Abstract
OBJECTIVES:
The aim of this study was to evaluate cardiovascular autonomic function in a rodent obesity model induced by monosodium glutamate injections during the first seven days of life.
METHOD:
The animals were assigned to control (control, n = 10) and monosodium glutamate (monosodium glutamate, n = 13) groups. Thirty-three weeks after birth, arterial and venous catheters were implanted for arterial pressure measurements, drug administration, and blood sampling. Baroreflex sensitivity was evaluated according to the tachycardic and bradycardic responses induced by sodium nitroprusside and phenylephrine infusion, respectively. Sympathetic and vagal effects were determined by administering methylatropine and propranolol.
RESULTS:
Body weight, Lee index, and epididymal white adipose tissue values were higher in the monosodium glutamate group in comparison to the control group. The monosodium glutamate-treated rats displayed insulin resistance, as shown by a reduced glucose/insulin index (-62.5%), an increased area under the curve of total insulin secretion during glucose overload (39.3%), and basal hyperinsulinemia. The mean arterial pressure values were higher in the monosodium glutamate rats, whereas heart rate variability (>7 times), bradycardic responses (>4 times), and vagal (∼38%) and sympathetic effects (∼36%) were reduced as compared to the control group.
CONCLUSION:
Our results suggest that obesity induced by neonatal monosodium glutamate treatment impairs cardiac autonomic function and most likely contributes to increased arterial pressure and insulin resistance.
Keywords: Monosodium Glutamate, Obesity, Insulin Resistance, Arterial Pressure, Autonomic Function
INTRODUCTION
The metabolic syndrome is an emerging global epidemic characterized by the clustering of metabolic abnormalities, such as glucose intolerance and/or type 2 diabetes, dyslipidemia, hypertension, and abdominal obesity, which lead to increased cardiovascular-related mortality (1). Weight gain usually leads to insulin resistance, compensatory hyperinsulinemia, and hypertension through parallel mechanisms that may be connected (2). In normotensive obese subjects, changes in sympathetic nervous system modulation correlate with insulin resistance, and decreased heart rate variability may partly account for the increased cardiovascular risk in these subjects (3).
In the experimental setting, animal models have been used to gain an understanding of obesity-related metabolic disorders, including models of surgical and pharmacological induction of obesity in mice (4) and rats (5,6). In mice, the neonatal administration of monosodium glutamate (MSG) causes damage in the hypothalamic and circumventricular areas and results in several neuroendocrine and metabolic abnormalities (7), including hypophagia, obesity, hypoactivity, delayed puberty, and elevated plasma corticosterone levels (8). Furthermore, stunted growth, increased adipose tissue stores, and a marked increase in plasma triglycerides have been noted in rats (9).
Although many of the above characteristics are similar to the characteristics of metabolic syndrome as it occurs in humans, the few studies that have evaluated cardiovascular changes in the MSG-induced obesity animal model have reported conflicting results. Female, but not male, MSG-treated rats exhibit systolic hypotension, and no changes in heart rate were obtained using the indirect tail-cuff method (10). These results suggest that gender may be important in the MSG response. Moreover, reduced vascular responsiveness to alpha-adrenergic stimulation was observed in MSG-treated rats (11). In a similar animal model, ventromedial hypothalamic lesions displayed massive obesity, insulin resistance, low sympathetic activity, high parasympathetic activity, and no changes in blood pressure (6). Although the metabolic aspects of MSG-induced obesity have been extensively examined, it has not been studied in detail concerning cardiovascular autonomic function. Thus, the present study was designed to evaluate cardiovascular autonomic function in rats that received MSG neonatal treatment and the potential relationship between this treatment and metabolic abnormalities.
METHODS
Animals
The experiments were performed using 23 male Wistar rats obtained from the animal facility at the Federal University of Health Sciences of Porto Alegre (Porto Alegre, Brazil). The experimental protocol was approved by the Institutional Animal Care and Use Committee at the Federal University of Health Sciences of Porto Alegre, and this investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1985).
