Abstract
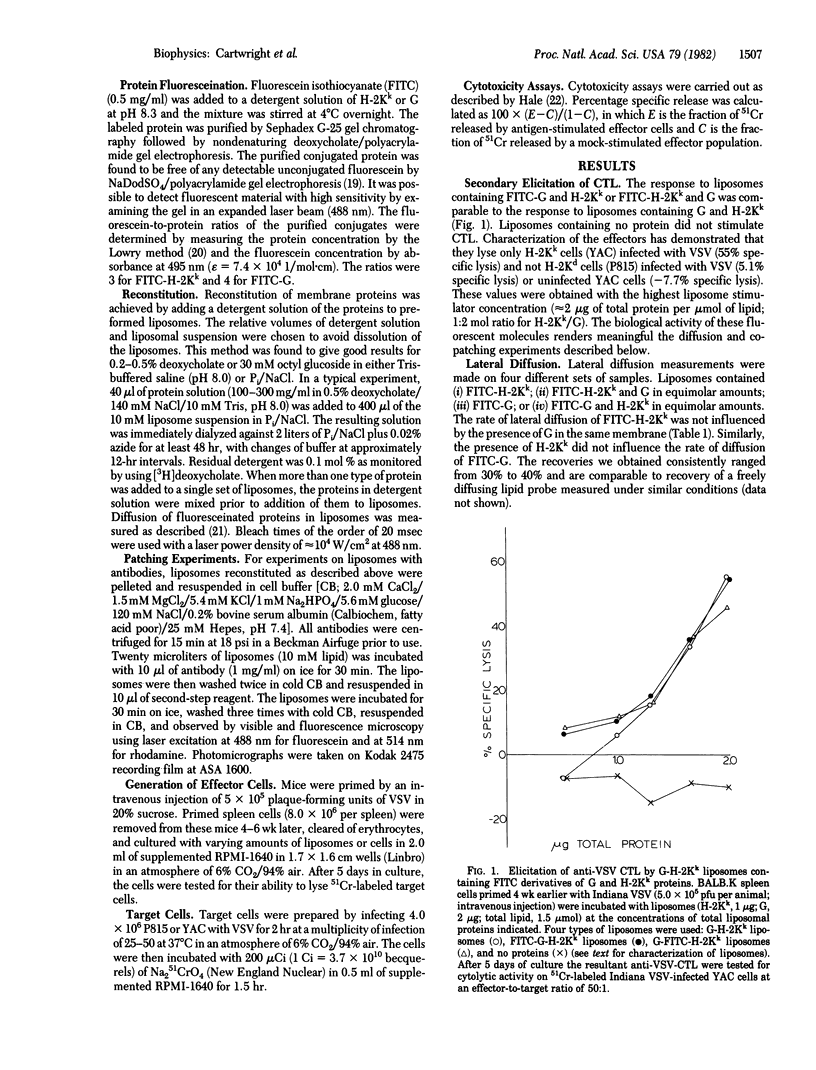
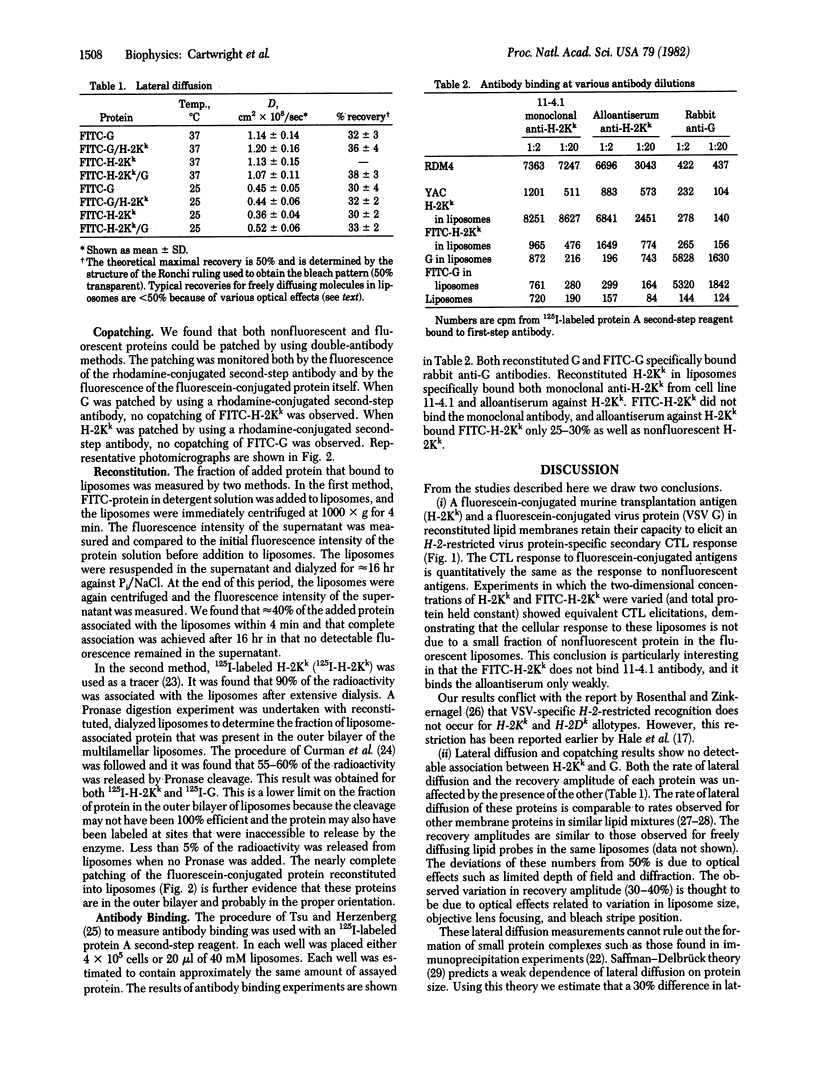
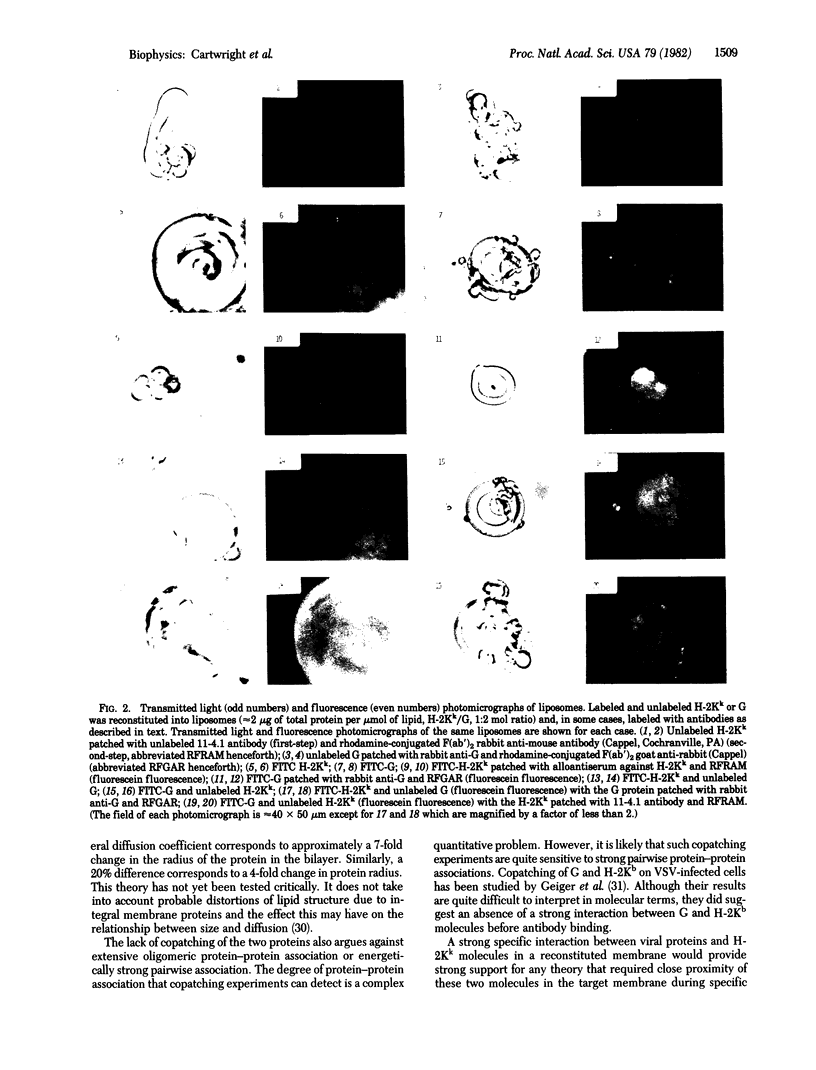
It is shown that liposomes containing (i) a fluorescein-labeled murine histocompatibility antigen (FITC-H-2Kk) and the G protein of vesicular stomatitis virus or (ii) H-2Kk and fluorescein-labeled viral protein (FITC-G) can elicit H-2-restricted syngeneic antiviral cytotoxic T cells as assayed by 51Cr release from appropriate virus-infected target cells. Fluorescence recovery after photobleaching was used to measure the diffusion coefficients of these reconstituted proteins in four different samples: (i) FITC-H-2Kk; (ii) FITC-H-2Kk and G; (iii) FITC-G; and (iv) FITC-G and H-2Kk. The same rate of lateral diffusion (D = 1 x 10(-8) cm2/sec at 37 degrees C in 25% cholesterol/75% dimyristoylphosphatidylcholine) was obtained in every case. Both proteins, fluorescent as well as nonfluorescent, could be patched by using specific antibodies. When G was patched with antibody, FITC-H-2Kk did not copatch. When H-2Kk was patched with antibody FITC-G did not copatch. These diffusion and patching measurements rule out the possibility that these proteins have either extensive oligomeric associations or strong specific pairwise associations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bubbers J. E., Chen S., Lilly F. Nonrandom inclusion of H-2K and H-2D antigens in Friend virus particles from mice of various strains. J Exp Med. 1978 Feb 1;147(2):340–351. doi: 10.1084/jem.147.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavarra R. P., Kang C. Y., Forman J. Vesicular stomatitis antigens recognized by cytotoxic cells: analysis with defective interfering particles and reconstituted membrane vesicles. J Immunol. 1980 Jul;125(1):336–343. [PubMed] [Google Scholar]
