Abstract
Objective
Physical activity (PA) is essential for successful aging and for the prevention and management of common chronic diseases. The empirical support for the beneficial effects of PA on vasomotor symptoms has however been mixed. The purpose of this study was to assess the effects of acute aerobic exercise and daily PA on menopausal vasomotor symptoms.
Methods
Community-dwelling midlife women (N = 121; age range 40–60 years) not on hormone therapy were recruited for a 15-day daily diary study. Women completed psychological, cardiorespiratory fitness, body composition, and hormonal status screening, followed by a 15-day prospective assessment in a “real-life” setting using a personal digital assistant device. Participants also completed a 30 minute moderate-intensity aerobic exercise bout on a treadmill between days 5–8. Daily PA was assessed objectively through accelerometry and all symptomatic women (n = 92) completed two 24-hour Biolog sternal skin conductance recordings of hot flashes (HFs), one at baseline and one immediately following treadmill exercise.
Results
Both total objective (p = .054) and total subjective (p < .05) HFs decreased following the acute exercise bout. At the between-person level, daily PA was not associated with self-reported HFs. However, at the within-person level, performing more moderate physical activity than usual was associated with more self-reported HFs in women with lower fitness levels.
Conclusions
Moderate aerobic exercise decreases objective and subjective HFs 24 hours following exercise, however, in women with lower fitness levels, more daily moderate PA leads to more self-reported symptoms.
Keywords: Menopause, Vasomotor Symptoms, Hot Flashes, Physical Activity, Exercise
Regular physical activity (PA) has numerous health benefits, many of which are directly relevant to menopausal women such as reduction in the risk for cardiovascular disease, diabetes, osteopenia and osteoporosis, some cancers, as well as depression.1 One area in which support for the beneficial influence of PA remains unconvincing is its effect on vasomotor symptoms. Vasomotor symptoms affect the majority of American women as they transition through menopause, and for some these symptoms become severe enough to negatively impact daily functioning and quality of life.2–5 Disheartening for many women is also the finding that vasomotor symptoms last much longer than previously thought (>5–8 years on average), with 10% of women experiencing these symptoms as long as 12 years post menopause.6,7 Most women are reluctant to treat their symptoms with pharmacological approaches, and opt instead for more natural methods which may include behavior modification through PA.
Reviews of research examining the effects of exercise and other forms of PA on vasomotor symptoms attest to the growing interest in pursuing PA as a modality for symptom management.8–11 The overall conclusion nonetheless remains that there is insufficient evidence that PA alleviates vasomotor symptoms. Moreover, one study documented an increase in self-reported vasomotor symptoms in some women as part of a year-long exercise intervention trial.12 In another laboratory-based study, objectively measured vasomotor symptoms increased within 24 hours following a moderate-intensity exercise bout.13 Perceived physical exertion also preceded hot flashes (HFs) in a study involving two days of ambulatory HF monitoring in a real-life setting.14
HFs are believed to serve a heat dissipating function. Specifically, the thermoregulatory model posits that HFs are caused by altered neurotransmitter release in the hypothalamus, disrupting the regulation of the thermoregulatory center such that the core body temperature threshold for sweating is lowered, reducing the thermoneutral zone (i.e, sweating occurs at lower body core temperature).15,16 PA increases body core temperature and might thus stimulate more HFs, especially when performed at high exercise intensities or for long durations. On the other hand, a protective role of PA for alleviating HFs may be expected given its other neuroendocrine17,18,19, body composition, thermoregulation, fitness and psychological effects 20–22.
There are no sufficiently powered randomized controlled trials of aerobic exercise targeting HFs as the primary outcome.11 The remaining studies are limited by the absence of both subjective and objective measures of PA and menopausal HFs. Consequently, it is unclear whether acute or chronic PA impacts primarily processes involved in symptom perception and reporting or whether any of the aforementioned physiological mechanisms are at play and having any discernible impact on objectively measured HFs. Thus, the present study had two main purposes. First, we sought to evaluate whether an acute bout of moderate-intensity aerobic exercise increases subjective and objective vasomotor symptoms, as previously reported by Freedman and Krell.13 Second, we examined whether objectively measured PA is associated with subjective HFs across a two-week monitoring period “in a real life setting” during which HFs were self-reported on a personal digital assistant (PDA) device. At the same time, we evaluated whether there are individual differences in the responses to acute exercise and daily PA associated with characteristics (cardiorespiratory fitness, weight status, or menopausal status) likely to influence the relation between PA and HFs.9,23–25
METHOD
Participants
Community-dwelling midlife women (N = 121; age range 40–60 years) not on hormone therapy for at least 6 months were recruited for a prospective daily diary study on PA and psychological function, which included an experimental acute exercise bout. The study was advertised as a study of psychological and physiological effects of exercise in middle-aged women in a variety of community-based settings such as public bulletin boards, libraries, physician’s offices, and through online listserves. Ninety two women from this sample reported menopause-related vasomotor symptoms (night sweats or HFs) within the last two weeks, and their data were used in the analysis for this study.