During the first seven days of life, 2 mg/kg of MSG (monosodium glutamate, Sigma, USA) or saline in a similar volume was injected subcutaneously into the rats once per day, as previously described (4). The rats were randomly assigned to either the control (n = 10) or MSG-injected (MSG, n = 13) group. The pups were weaned on the 21st day of life and kept in plastic cages (five per cage) in a temperature-controlled room (22°C) with a 12-hour dark-light cycle (light 07:00-19:00 h). The animals were fed standard laboratory chow and water ad libitum, and their body weights were monitored weekly for 33 weeks. The Lee index (b.w.1/3 in g/naso-anal length in mm) was measured during the final evaluation.
Hemodynamic assessments
Thirty-three weeks after birth, two catheters filled with 0.06 mL of saline were implanted into the femoral artery of each anesthetized rat (80 mg/kg ketamine and 12 mg/kg xylazine, i.p.). Twenty-four hours later, the arterial cannula was connected to a strain gauge transducer (Blood Pressure XDCR; Kent Scientific, Torrington, CT, USA), and the arterial pressure (AP) signals were recorded over a 30-minute period in conscious animals using a microcomputer equipped with an analog-to-digital converter board (WinDaq, 2 kHz, DATAQ, Springfield, OH, USA). The recorded data were analyzed on a beat-by-beat basis to quantify any changes in the mean AP (MAP) and heart rate (HR). The overall variability of the pulse interval and systolic AP in the time domain was assessed by computing the standard deviation (SD) of the time series (12)-(14).
Baroreflex sensitivity
Sequential bolus injections (0.1 mL) of increasing doses of phenylephrine (0.25 to 32 µg/kg) and sodium nitroprusside (0.05 to 1.6 µg/kg) were given to induce increases or decreases in MAP pressure responses (for each drug), ranging from 5 to 40 mm Hg. Baroreflex sensitivity is expressed as bradycardic (BR) and tachycardic (TR) responses in beats per minute per millimeter of mercury, as described elsewhere (12)-(14).
Autonomic evaluation
The vagal and sympathetic effects were both studied by administering injections of methylatropine (3 mg/kg IV, Sigma) and propranolol (4 mg/kg IV, Sigma) at a maximal volume per injection of 0.2 mL. On the first day of the study, the resting HR was recorded in unrestrained rats that were kept in their own cages. Immediately after the resting HR was recorded, methylatropine was injected. Because the HR response to methylatropine reaches its peak within 10 to 15 minutes, this time interval was standardized before the HR measurement was taken. On the second day of the study, propranolol was administered. The vagal effect (VE) was evaluated as the difference between the maximum HR after the methylatropine injection and the resting HR. The sympathetic effect (SE) was evaluated as the difference between the resting HR and the minimum HR after the propranolol injection (14,15).
Metabolic measurements
One day after the last cardiovascular evaluation (6-h fasting), blood was collected from the arterial cannula for plasma glucose and insulin evaluation. An oral glucose tolerance test (OGTT, 0.5 g/kg oral) was also performed (16). The blood samples (0.5 mL) were collected through the arterial catheter at baseline and 5, 15, 30 and 60 min after the oral glucose test. Using a glucose oxidase kit (Boehringer Mannheim, Mannheim, Germany), plasma samples were obtained for further glucose analysis. For insulin measurements, a radioimmunoassay was performed using anti-human insulin antibodies (Sigma) and 125I-human insulin as the radiolabel (Amersham). After the evaluations, the animals were sacrificed, and a fat mass (white adipose tissue) and lean mass (gastrocnemius and heart muscle) were weighed from each animal.
Statistical analysis
The data are reported as the mean±SEM, and Student's unpaired t-test was used to compare the values obtained between groups. The insulin response to the glucose overload was evaluated using the area under the curve. Baroreflex sensitivity was evaluated using a regression analysis of different groups, and the slopes were compared using Tukey's ANOVA as a post-hoc test. Pearson's correlation coefficient was used to identify associations between variables. Differences were considered statistically significant when p<0.05.
RESULTS
The body weight measurements increased in the MSG group (375±6 g) after 33 weeks of follow-up in comparison to the control group (355±5 g) (p = 0.03). The Lee index values were also higher in the MSG group as compared to the control group (325±2 vs. 315±2 body weight1/3 in g/naso-anal length in mm, respectively) (p = 0.04), and the MSG group had a greater fat mass (evaluated by the epididymal white adipose tissue) than the control group (1.58±0.02 vs. 1.7±0.07 g, respectively) (p = 0.01). However, the lean mass was similar between the control and MSG groups, as evaluated by the weights of the gastrocnemius (1.92±0.11 vs. 2.02±0.08 g in MSG and control, respectively) (p = 0.451) and heart muscles (1.17±0.02 vs. 1.17±0.03 g in MSG and control, respectively) (p = 1.00).
