complications and thus undermine the healing process (12). The large amounts of crystalloids that were administered in the GD-RL group may have offset the beneficial effects of the goal-directed therapy during the surgery. Subsequently, the elimination of flatus and the postoperative discharge times were delayed in the goal-directed crystalloid therapy group compared with the restrictive regimen group (23).
Two studies have compared the outcomes between intraoperative restrictive and goal-directed fluid management strategies in animal experiments. Kimberger et al. (28) demonstrated that goal-directed therapy with colloids significantly increased perianastomotic tissue oxygen tension compared with goal-directed crystalloid therapy and restrictive crystalloid therapy after 4 hours of treatment in a pig model of colon anastomosis surgery. In contrast to the mucosal tissue, the microcirculatory blood flow in the perianastomotic muscularis tissue was significantly higher in the GD-C group compared with the GD-RL group. Another study with the same fluid protocol found that the hemodynamic parameters were significantly better in the GD-C and GD-RL groups than in the R-RL group (29). The microcirculatory flow and tissue oxygen tension in the intestinal mucosa increased in the GD-C group but remained unchanged or decreased in the GD-RL and R-RL groups. These results suggest that goal-directed therapy with colloids during gastrointestinal surgery can lead to more stable intraoperative hemodynamics, faster bowel function recovery and a shorter postoperative discharge time compared with the goal-directed therapy with crystalloids in this study. However, several authors have questioned the beneficial role of goal-directed fluid therapy and indicated that the better outcomes in the goal-directed groups can be solely attributed to the larger amounts of colloids that are used in these groups compared with the control groups (30). Studies have demonstrated that the beneficial local tissue effects are likely due to the anti-inflammatory and antioxidant properties of colloids (HES 130/0.4), which augment the healing of anastomosis (31)-(34). A restrictive colloid group was not included in our fluid protocols; therefore, we cannot completely rule out the possibility that additional colloids may have contributed to our findings. However, in another experimental study with a small bowel anastomosis rat model, Marjanovic et al. (35) demonstrated that the animals that were treated with an intraoperative crystalloid volume overcharge with a fixed high flow rate exhibited worse anastomotic bursting pressure and poor histological results (submucosal edema) compared with the colloid overload group and the crystalloid or colloid volume restriction group that had a fixed low flow rate. However, the colloid restriction group had only comparable, but not better, results than the colloid overload and crystalloid restriction groups.
There are several limitations to this study. The first limitation is that an accelerated surgical recovery program was not adopted. The morphine administration for postoperative rescue analgesia may have affected the results because this treatment can delay bowel function recovery and cause PONV. Moreover, nasogastric tubes are routinely applied during gastrointestinal surgery at our hospital. However, the three groups in our series were similar with respect to the surgeons (the same surgical team), clinical characteristics and postoperative quality of care (conventional but standardized). Second, we did not establish a restrictive colloid group; therefore, the improvement in the outcomes could be due to the fluid type rather than the fluid therapy strategy. However, colloid replacement alone is not recommended in the textbooks and guidelines on intravenous fluid therapy in surgical patients. Finally, we did not evaluate the 30-day mortality; however, there were no deaths during the hospital stay of each patient. Additionally, this study did not have the power to detect significant differences in the cardiorespiratory outcomes because of the small number of patients. Therefore, the clinical benefits of the intraoperative goal-directed colloid strategy require further assessment.
In summary, the PPV, which can be obtained using a simple and inexpensive method, could be helpful in the implementation of intraoperative goal-directed therapy. Our data demonstrate that intraoperative treatment with PPV-directed 6% HES (130/0.4) leads to more stable hemodynamics, faster bowel function recovery and a shorter postoperative hospital stay than treatment with restrictive or PPV-directed lactated Ringer's solution, which suggests that intraoperative goal-directed therapy with colloids may be beneficial in ASA I/II patients during major gastrointestinal surgery. In addition, our results support the use of intraoperative fluid therapy with the accurate targeting of colloid fluid boluses, which prevents excessive crystalloid administration, even in goal-directed therapy. Relatively healthy and young adult patients participated in this study; however, the effects of fluid therapy strategies on the outcomes in elderly patients with comorbidities (ASA III-IV) who undergo major abdominal surgery remain unclear. Therefore, the explicit outcome advantages of goal-directed colloid therapy in major surgery warrant further study.
Figure 3.