Study Design
As part of screening into the study, all women completed cardiorespiratory fitness (maximal graded exercise test using TrueMax2400 Parvomedics Inc. metabolic system, Salt Lake City, Utah), body composition (dual-energy X-ray absorptiometry or DEXA), menopausal (self-reported menstrual bleeding history) and hormonal status (blood draw) screening. All women also completed a baseline battery of psychological questionnaires, three laboratory sessions, and prospective monitoring across a 15-day period. All symptomatic women additionally completed two 24-hour Biolog (UFI, model 3991/1-SCL; Morro Bay, CA) sternal skin conductance recordings of HFs, one at baseline (day 1) and one immediately following the acute exercise bout, which occurred during their second lab visit, usually between days 5 and 8 of the 15-day study. PA was monitored objectively with an activity monitor that used a uniaxial accelerometer (Actigraph model GT1M, Pensacola, FL) and daily HF symptoms were self-reported in real-time using the PDA (Tungsten E2) device.
Lab session 1 was scheduled on the first day of the study (usually in the morning) and consisted of a familiarization procedure to the study protocol and a tutorial on PDA and activity monitor use. All symptomatic women were subsequently fitted with a Biolog sternal skin conductance monitor, which they were asked to wear for a 24-hour period. The first lab session was followed by a 15-day prospective assessment of PA and vasomotor symptoms.
During week 1 of this prospective monitoring, women attended their second lab visit, during which they completed a 30-minute moderate-intensity exercise bout. This exercise bout was performed (in the morning or early afternoon) on a treadmill at a self-selected pace and consisted of a 5 minute warm-up, 20 minute steady state exercise, and a 5 minute cool-down. Women were instructed to self-select a pace corresponding to ratings of perceived exertion between ‘12’ (light/somewhat hard) to ‘15’ (somewhat hard/hard) using the Borg’s RPE scale.26 The moderate intensity was corroborated objectively through intermittent assessment of oxygen uptake during exercise and corresponded on average to 60% of peak oxygen uptake determined during screening, a level considered moderate based on the guidelines of the American College of Sports Medicine.27 Following the exercise bout, all symptomatic women were again fitted with the skin conductance monitor for another 24-hour ambulatory HF monitoring session.
Measures
Background information
Basic demographic and health history information included menopausal status based on self-reported bleeding patterns and categorized according to the Stages of Reproductive Aging Workshop (STRAW) criteria.28 During initial telephone screening women were asked about their menstrual bleeding patterns and subsequently completed a questionnaire which presented several yes/no statements (e.g., I have had normal menses during the last 12 months) corresponding to the STRAW menopausal categories.
Physical activity
Habitual PA was assessed objectively through continuous monitoring with the accelerometer, which was positioned over the participants’ non-dominant hip with an adjustable elastic belt. Participants wore the accelerometer for the entire data collection period (i.e, 15 days) and were asked to take off the accelerometer when in contact with water (e.g., bathing or swimming). The accelerometer collected data in 1 minute epochs, and the data were processed and analyzed using the ActiLife data analysis software from Actigraph (version 5.7.4). Wear-time was validated through the ActiLife software procedures, with valid hour considered to have at least 10% of non-zero activity counts and valid day reflecting at least 10 valid hours. Only valid data were analyzed. The primary PA measures in this study were total daily PA counts, percent time of daily activity spent sedentary (0–100 counts per minute), or engaged in lifestyle (101–759 counts per minute), light (760–1952 counts per minute), moderate (1953–5724 counts per minute), and vigorous (5725+ counts per minute) PAs. The selected cutpoints are based on criteria previously established by Freedson et al. (1998). One of the 92 women lost her activity monitor, thus data from only 91 participants were used in the analysis involving daily PA. In addition to PA, body mass index (BMI) was computed from weight and height measured in the lab using standardized procedures.
Hot flashes
The Purdue Momentary Assessment platform was used to collect the data on HF symptoms self-reported in real-time on the PDA.29 Women entered each HF as a separate event (time-stamped automatically by the PDA) and each time answered questions regarding how long ago the HF occurred, its duration, severity, and some relevant contextual information regarding location, approximate temperature, type of activity performed at the moment of the HF, and previous intake of HF-triggering types of foods and beverages. The data were downloaded on to a computer and processed to determine the total number of reported unique HF events per day.