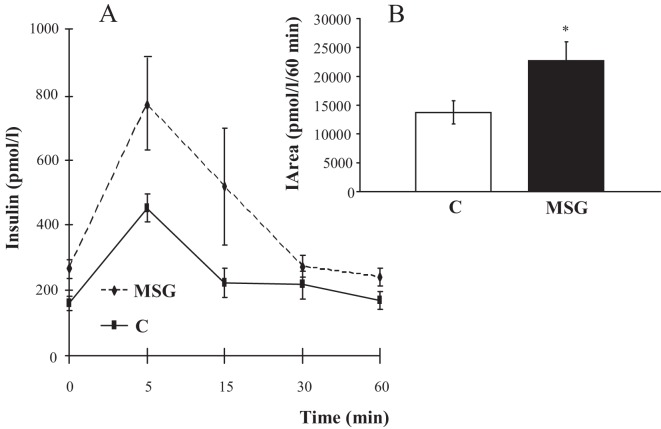
MSG treatment induced a state of insulin resistance, as demonstrated by the plasma insulin levels during the OGTT (Figure 1A). Furthermore, increased levels of fasting plasma glucose (5.13±0.79 mmol/L, p = 0.03) and plasma insulin (267±29 pmol/L, p = 0.02) were observed in the MSG-treated animals as compared to the control group (3.18±0.24 mmol/l, and 159±22 pmol/L, respectively). As a result, the glucose/insulin index values were significantly lower in the MSG group as compared to the control group (11.9±0.12 vs. 32.2±0.08 mmol/pmol x1000, respectively) (p = 0.02). The area under the curve for plasma insulin during the OGTT (Figure 1B) was higher for MSG-treated rats (22,685±3,184 pmol/L/60 min) as compared to control animals (13,729±2,527 pmol/L/60 min), (p = 0.05), confirming a higher level of insulin secretion in these animals in response to the glucose overload.
Figure 1.
Plasma insulin levels (A) during the OGTT in the control (C) and monosodium glutamate-treated (MSG) rats. In detail, the insulin area under the curve (IArea) for the control and MSG groups (B). * p = 0.05.
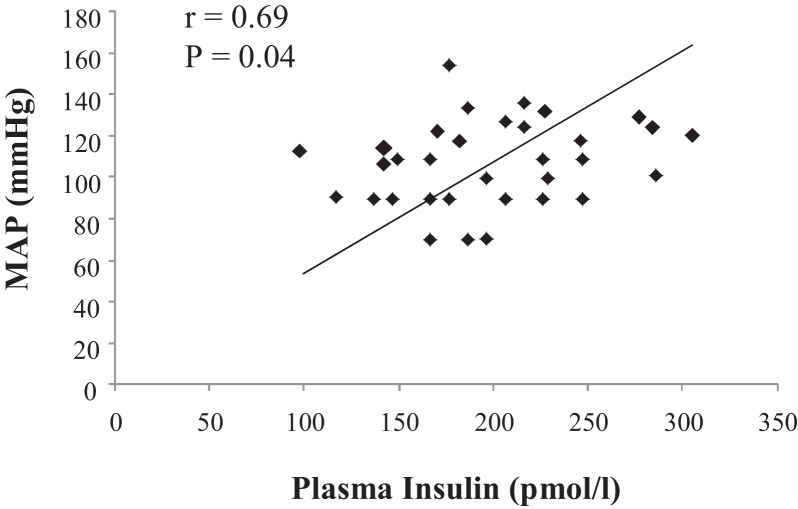
Regarding the hemodynamic measurements, the MSG-treated group displayed increased systolic, diastolic, and mean arterial pressures as compared to the control group; however, the systolic arterial pressure variability was similar between groups (Table 1). Although the heart rate was similar between the experimental groups, heart rate variability was reduced in the MSG-treated animals in comparison to the control animals (Table 1). By plotting the results obtained from all of the control and MSG animals, it was possible to identify a positive correlation between plasma insulin and mean AP (r = 0.69, p = 0.04), as shown in Figure 2. However, there was no relationship between plasma glucose and mean AP (r = 0.19, p = 0.47).