Postoperative days of hospital stay.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–8. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326–41. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKay G, Fearon K, McConnachie A, Serpell MG, Molloy RG, O'Dwyer PJ. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. Br J Surg. 2006;93(12):1469–74. doi: 10.1002/bjs.5593. [DOI] [PubMed] [Google Scholar]
- 4.Gobindram A, Gowrie-Mohan S. Major elective gastrointestinal surgery: does fluid restriction improve outcome. Br J Hosp Med (Lond) 2007;68(3):168. doi: 10.12968/hmed.2007.68.3.22860. [DOI] [PubMed] [Google Scholar]
- 5.Futier E, Constantin JM, Petit A, Chanques G, Kwiatkowski F, Flamein R, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: A prospective randomized trial. Arch Surg. 2010;145(12):1193–200. doi: 10.1001/archsurg.2010.275. [DOI] [PubMed] [Google Scholar]
- 6.Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97(4):820–6. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Qiao H, Zhang J, Liang WM. Validity of pulse pressure and systolic blood pressure variation data obtained from a Datex Ohmeda S/5 monitor for predicting fluid responsiveness during surgery. J Neurosurg Anesthesiol. 2010;22(4):316–22. doi: 10.1097/ANA.0b013e3181e41299. [DOI] [PubMed] [Google Scholar]
- 8.He Z, Qiao H, Zhou W, Wang Y, Xu Z, Che X, et al. Assessment of cardiac preload status by pulse pressure variation in patients after anesthesia induction: comparison with central venous pressure and initial distribution volume of glucose. J Anesth. 2011;25(6):812–7. doi: 10.1007/s00540-011-1225-1. [DOI] [PubMed] [Google Scholar]
- 9.Pestel GJ, Hiltebrand LB, Fukui K, Cohen D, Hager H, Kurz AM. Assessing intravascular volume by difference in pulse pressure in pigs submitted to graded hemorrhage. Shock. 2006;26(4):391–5. doi: 10.1097/01.shk.0000228792.10550.ed. [DOI] [PubMed] [Google Scholar]
- 10.Westphal GA, Gonçalves AR, Bedin A, Steglich RB, Silva E, Poli-de-Figueiredo LF. Vasodilation increases pulse pressure variation, mimicking hypovolemic status in rabbits. Clinics. 2010;65(2):189–94. doi: 10.1590/S1807-59322010000200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO, Jr, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11(5):R100. doi: 10.1186/cc6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett-Guerrero E, Welsby I, Dunn TJ, Young LR, Wahl TA, Diers TL, et al. The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery. Anesth Analg. 1999;89(2):514–9. doi: 10.1097/00000539-199908000-00050. [DOI] [PubMed] [Google Scholar]
- 13.Holte K, Foss NB, Andersen J, Valentiner L, Lund C, Bie P, et al. Liberal or restrictive fluid administration in fast-track colonic surgery: a randomized, double-blind study. Br J Anaesth. 2007;99(4):500–8. doi: 10.1093/bja/aem211. [DOI] [PubMed] [Google Scholar]
- 14.de Aguilar-Nascimento JE, Diniz BN, do Carmo AV, Silveira EA, Silva RM. Clinical benefits after the implementation of a protocol of restricted perioperative intravenous crystalloid fluids in major abdominal operations. World J Surg. 2009;33(5):925–30. doi: 10.1007/s00268-009-9944-2. [DOI] [PubMed] [Google Scholar]
- 15.Conway DH, Mayall R, Abdul-Latif MS, Gilligan S, Tackaberry C. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia. 2002;57(9):845–9. doi: 10.1046/j.1365-2044.2002.02708.x. [DOI] [PubMed] [Google Scholar]
- 16.Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445] Crit Care. 2005;9(6):R687–93. doi: 10.1186/cc3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth. 2002;88(1):65–71. doi: 10.1093/bja/88.1.65. [DOI] [PubMed] [Google Scholar]
- 18.Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95(5):634–42. doi: 10.1093/bja/aei223. [DOI] [PubMed] [Google Scholar]
- 19.Senagore AJ, Emery T, Luchtefeld M, Kim D, Dujovny N, Hoedema R. Fluid management for laparoscopic colectomy: a prospective, randomized assessment of goal-directed administration of balanced salt solution or hetastarch coupled with an enhanced recovery program. Dis Colon Rectum. 2009;52(12):1935–40. doi: 10.1007/DCR.0b013e3181b4c35e. [DOI] [PubMed] [Google Scholar]