Objective HFs were determined from continuous skin conductance monitoring using the Biolog HF monitor, a battery-powered, portable device (14cm × 7cm × 3cm) that measures skin conductance through two electrodes (1.5 cm in diameter) attached to the sternum. In addition to continuous monitoring, participants are also instructed to press two event mark buttons simultaneously when they perceived a HF, providing time-stamped self-reported HF data. HFs entered through event marks were compared to PDA entries and counted as the same event if within 5 minutes of each other. Skin conductance data were processed using the FlashTrax software (version 1.2e; UFI, Morro Bay, CA). Standard criteria were used to detect HFs using the FlashTrax auto analysis features. Increases in skin conductance of ≥ 2 micro-mho (μmho) in a 30-second period were electronically marked and considered as potential HFS. Two trained research assistants then independently visually inspected the data to verify the events and distinguish them from noise or artifact. Both sets of analyses were then compared, and differences were reconciled by mutual agreement among the assistants and the principal investigator. Periods of noisy data that were deemed non-interpretable were excluded from the data, and the daily totals were adjusted for valid wear time. For descriptive purposes, the data are reported as rates representing number of HF events per valid hour of recording on each monitoring day. Rate of true positive, false negative, and false positive HFs was established by comparing subjective HF reports (either on PDA or through Biolog event marks) with objective HFs (Biolog skin conductance). A physiologically objective event that was accompanied by a subjective report within 5 minutes was considered a true positive HF. An objective event not accompanied by a subjective report within 5 minutes was considered a false negative HF, and a subjective report without an objective event within 5 minutes was considered a false positive HF.14,30 HF events detected or reported between 11:00 pm and 6:00 am were considered night sweats. Because women were not required to self-report these events while asleep, analysis was performed without these events in the daily rates at first. For objective HFs, we then repeated the analysis with these night time events (i.e., presumptive night sweats). Ultimately, complete pre and post data for both objective and subjective symptoms were available for 84 out of the 92 symptomatic women. The main reasons for the missing data were technical problems with the Biolog monitors. A series mean imputation method was used to replace the missing values. Where results differed as a function of this imputation, the findings were noted for both sets of analyses (i.e., with imputed missing values versus with missing cases present).
Statistical analysis
Acute exercise effects
Descriptive statistics were used to describe the sample and a set of generalized estimating equation (GEE) analyses 31 were conducted to determine whether there were changes on the sets of HF variables from PRE to POST exercise, and to determine whether there was a time (PRE to POST) by grouping variable (i.e., fitness, weight, menopausal, and overall symptom burden status) interaction on the HF variables. The GEE method was used because: (1) the dependent variables (i.e., HF variables) were count data (violating the assumption of normal distribution); (2) GEE has the option to analyze count data with a log link function, thus providing more accurate results than other conventional statistical methods that may underestimate standard errors; (3) GEE is the more appropriate method to use for the analysis of correlated data (participants’ pre HFs were moderately correlated with their post HFs in this study). 31 The GEE analyses were performed separately for total objective and subjective HFs, and for true positive, false negative, and false positive HFs. For total objective HFs, the analysis was repeated including both daytime HFs and night sweats. Total hours of valid wear time was used as an offset variable in the GEE analyses to adjust for differences in valid HF recording hours. To evaluate differences in response based on fitness, weight, menopausal, and overall symptom burden status, women were categorized into high (higher fit women; >50th percentile age-matched) and low (lower fit women; ≤50th percentile age-matched) fitness categories based on peak oxygen uptake achieved during the graded exercise test at screening.27 BMI was used to categorize women into normal (BMI<25) and overweight/obese (BMI≥25) categories. Menopausal status reflected three categories, early perimenopausal, late perimenopausal, and postmenopausal.28 To examine whether the acute bout had differential effect on symptoms depending on overall symptom burden, we categorized women into tertiles based on the average frequency of symptoms reported across the entire 15-day monitoring period. The analyses were performed using SPSS 19.0 statistical software package.
Daily physical activity effects
Daily PA and HF reports were nested within people; thus multilevel modeling was used to test the association between daily PA and self-reported HFs. This approach allows for examining both between- and within-person associations between PA and HFs.32 To separately test between- and within-person associations, daily PA measures and HFs were person centered.33,34, 35 For example, person i’s overall level of PA was calculated as the within-person mean of her PA counts across the 15-day study period and each person’s daily PA was calculated as the deviation of day d’s score from the individual mean. Thus, this score represents PA fluctuations from individual mean, equivalent to being more or less active that day than on usual days. Separate models were run for total PA (daily activity counts), lifestyle PA, light intensity PA, moderate intensity PA, vigorous intensity PA, and sedentary time as the independent (exposure) variables. SAS 9.2 PROC MIXED35 was used to test the following multilevel model by regressing daily HF on overall and daily PA:
where γ00 represents the average level of HFs in the sample, γ01 represents the influence of PA and HFs (i.e., between-person effect), γ10 represents the average effect of PA fluctuations (i.e., average within-person effects) on HFs, and u0i are individual-level residual deviations that are uncorrelated with the day-level residuals edi. Of particular interest for our research questions are the γ01 and γ10 parameters quantifying the between-person and within-person associations between PA and HFs.