Table 1.
Hemodynamic and autonomic measurements in the control (C) and MSG-treated (MSG) groups.
| Control | MSG | |
| SAP (mm Hg) | 139±2 | 147±2* |
| DAP (mm Hg) | 92±2 | 99±2* |
| MAP (mm Hg) | 112±1 | 121±2* |
| HR (bpm) | 352±11 | 330±11 |
| SAP VAR (mm Hg) | 6.0±0.3 | 6.1±0.6 |
| HR VAR (bpm) | 31.8±1.8 | 4.3±1.6* |
| TR (bpm/mm Hg) | 2.5±0.6 | 2.1±0.5 |
| BR (bpm/mm Hg) | -1.4±0.3 | -0.3±0.5* |
Values are expressed as the mean±SEM. SAP - Systolic arterial pressure; DAP - Diastolic arterial pressure; MAP - Mean arterial pressure; SAP VAR - Systolic arterial pressure variability evaluated according to the standard deviation; HR - Heart rate; HR VAR – Heart rate variability evaluated according to the standard deviation. *p<0.05 vs. control group.
Figure 2.
Correlation between plasma insulin and mean arterial pressure (MAP) in the control (C) and monosodium glutamate-treated (MSG) rats (r = 0.69, p = 0.04).
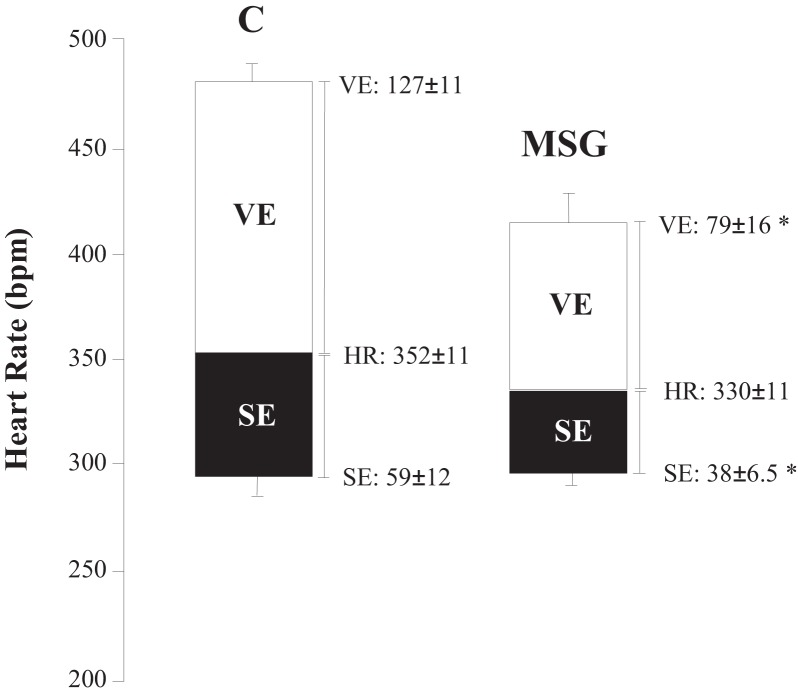
Baroreflex sensitivity, as evaluated by BR, was reduced in the MSG-treated animals as compared to the control animals, although no differences were observed in the TR between the experimental groups (Table 1). Furthermore, the vagal and sympathetic effects were reduced in the MSG-treated rats as compared to the control rats, as shown in Figure 3.
Figure 3.
Heart rate (HR) and sympathetic (SE) and vagal (VE) effects in the control (C) and monosodium glutamate-treated (MSG) rats (see "Methods" for calculation). Adjacent to the plots, the mean±SEM. *Significant difference between the control and MSG groups (p<0.05).
DISCUSSION
The main finding of the present study was that MSG treatment induced metabolic (increases in body weight, the Lee index, fat mass, hyperinsulinemia, and the insulin response to OGTT) and cardiovascular (increased baseline arterial pressure) changes that were most likely associated with impairments in the autonomic control of circulation and were manifested as reduced heart rate variability, baroreflex sensitivity, and vagal and sympathetic effects. In addition, the high blood pressure values observed were positively correlated with insulin levels.