- Ciavarra R., Kang C. Y., Forman J. Mechanisms for generating cell membrane antigens that are recognized by cytotoxic T lymphocytes. Fed Proc. 1981 Feb;40(2):222–227. [PubMed] [Google Scholar]
- Curman B., Klareskog L., Peterson P. A. On the mode of incorporation of human transplantation antigens into lipid vesicles. J Biol Chem. 1980 Aug 25;255(16):7820–7826. [PubMed] [Google Scholar]
- Doherty P. C., Bennink J. R. Monitoring the integrity of self: biology of MHC-restriction of virus-immune T cells. Fed Proc. 1981 Feb;40(2):218–221. [PubMed] [Google Scholar]
- Finberg R., Mescher M., Burakoff S. J. The induction of virus-specific cytotoxic T lymphocytes with solubilized viral and membrane proteins. J Exp Med. 1978 Dec 1;148(6):1620–1627. doi: 10.1084/jem.148.6.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Rosenthal K. L., Klein J., Zinkernagel R. M., Singer S. J. Selective and unidirectional membrane redistribution of an H-2 antigen with an antibody-clustered viral antigen: relationship to mechanisms of cytotoxic T-cell interactions. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4603–4607. doi: 10.1073/pnas.76.9.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., Lewis J. T., McConnell H. M. Triggering of the macrophage and neutrophil respiratory burst by antibody bound to a spin-label phospholipid hapten in model lipid bilayer membranes. Biochemistry. 1980 Nov 11;19(23):5387–5394. doi: 10.1021/bi00564a037. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., Parce J. W., McConnell H. M. Specific antibody-dependent activation of neutrophils by liposomes containing spin-label lipid haptens. Biochem Biophys Res Commun. 1979 Feb 14;86(3):522–528. doi: 10.1016/0006-291x(79)91745-5. [DOI] [PubMed] [Google Scholar]