RESULTS
Sample Description
Descriptive statistics for ninety two symptomatic women are presented in Table 1. Participants were on average 52 years old and predominantly Non-Hispanic White. Most were married or in committed relationships, well educated, had income above $65,000 (68%), and had at least one child. There was a representation across different menopause stages. During a 24-hour baseline assessment, women self-reported a total of 3.26 HFs (.17 per valid hour) and had 8.6 objectively recorded HFs or night sweats (.41 per valid hour) on average. Across the entire 15-day monitoring period women self-reported on average 1.1 HFs per day.
Table 1.
Descriptive statistics of the study sample
| Variable | Mean | SD | Range |
|---|---|---|---|
| Age | 51.8 | 3.9 | 40–59 |
| Height (cm) | 163.7 | 6.7 | 148–188.5 |
| Weight (kg) | 68.8 | 13.1 | 46.2–110 |
| BMI | 25.7 | 4.4 | 18.4–39.8 |
| VO2 Peak (ml/kg/min) | 31.0 | 6.3 | 17.7–54.3 |
| Number of children | 2.3 | 1.2 | 0–8 |
| Baseline self-reported HFs (per hour) | 0.2 | 0.3 | 0–2 |
| Baseline objective HFs (per hour) | 0.4 | 0.4 | 0–2 |
| Average PDA-reported HFs (per day) | 1.1 | 1.2 | 0.1–5.8 |
| Number (%) | |||
| Early perimenopausal | 18(19.6) | ||
| Late perimenopausal | 31(33.7) | ||
| Postmenopausal | 43(46.7) | ||
| Surgical menopause | 9(9.8) | ||
| Prior HRT use (not within 6 months) | 11(12.0) | ||
| Education | |||
| High school or some college | 26(28.3) | ||
| College graduate | 66(71.7) | ||
| Income (reported by 99%) | |||
| < $42,360 | 11(12.1) | ||
| Between $42,360–$65,727 | 17(18.7) | ||
| > $65,727 | 63(69.2) | ||
| Marital Status | |||
| Single | 2(2.2) | ||
| Married | 79(85.9) | ||
| Committed Relationship | 5(5.4) | ||
| Divorced | 2(2.2) | ||
| Widow | 4(4.3) | ||
| Race/Ethnicity (reported by 97%) | |||
| Hispanic or Latino | 1(1.1) | ||
| Not Hispanic or Latino | 88(95.7) | ||
| Black or African-American | 3(3.3) | ||
| White | 88(95.7) | ||
Acute Exercise Effects on Hot Flashes
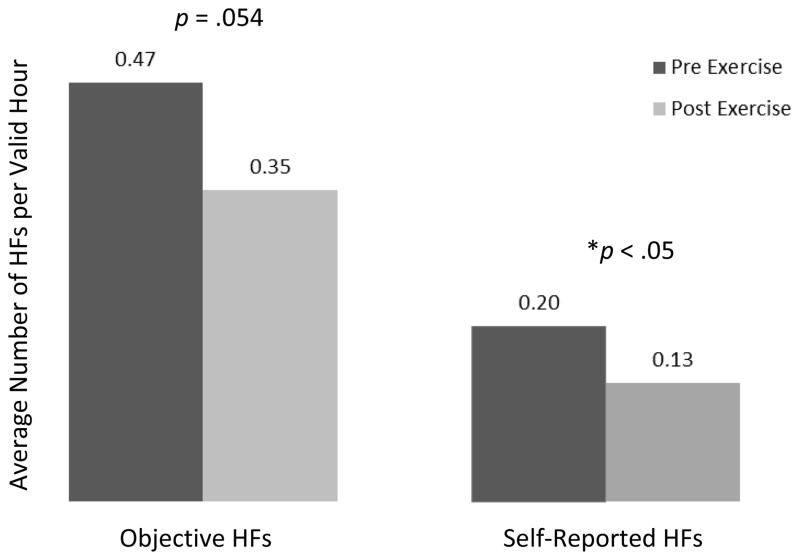
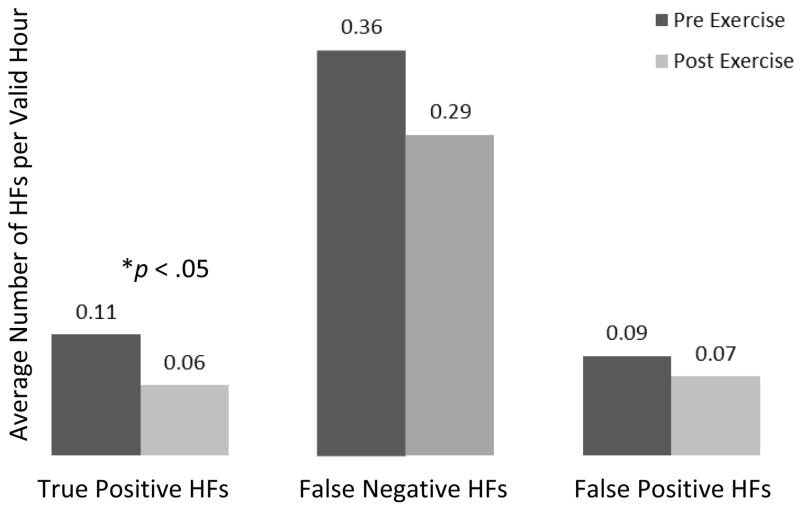
When analyzing daytime HFs only, the analysis indicated that both total objective and total subjective HFs decreased following the acute exercise bout, but the change was statistically significant only for subjective HFs (β = −.257, p = .054 for objective, and β = −.305, p = .010 for subjective HFs) (Figure 1). On average, objective daytime HF count following exercise (POST) was about 77% of the count before exercise (PRE). For subjective HFs, the POST count was about 74% of the count before exercise (PRE). When analyzing acute exercise effects on symptoms expressed as counts of true positive, false negative, and false positive symptoms, there was a significant reduction in true positive HFs from PRE to POST exercise (β = −.53, p = .008). On average, true positive daytime HF count following exercise (POST) was about 59% of the count before exercise (PRE). There was no significant difference between PRE and POST false negative HFs (p = .231) or false positive HFs (p = .923). (Figure 2). When including both daytime HFs and night sweats, there was a significant reduction in total objective vasomotor symptoms (both HFs and night sweats), β = −.241, p = .028. On average, objective vasomotor symptom count following exercise (POST) was about 78.6% of the count before exercise (PRE). Reflecting objective night sweats only, there was no significant change from pre to post exercise.