Insights into the etiology, pathophysiology, and pharmacological treatment of human obesity have arisen from the study of animal models. However, animal obesity models differ widely in the type and extent of obesity. For example, these models are based on environmental factors, (e.g., high-fat diet-induced obesity), spontaneous mutants (i.e., ob/ob and/or db/db mice), genetically engineered animals (e.g., mice with melanocortin receptor subtype-4 gene disruption (knock-out), or mechanical intervention (e.g., chemical or electrical lesions in the ventromedial hypothalamus) and demonstrate different metabolic, hemodynamic, and hormonal results (17).
The neonatal administration of MSG induces obesity in rodents through neuronal damage to the arcuate nucleus-median eminence region (4). This experimental model behaves differently from genetically obese and aurothioglucose-induced (auTG) obese rats (18), as it results in increased lipid mass and mild body weight increases without hyperphagia. In our experiments, increases in body weight, Lee index values, and adipose tissue were observed in MSG-treated rats as compared to control animals, although the lean mass measurements were similar between groups. Consistent with these results, other studies have shown similar changes in these parameters. Ribeiro Braga et al. (19) showed that higher doses of MSG (4 mg/g of body weight) induced increases in carcass fat, cellular size, and weight of the epididymal adipose tissue. In another study (20), MSG treatment (2 mg/g of body weight) during the first four days after birth induced greater retroperitoneal fat mass in comparison to control rats. Moreover, recently published data demonstrated that MSG treatment during the first five postnatal days induced increased fat deposits and hypertension in rats (21).
In the early phase of obesity establishment, normal plasma glucose associated with hyperinsulinemia (22) has been observed, which indicates that insulin resistance is present and that the high insulin levels may be compensatory (2,23). Other authors have shown similar results concerning hyperinsulinemia and insulin resistance, suggesting that there is an important decrease in the insulin-stimulated IRS/PI3K/Akt pathway in muscle, which may have an important role in the insulin resistance state (24). In a similar vein, de Souza et al. (25) showed that the hyperinsulinemia observed in MSG-obese rats is, at least in part, a consequence of the direct hypersecretion of ß cells and that chronic aerobic exercise is able to partially counteract the hyperinsulinemic state.
The present study found that MSG treatment during the neonatal period induced a slight increase in arterial pressure (∼8%) in conscious adult rats. In accordance with these data, the study by Cunha et al. (21) demonstrated that the administration of MSG to rats resulted in neuroendocrine obesity and a moderate level of hypertension. Interestingly, in this work, the mean arterial pressure values were positively correlated with the insulin plasma levels, suggesting a cardio-metabolic interaction. Several hypotheses have been postulated to explain the link between hypertension and insulin resistance. Rodents chronically fed high-carbohydrate diets (26,27) or chronically infused with exogenous insulin to produce hyperinsulinemia (28) showed increased blood pressure levels, although chronic insulin infusion failed to increase blood pressure measurements in dogs (29). However, acute (1 to 2 h) insulin infusion in rats (30) and dogs (31) has been shown to increase blood pressure, which is most likely the result of sympathetic activation (32) and sodium retention during acute hyperinsulinemia. This rise in blood pressure is not generally sustained because it is overridden by the vasodilator action of insulin, which is rapidly sensed by baroreceptors, and a counterbalancing increase in vasodilation returns the blood pressure to normal. Although these studies may provide insight into the potential role of insulin in hypertension, the quantitative importance of hyperinsulinemia in the etiology of hypertension has not yet been established.
To further explore the mechanisms involved in the cardio-metabolic changes observed in animals treated with MSG, we analyzed the autonomic control of circulation. It is well known that decreased baroreflex sensitivity is a marker of an increased risk of sudden death in patients after myocardial infarction (33), and this measurement has also been associated with increased arterial pressure, myocardial damage, renal lesions, and arterial remodeling (34,35). Moreover, there is some evidence correlating the decrease in baroreflex function with body fat distribution, as patients with abdominal obesity demonstrate a greater reduction in baroreflex sensitivity than those with peripheral obesity (35). In fact, children with primarily low baroreflex sensitivity could have a greater predisposition to pathological arterial pressure elevation, and obesity has been shown to be an additive factor to low baroreflex sensitivity in the development of hypertension (36).