- 20.Lahner D, Kabon B, Marschalek C, Chiari A, Pestel G, Kaider A, et al. Evaluation of stroke volume variation obtained by arterial pulse contour analysis to predict fluid responsiveness intraoperatively. Br J Anaesth. 2009;103(3):346–51. doi: 10.1093/bja/aep200. [DOI] [PubMed] [Google Scholar]
- 21.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000–8. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 22.Westphal G, Garrido Adel P, de Almeida DP, Rocha e Silva M, Poli-de-Figueiredo LF. Pulse pressure respiratory variation as an early marker of cardiac output fall in experimental hemorrhagic shock. Artif Organs. 2007;31(4):284–9. doi: 10.1111/j.1525-1594.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 23.Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103(1):25–32. doi: 10.1097/00000542-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Lobo DN, Stanga Z, Aloysius MM, Wicks C, Nunes QM, Ingram KL, et al. Effect of volume loading with 1 liter intravenous infusions of 0.9% saline, 4% succinylated gelatine (Gelofusine) and 6% hydroxyethyl starch (Voluven) on blood volume and endocrine responses: a randomized, three-way crossover study in healthy volunteers. Crit Care Med. 2010;38(2):464–70. doi: 10.1097/CCM.0b013e3181bc80f1. [DOI] [PubMed] [Google Scholar]
- 25.McIlroy DR, Kharasch ED. Acute intravascular volume expansion with rapidly administered crystalloid or colloid in the setting of moderate hypovolemia. Anesth Analg. 2003;96(6):1572–7. doi: 10.1213/01.ANE.0000061460.59320.B0. [DOI] [PubMed] [Google Scholar]
- 26.Hartog C, Reinhart K. CONTRA: Hydroxyethyl starch solutions are unsafe in critically ill patients. Intensive Care Med. 2009;35(8):1337–42. doi: 10.1007/s00134-009-1521-5. [DOI] [PubMed] [Google Scholar]
- 27.Mythen M, Vercueil A. Fluid balance. Vox Sang. 2004;87 Suppl 1:77–81. doi: 10.1111/j.1741-6892.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- 28.Kimberger O, Arnberger M, Brandt S, Plock J, Sigurdsson GH, Kurz A, et al. Goal-directed colloid administration improves the microcirculation of healthy and perianastomotic colon. Anesthesiology. 2009;110(3):496–504. doi: 10.1097/ALN.0b013e31819841f6. [DOI] [PubMed] [Google Scholar]
- 29.Hiltebrand LB, Kimberger O, Arnberger M, Brandt S, Kurz A, Sigurdsson GH. Crystalloids versus colloids for goal-directed fluid therapy in major surgery. Crit Care. 2009;13(2):R40. doi: 10.1186/cc7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horowitz PE, Kumar A. It's the colloid, not the esophageal Doppler monitor. Anesthesiology. 2003(1);99:238–39. doi: 10.1097/00000542-200307000-00045. [DOI] [PubMed] [Google Scholar]
- 31.Collis RE, Collins PW, Gutteridge CN, Kaul A, Newland AC, Williams DM, et al. The effect of hydroxyethyl starch and other plasma volume substitutes on endothelial cell activation; an in vitro study. Intensive Care Med. 1994;20(1):37–41. doi: 10.1007/BF02425053. [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer R, Moser D, Hornykewycz S, Frass M, Kapiotis S. Hydroxyethyl starch reduces the chemotaxis of white cells through endothelial cell monolayers. Transfusion. 1999;39(3):289–94. doi: 10.1046/j.1537-2995.1999.39399219286.x. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann JN, Vollmar B, Laschke MW, Inthorn D, Schildberg FW, Menger MD. Hydroxyethyl starch (130 kD), but not crystalloid volume support, improves microcirculation during normotensive endotoxemia. Anesthesiology. 2002;97(2):460–70. doi: 10.1097/00000542-200208000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Gong G, Li Y, Li J. Hydroxyethyl starch 130/0.4 augments healing of colonic anastomosis in a rat model of peritonitis. Am J Surg. 2010;199(2):232–9. doi: 10.1016/j.amjsurg.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Marjanovic G, Villain C, Timme S, zur Hausen A, Hoeppner J, Makowiec F, et al. Colloid vs. crystalloid infusions in gastrointestinal surgery and their different impact on the healing of intestinal anastomoses. Int J Colorectal Dis. 2010;25(4):491–8. doi: 10.1007/s00384-009-0854-4. [DOI] [PubMed] [Google Scholar]