Figure 1.
Effects of an acute bout of moderate-intensity aerobic exercise on rates of objective and self-reported hot flashes
*This figure has been generated for descriptive purposes only. The data are displayed as HF count per valid HF recording hour and represent a general trend of changes from pre to post exercise. This trend has been verified by GEE analysis.
Figure 2.
Effects of an acute bout of moderate-intensity aerobic exercise on rates of true positive, false positive, and false negative hot flashes
*This figure has been generated for descriptive purposes only. The data are displayed as HF count per valid HF recording hour and represent a general trend of changes from pre to post exercise. This trend has been verified by GEE analysis.
When evaluating variation in symptom responses by fitness, weight, menopausal, and overall symptom burden status, respectively, there were no statistically significant time by group interactions for any of the HFs variables when analyzing the data with imputed missing values. In the analysis of data with missing values (<10% missing), significant differences by menopausal and weight status were observed on selected HF variables. Specifically, when controlling any other grouping variables, significant weight status by time interaction on false positive daytime HF count was observed (p = .039). Normal weight women were more likely to report less false positive HF count after exercise, while overweight women reported a slightly more false positive HFs after exercise. There was also a significant menopausal status by time interaction on false positive daytime HF count (p = .020), with late perimenopausal women reporting significantly more false positive HFs after exercise compared to pre-exercise, while early postmenopausal and late postmenopausal women reported similar rates of false positive HFs after exercise compared to pre-exercise. Finally, when controlling any other grouping variables, significant weight status by time interaction on total subjective daytime HF count was revealed (p = .046). Normal weight women had greater reduction in subjective HFs following exercise as compared to overweight women. It must be noted that the afore-mentioned interaction effects were only present when all other grouping variables were included in the model. For descriptive purposes, rates of HFs per valid wear time hour by all grouping categories are presented in Table 2.
Table 2.
Descriptive statistics (from pre to post exercise) for HF variables for total sample and by weight, fitness, symptom burned, and menopausal status category.