In the present study, the reduced bradycardic reflex in MSG-treated rats could have been due to a decrease in the vagal effect on the heart, which may have indicated impairments in the vagal reserve used during heart rate responses evoked by baroreceptors. Indeed, baroreflex-mediated bradycardia is predominantly associated with vagal actions on the heart. If a reduction in this activity occurs, bradycardia is also reduced. Therefore, the attenuation of the bradycardic response may have resulted from reduced responsiveness of the sinus node to parasympathetic modulation, as the vagal effect and (consequently) the HR variability were reduced in MSG-treated rats. In addition, HR variability has been strongly correlated with the vagal indices of HR variability (37). Furthermore, similar results were observed by Miller et al. (38), as rats fed a fructose-rich diet displayed increased arterial pressure levels and reduced baroreflex sensitivity associated with a large reduction in vagal activity. Moreover, we previously observed arterial pressure increases in female fructose-fed rats as well as was a positive correlation between insulin resistance and cardiac vagal effect attenuation (15).
In the present study, we observed a reduced sympathetic effect that was not accompanied by changes in the basal HR or tachycardic responses to AP changes. Moreover, Rehorek et al. (39) suggested that reduced sympathetic activity, as expressed by decreased basal levels of norepinephrine, in MSG-treated rats may be associated with reduced thermogenic responses, which could contribute to the development of obesity. Some evidence has suggested a positive correlation between excess weight gain and sympathetic hyperactivity, which contributes to the development of hypertension in both human and animal models (29,40). In fact, Hellstrom (41) presented convincing evidence that the development of a diverse group of diseases, such as hypertension, diabetes and heart disease, is triggered by increased sympathetic neural outflow, which results in endothelial dysfunction, dyslipidemia, inflammation and insulin resistance. Moreover, other data have suggested that autonomic changes modulate hormonal and immune function by inducing the release of bioactive molecules, which are likely involved in the development of cardiometabolic profile changes (42,43). In the present work, the reduction in parasympathetic cardiac activity predominated over other sympathetic changes in the MSG group, as only the bradycardic reflex (associated with impaired cardiac vagal effect and variability) was reduced in this group. Together, these results suggest that a relative sympathetic predominance may be associated with the increased arterial pressure observed in MSG-treated rats.
Therefore, our results suggest that the obesity induced by neonatal MSG treatment reduces cardiac autonomic function by most likely contributing to insulin resistance and an increase in arterial pressure.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE, Summers RL, Brands MW, Keen H, Alonso-Galicia M. Resistance to metabolic actions of insulin and its role in hypertension. Am J Hypertens. 1994;7(8):772–88. doi: 10.1093/ajh/7.8.772. [DOI] [PubMed] [Google Scholar]
- 3.Quilliot D, Fluckiger L, Zannad F, Drouin P, Ziegler O. Impaired autonomic control of heart rate and blood pressure in obesity: role of age and of insulin-resistance. Clin Auton Res. 2001;11(2):79–86. doi: 10.1007/BF02322050. [DOI] [PubMed] [Google Scholar]
- 4.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164(3880):719–21. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 5.Marmo MR, Dolnikoff MS, Kettelhut IC, Matsushita DM, Hell NS, Lima FB. Neonatal monosodium glutamate treatment increases epididymal adipose tissue sensitivity to insulin in three-month old rats. Braz J Med Biol Res. 1994;27(5):1249–53. [PubMed] [Google Scholar]
- 6.Valensi P, Doare L, Perret G, Germack R, Paries J, Mesangeau D. Cardiovascular vagosympathetic activity in rats with ventromedial hypothalamic obesity. Obes Res. 2003;11(1):54–64. doi: 10.1038/oby.2003.10. [DOI] [PubMed] [Google Scholar]