- Hale A. H., Witte O. N., Baltimore D., Eisen H. N. Vesicular stomatitis virus glycoprotein is necessary for H-2-restricted lysis of infected cells by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):970–974. doi: 10.1073/pnas.75.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Interactions of vesicular stomatitis virus with murine cell surface antigens. J Virol. 1976 Sep;19(3):833–845. doi: 10.1128/jvi.19.3.833-845.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. Purification of the H-2Kk molecule of the murine major histocompatibility complex. J Biol Chem. 1979 Sep 25;254(18):8713–8716. [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. Secondary cytolytic T lymphocyte stimulation by purified H-2Kk in liposomes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2488–2492. doi: 10.1073/pnas.78.4.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemonnier F., Mescher T. M., sherman L., Burakoff S. The induction of cytolytic T lymphocytes with purified plasma membranes. J Immunol. 1978 Apr;120(4):1114–1120. [PubMed] [Google Scholar]
- Loh D., Ross A. H., Hale A. H., Baltimore D., Eisen H. N. Synthetic phospholipid vesicles containing a purified viral antigen and cell membrane proteins stimulate the development of cytotoxic T lymphocytes. J Exp Med. 1979 Nov 1;150(5):1067–1074. doi: 10.1084/jem.150.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., McConnell H. M. Specificity of memory cells raised against trinitrophenyl-conjugated syngeneic cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1537–1541. doi: 10.1073/pnas.76.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M. F., Jose M. J., Balk S. P. Actin-containing matrix associated with the plasma membrane of murine tumour and lymphoid cells. Nature. 1981 Jan 15;289(5794):139–144. doi: 10.1038/289139a0. [DOI] [PubMed] [Google Scholar]