| Symptoms | Total Objective HFs | Total Subjective HFs | True Positive HFs | False Negative HFs | False Positive HFs | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PRE Mean (SD) Range Skew |
POST Mean (SD) Range Skew |
PRE Mean (SD) Range Skew |
POST Mean (SD) Range Skew |
PRE Mean (SD) Range Skew |
POST Mean (SD) Range Skew |
PRE Mean (SD) Range Skew |
POST Mean (SD) Range Skew |
PRE Mean (SD) Range Skew |
POST Mean (SD) Range Skew |
|
| Total sample | .47 (.51) | .35 (.51) | .20 (.29) | .13 (.20) | .11 (.17) | .06 (.16) | .36 (.46) | .29 (.45) | .09 (.24) | .07 (.13) |
| .00–2.40 | .00–2.5 | .00–2.00 | .00–1.09 | .00–.76 | .00–1.09 | .00–2.40 | .00–2.50 | .00–2.00 | .00–.59 | |
| 1.51 | 2.13 | 3.14 | 2.07 | 1.85 | 4.04 | 2.11 | 2.78 | 6.64 | 2.41 | |
| Normal weight (n=45) | .41 (.39) | .36 (.50) | .15 (.18) | .11 (.16) | .09 (.14) | .06 (.13) | .30 (.35) | .29 (.45) | .06 (.10) | .05 (.11) |
| .00–1.71 | .00–2.14 | .00–.88 | .00–.71 | .00–.65 | .00–.65 | .00–1.29 | .00–2.14 | .00–.50 | .00–.50 | |
| 1.39 | 1.86 | 1.68 | 1.91 | 1.995 | 2.94 | 1.53 | 2.42 | 2.57 | 2.89 | |
| Overweight/obese (n=39) | .60 (.62) | .38 (.54) | .28 (.37) | .17 (.24) | .13 (.21) | .07 (.19) | .43 (.55) | .29 (.46) | .13 (.33) | .09 (.15) |
| .00–2.40 | .00–2.50 | .00–2.00 | .00–1.09 | .00–.76 | .00–1.09 | .00–2.4 | .00–2.50 | .00–2.00 | .00–.59 | |
| 1.24 | 2.445 | 3.01 | 1.88 | 1.67 | 4.09 | 1.91 | 3.26 | 5.08 | 2.06 | |
| Higher fit (n=44) | .49 (.54) | .31 (.43) | .19 (.33) | .13 (.20) | .07 (.12) | .06 (.18) | .41 (.51) | .24 (.33) | .11 (.31) | .07 (.12) |
| .00–2.40 | .00–1.73 | .00–2.00 | .00–1.09 | .00–.65 | .00–1.09 | .00–2.40 | .00–1.59 | .00–2.00 | .00–.50 | |
| 1.74 | 2.08 | 3.75 | 2.86 | 2.34 | 4.58 | 2.32 | 2.27 | 5.585 | 2.32 | |
| Lower fit (n=39) | .51 (.49) | .44 (.61) | .23 (.24) | .15 (.21) | .15 (.21) | .07 (.13) | .32 (.39) | .34 (.56) | .06 (.12) | .08 (.15) |
| .00–1.76 | .00–2.50 | .00–.76 | .00–.71 | .00–.76 | .00–.65 | .00–1.47 | .00–2.50 | .00–.53 | .00–.59 | |
| 1.14 | 1.99 | 0.84 | 1.29 | 1.42 | 2.59 | 1.33 | 2.50 | 2.94 | 2.35 | |
| Low symptom burden | .43 (.54) | .31 (.41) | .19 (.44) | .05 (.10) | .04 (.08) | .03 (.09) | .39 (.52) | .28 (.40) | .16 (.44) | .02 (.04) |
| .00–2.00 | .00–1.59 | .00–2.00 | .00–.41 | .00–.24 | .00–.35 | .00–2.00 | .00–1.59 | .00–2.00 | .00–.12 | |
| 1.65 | 1.65 | 4.05 | 2.11 | 2.18 | 2.35 | 1.84 | 2.13 | 4.29 | 1.49 | |
| Moderate symptom burden | .53 (.60) | .31 (.36) | .20 (.19) | .15 (.17) | .13 (.20) | .05 (.06) | .40 (.56) | .26 (.33) | .07 (.08) | .10 (.18) |
| .00–2.40 | .00–1.24 | .00–.76 | .00–.65 | .00–.76 | .00–.21 | .00–2.40 | .00–1.18 | .00–.24 | .00–.59 | |
| 1.83 | 1.60 | 1.68 | 1.56 | 2.45 | 1.44 | 2.54 | 1.68 | .72 | 1.86 | |
| High symptom burden | .67 (.51) | .65 (.75) | .36 (.22) | .30 (.26) | .25 (.20) | .16 (.27) | .43 (.42) | .49 (.68) | .11 (.15) | .14 (.16) |
| .00–1.76 | .00–2.50 | .00–.88 | .00–1.09 | .00–.65 | .00–1.09 | .00–1.47 | .00–2.50 | .00–.53 | .00–.59 | |
| 0.71 | 1.26 | 0.17 | 1.49 | .40 | 2.44 | 1.15 | 2.07 | 1.95 | 1.62 | |
| Early perimenopausal (n =17) | .36 (.36) | .34 (.51) | .07 (.14) | .07 (.15) | .02 (.05) | .02 (.08) | .30 (.35) | .27 (.45) | .05 (.13) | .04 (.12) |
| .00–1.29 | .00–1.59 | .00–.50 | .00–.50 | .00–.47 | .00–.33 | .00–1.29 | .00–1.59 | .00–.50 | .00–.50 | |
| 1.30 | 1.95 | 1.93 | 2.23 | 3.42 | 3.53 | 1.77 | 2.04 | 3.33 | 3.55 | |
| Late perimenopausal (n =27) | .42 (.49) | .20 (.24) | .17 (.40) | .10 (.15) | .06 (.11) | .03 (.13) | .34 (.45) | .16 (.19) | .11 (.38) | .07 (.08) |
| .00–2.00 | .00–.88 | .00–2.00 | .00–.71 | .00–.65 | .00–.65 | .00–2.00 | .00–.59 | .00–2.00 | .00–.29 | |