- 7.Olney JW. Status of monosodium glutamate revisited. Am J Clin Nutr. 1973;26(7):683–85. doi: 10.1093/ajcn/26.6.683. [DOI] [PubMed] [Google Scholar]
- 8.Lorden JF, Caudle A. Behavioral and endocrinological effects of single injections of monosodium glutamate in the mouse. Neurobehav Toxicol Teratol. 1986;8(5):509–19. [PubMed] [Google Scholar]
- 9.Oida K, Nakai T, Hayashi T, Miyabo S, Takeda R. Plasma lipoproteins of monosodium glutamate-induced obese rats. Int J Obes. 1984;8(5):385–91. [PubMed] [Google Scholar]
- 10.Clough RW, Aravich PF, Sladek CD. Monosodium glutamate neurotoxicity: a sex-specific impairment of blood pressure but not vasopressin in developing rats. Brain Res Bull. 1986;17(1):51–8. doi: 10.1016/0361-9230(86)90160-7. [DOI] [PubMed] [Google Scholar]
- 11.Tokarev D, Kristova V, Kriska M, Jezova D. Treatment of neonatal rats with monosodium glutamate attenuates the cardiovascular reactivity to phenylephrine and angiotensin II. 1997;46(3):165–71. Physiol Res. [PubMed] [Google Scholar]
- 12.Zamo FS, Lacchini S, Mostarda C, Chiavegatto S, Silva IC, Oliveira EM, et al. Hemodynamic, morphometric and autonomic patterns in hypertensive rats - Renin-Angiotensin system modulation. Clinics. 2010;65(1):85–92. doi: 10.1590/S1807-59322010000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues B, Rosa KT, Medeiros A, Schaan BD, Brum PC, De Angelis K, et al. Hyperglycemia can delay left ventricular dysfunction but not autonomic damage after myocardial infarction in rodents. Cardiovasc Diabetol. 2011;10:26. doi: 10.1186/1475-2840-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva RJ, Bernardes N, Brito Jde O, Sanches IC, Irigoyen MC, De Angelis K. Simvastatin-induced cardiac autonomic control improvement in fructose-fed female rats. Clinics. 2011;66(10):1793–6. doi: 10.1590/S1807-59322011001000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brito JO, Ponciano K, Figueroa D, Bernardes N, Sanches IC, Irigoyen MC, De Angelis K. Parasympathetic dysfunction is associated with insulin resistance in fructose-fed female rats. Braz J Med Biol Res. 2008;41(9):804–8. doi: 10.1590/s0100-879x2008005000030. [DOI] [PubMed] [Google Scholar]
- 16.Triadou N, Portha B, Picon L, Rosselin G. Experimental chemical diabetes and pregnancy in the rat. Evolution of glucose tolerance and insulin response. Diabetes. 1982;31(1):75–9. doi: 10.2337/diab.31.1.75. [DOI] [PubMed] [Google Scholar]
- 17.Von Diemen V, Trindade EN, Trindade MR. Experimental model to induce obesity in rats. Acta Cir Bras. 2006;21:425–9. doi: 10.1590/s0102-86502006000600013. [DOI] [PubMed] [Google Scholar]
- 18.Bunyan J, Murrell EA, Shah PP. The induction of obesity in rodents by means of monosodium glutamate. 1976;35(1):25–39. doi: 10.1079/bjn19760005. Br J Nutr. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro Braga L, de Mello MA, Gobatto CA. Continuous and intermittent exercise: effects of training and detraining on body fat in obese rats. Arch Latinoam Nutr. 2004;54(1):58–65. [PubMed] [Google Scholar]
- 20.Mozes S, Sefcíkov Z, Lenhardt L, Racek L. Effect of adrenalectomy on the activity of small intestine enzymes in monosodium glutamate obese rats. Physiol Res. 2004;53(4):415–22. [PubMed] [Google Scholar]
- 21.Cunha NV, de Abreu SB, Panis C, Grassiolli S, Guarnier FA, Cecchini R, et al. Cox-2 inhibition attenuates cardiovascular and inflammatory aspects in monosodium glutamate-induced obese rats. Life Sci. 2010;87(11-12):375–81. doi: 10.1016/j.lfs.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 22.de Carvalho Papa P, Vargas AM, da Silva JL, Nunes MT, Machado UF. GLUT4 protein is differently modulated during development of obesity in monosodium glutamate-treated mice. Life Sci. 2002;71(16):1917–28. doi: 10.1016/s0024-3205(02)01948-3. [DOI] [PubMed] [Google Scholar]
- 23.Defronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979;28(12):1095–101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- 24.Thirone AC, Carvalheira JB, Hirata AE, Velloso LA, Saad MJ. Regulation of Cbl-associated protein/Cbl pathway in muscle and adipose tissues of two animal models of insulin resistance. Endocrinology. 2004;145(1):281–93. doi: 10.1210/en.2003-0575. [DOI] [PubMed] [Google Scholar]