- Mescher M., Sherman L., Lemonnier F., Burakoff S. The induction of secondary cytolytic T lymphocytes by solubilized membrane proteins. J Exp Med. 1978 Mar 1;147(3):946–951. doi: 10.1084/jem.147.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. K., Feuer B. I., Vanderoef R., Lenard J. Reconstituted G protein-lipid vesicles from vesicular stomatitis virus and their inhibition of VSV infection. J Cell Biol. 1980 Feb;84(2):421–429. doi: 10.1083/jcb.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Owicki J. C., McConnell H. M. Theory of protein-lipid and protein-protein interactions in bilayer membranes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4750–4754. doi: 10.1073/pnas.76.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty H. R., Smith L. M., Fearon D. T., McConnell H. M. Lateral distribution and diffusion of the C3b receptor of complement, HLA antigens, and lipid probes in peripheral blood leukocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6587–6591. doi: 10.1073/pnas.77.11.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Inability of mice to generate cytotoxic T lymphocytes to vesicular stomatitis virus restricted to H-2Kk or H-2Dk. J Immunol. 1981 Feb;126(2):446–451. [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., McConnell H. M. Surface areas of lipid membranes. Biochemistry. 1978 Mar 7;17(5):837–840. doi: 10.1021/bi00598a014. [DOI] [PubMed] [Google Scholar]
- Senik A., Neauport-Sautes C. Association between H-2 and vaccinia virus-induced antigens on the surface of infected cells. J Immunol. 1979 Apr;122(4):1461–1467. [PubMed] [Google Scholar]
- Smith L. M., Rubenstein J. L., Parce J. W., McConnell H. M. Lateral diffusion of M-13 coat protein in mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1980 Dec 9;19(25):5907–5911. doi: 10.1021/bi00566a037. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Jacobson K., Wu E. S., Derzko Z. Lateral mobility of an amphipathic apolipoprotein, ApoC-III, bound to phosphatidylcholine bilayers with and without cholesterol. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5645–5649. doi: 10.1073/pnas.76.11.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]