| 2.00 | 1.25 | 3.76 | 2.34 | 2.72 | 3.79 | 2.41 | 1.06 | 5.23 | 1.05 | |
| Postmenopausal (n =40) | .60 (.57) | .49 (.62) | .28 (.23) | .19 (.24) | .18 (.21) | .10 (.19) | .40 (.50) | .38 (.56) | .09 (.13) | .09 (.16) |
| .00–2.40 | .00–2.50 | .00–.76 | .00–1.09 | .00–.76 | .00–1.09 | .00–2.40 | .00–2.50 | .00–.53 | .00–.59 | |
| 1.17 | 1.73 | .55 | 1.77 | 1.31 | 3.78 | 1.96 | 2.36 | 1.96 | 2.05 | |
Note. Symptoms are presented as rates per valid waking hour (i.e., do not reflect night sweats). The Means and Standard Deviations (SDs) should be interpreted with caution given the non-normal distribution of the data.
Effects of Daily Physical Activity on Self-Reported Hot Flashes
The longitudinal multilevel analysis revealed substantial variation in both daily PA and HFs at the between- and within-person levels, respectively. The intraclass correlation cofficients reflecting between-person variation were 41% and 42% for PA and HFs, respectively, suggesting that 59% of the PA and 58% of the HF data varied within-persons over time. There were no statistically significant associations between daily HFs and overall PA or in categories of PA (sedentary time, lifestyle PA, light intensity PA, or vigorous intensity PA) at either the between-or within-person level. At the between-person level, there was no relationship between a woman’s overall level of moderate intensity activity and HF frequency (t73 = −1.22, p=0.22). At the within-person level, however, a woman’s daily fluctuations in moderate intensity activity covaried significantly with self-reported HF frequency (t949=2.98, p<0.01). That is, on days when a woman was more active than usual, she also reported more HFs. Daily within-person fluctuations in HFs did not differ for women with different overall activity level (t949=−1.53, p=0.13). Put differently, reporting more or less HFs on a given day did not depend on how active a woman was overall (Table 3).
Table 3.
Key multilevel model statistics for the basic model testing effects of time spent in daily moderate intensity physical activity on daily self-reported hot flashes.
| Parameter Estimate (Standard Error) | |
|---|---|
| Fixed Effects | |
| Intercept, γ00 | 1.45 (.29) |
| Overall PA, γ01 | −.12 (.10) |
| Daily PA, γ10 | .15* (.05) |
| Daily PA × overall PA, γ11 | −.02 (.01) |
| −2LL | 3716.9 |
| AIC | 3720.9 |
Note. Unstandardized estimates and standard errors. Model is based on data reflecting an average of 15 occasions nested within 91 participants for a total of 1447 observations. Due to some missing data (e.g., activity monitor malfunction), a total of 1026 observations were used to test this model. AIC = Akaike Information Criterion (measure of the relative goodness of fit of the statistical model). −2LL = −2 Log Likelihood.
p < .05.
We also evaluated the influence of fitness and BMI as additional between-person factors in the same multilevel model. The within-person association between daily HFs and fluctuations in moderate intensity PA varied significantly by fitness level (t942=−2.20, p<.05), revealing that this positive within-person association between daily HFs and moderate PA could be explained by differences in fitness such that lower fit women tended to self-report more HFs with increasing amount of moderate PA (Table 4). The interaction terms with BMI and menopausal status were not statistically significant, suggesting that differences in weight or menopausal status did not account for the positive within-person association between self-reported HFs and moderate PA.
Table 4.