- 25.de Souza CT, Nunes WM, Gobatto CA, de Mello MA. Insulin secretion in monosodium glutamate (MSG) obese rats submitted to aerobic exercise training. Physiol Chem Phys Med NMR. 2003;35(1):43–53. [PubMed] [Google Scholar]
- 26.Farah V, Elased KM, Chen Y, Key MP, Cunha TS, Irigoyen MC, et al. Nocturnal hypertension in mice consuming a high fructose diet. Auton Neurosci. 2006;130(1-2):41–50. doi: 10.1016/j.autneu.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Martinez FJ, Rizza RA, Romero JC. High-fructose feeding elicits insulin resistance, hyperinsulinism, and hypertension in normal mongrel dogs. Hypertension. 1994;23(4):456–63. doi: 10.1161/01.hyp.23.4.456. [DOI] [PubMed] [Google Scholar]
- 28.Brands MW, Hildebrandt DA, Mizelle HL, Hall JE. Sustained hyperinsulinemia increases arterial pressure in conscious rats. :R764–8. doi: 10.1152/ajpregu.1991.260.4.R764. Am J Physiol 1991;260(4 Pt 2) [DOI] [PubMed] [Google Scholar]
- 29.Hall JE, Brands MW, Zappe DH, Dixon WN, Mizelle HL, Reinhart GA, et al. Hemodynamic and renal responses to chronic hyperinsulinemia in obese, insulin-resistant dogs. Hypertension. 1995;25(5):994–1002. doi: 10.1161/01.hyp.25.5.994. [DOI] [PubMed] [Google Scholar]
- 30.Qadir E, Porter JP. Effect of insulin on regional vascular resistances in conscious rats. Am J Physiol. 1996;270(2 Pt 2):R450–5. doi: 10.1152/ajpregu.1996.270.2.R450. [DOI] [PubMed] [Google Scholar]
- 31.Richey JM, Poulin RA, Buchanan TA, Galperin E, Moore DM, Bergman RN. Failure of acute hyperinsulinemia to alter blood pressure is not due to baroreceptor feedback. Am J Hypertens. 1999;12(4 Pt 1):405–13. doi: 10.1016/s0895-7061(98)00272-6. [DOI] [PubMed] [Google Scholar]
- 32.Moreau P, Lamarche L, Laflamme AK, Calderone A, Yamaguchi N, de Champlain J. Chronic hyperinsulinaemia and hypertension: the role of the sympathetic nervous system. J Hypertens. 1995;13(3):333–40. [PubMed] [Google Scholar]
- 33.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 19998;14. 351(9101):478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 34.Su DF, Miao CY. Arterial baroreflex function in conscious rats. Acta Pharmacol Sin. 2002;23(8):673–9. [PubMed] [Google Scholar]
- 35.Grassi G, Dell'Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G. Effect of central peripheral obesity body fat distribution on sympathetic and baroreflex function in obese normotensive. J Hypertens. 2004;22(12):2363–9. doi: 10.1097/00004872-200412000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Krontorádová K, Honzíková N, Fiser B, Nováková Z, Závodná E, Hrstková H, et al. Overweight and decreased baroreflex sensitivity as independent risk factors for hypertension in children, adolescents, and young adults. Physiol Res. 2008;57(3):385–91. doi: 10.33549/physiolres.931250. [DOI] [PubMed] [Google Scholar]
- 37.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 38.Miller AW, Sims JJ, Canavan A, Hsu T, Ujhelyi MR. Impaired vagal reflex activity in insulin-resistant rats. J Cardiovasc Pharmacol. 1999;33(5):698–702. doi: 10.1097/00005344-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Rehorek A, Kerecsen L, Müller F. Measurement of tissue catecholamines of obese rats by liquid chromatography and electrochemical detection. Biomed Biochim Acta. 1987;46(11):823–7. [PubMed] [Google Scholar]
- 40.Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev. 2009;33(2):116–24. doi: 10.1016/j.neubiorev.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellstrom HR. The altered homeostatic theory: A hypothesis proposed to be useful in understanding and preventing ischemic heart disease, hypertension, and diabetes–including reducing the risk of age and atherosclerosis. Med Hypotheses. 2007;68(2):415–433. doi: 10.1016/j.mehy.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 42.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 43.Van Gaal LF, Mertens IL, De Block DE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]