Key multilevel model statistics for model testing the effects of time spent in daily moderate intensity physical activity on daily self-reported hot flashes by fitness, BMI, and menopausal status.
| Parameter Estimate (Standard Error) | |
|---|---|
| Fixed Effects | |
| Intercept | .67 (.64) |
| Overall PA | −.08 (.11) |
| Daily PA | .18 (.11) |
| Fitness | −.02 (.03) |
| BMI | −.02 (.03) |
| Menopausal status | .28 (.22) |
| Daily PA × overall PA | .01 (.01) |
| Daily PA × fitness | .01*(.01) |
| Daily PA × BMI | .01 (.03) |
| Daily PA × menopausal status | .01 (.04) |
| −2LL | 3728.3 |
| AIC | 3732.3 |
Note. Unstandardized estimates and standard errors. Model is based on data reflecting an average of 15 occasions nested within 91 participants for a total of 1447 observations. Due to some missing data (e.g., activity monitor malfunction and missing fitness data), a total of 1021 observations were used to test this model. AIC = Akaike Information Criterion (measure of the relative goodness of fit of the statistical model). −2LL = −2 Log Likelihood.
p < .05.
DISCUSSION
This study is the first to evaluate the links between PA and menopausal HFs using both objective and subjective measures. A key finding is that an acute bout of moderate intensity aerobic exercise decreases both objective and subjective HFs. Although overall PA was not associated with HF frequency in longitudinal analyses over a 15 day period, lower fit women reported more vasomotor symptoms on days when they engaged in more moderate intensity PA than usual. These findings extend our understanding of the relationship between PA and vasomotor symptoms, given the absence of sufficiently powered randomized controlled trials11 and the lack of studies corroborating self-report assessments with objective measures of both PA and vasomotor symptoms.
Our study demonstrated that an acute bout of exercise reduces both objectively and subjectively measured symptoms. This finding is inconsistent with the suppositions of the thermoregulatory model of HFs which suggests that more HFs should follow periods of activity that increase body core temperature such as moderate intensity exercise. The only other study that directly evaluated acute effects of exercise on objectively measured HFs was conducted by Freedman & Krell13 and noted a triggering of HFs following exercise in a laboratory setting. The most likely explanation for the differing results involves the overall level of HFs in our sample which was rather low as compared to the Freedman & Krell study. Interestingly, the calculated effect sizes in the present study indicate it was the women in the two lowest symptom burden categories that experienced symptom reductions, with women in the highest category showing little change (ds = −.25, −.47, and −.04, for low, moderate, and high symptom burden, respectively). Notably, the average number of HFs reported on the PDA across 15 days in the highest symptom burden category was 2.4 HFs per day, which is about half the symptoms used as a criterion for entry into the Freedman & Krell study (≥5 HFs per day) and is also below the FDA definition of moderate to severe symptoms (7–8 HFs per day).36 Additionally, the Freedman & Krell study differed from our study in target population (postmenopausal women versus peri and postmenopausal in our study), environment (HF monitoring in controlled laboratory environment versus in real-life setting in our study), mode and duration of exercise (cycle ergometer at 40% predicted VO2 until either a HF occurred, 60 minutes passed, or participants requested termination; versus a standard 30 minute exercise session on a treadmill in our study). The intensity of exercise was nonetheless comparable between the two studies when expressed as percent predicted VO2. Future studies are needed to evaluate potential increases in symptoms following exercise in women who are highly symptomatic. More symptomatic women may be also disproportionately represented by overweight (as opposed to normal weight) and late perimenopausal women, the two groups of women that tended to exhibit smaller reductions in subjective symptoms in our study when controlling for each other and the effects of other characteristics (i.e., fitness and symptom burden).
Our study also examined the relationship between daily (habitual) PA and self-reported HFs. At the between-person level, there was no association between any of the PA indicators and HFs. However, in within-person analyses time spent in moderate intensity activity was positively associated with subjective HFs among lower fit women. The effect of daily PA and HFs did not significantly vary by BMI or menopausal status. The involvement of cardiorespiratory fitness in the mechanisms of the association between PA and HFs is plausible given the numerous adaptations endurance trained persons exhibit in thermoregulatory control as compared to untrained persons.37 In addition to attenuated blood pressure and heart rate responses to exercise, aerobically-trained individuals exhibit blunted sympathetic vasomotor activation as compared to untrained individuals.38 Sympathetic activation is higher in symptomatic as compared to asymptomatic menopausal women; drugs that increase sympathetic activation provoke HFs and drugs that decrease this activation ameliorate them. It is believed that an increase in sympathetic activation narrows the thermoneutral zone, which increases the likelihood of HFs, whereas decreased sympathetic activation widens it.15 Additionally, decreases in cardiac vagal control were observed during HFs 39 and aerobically fit/endurance trained individuals have higher heart rate variability (an indirect measure of increased cardiac vagal control) than less fit/sedentary individuals.40 Given these effects involving both sympathetic and parasympathetic control, cardiorespiratory fitness may protect women from experiencing HFs in response to chronic PA.
Our analysis regarding the effects of daily PA on HFs evaluated only self-reported symptoms. Unfortunately, most devices that measure HFs objectively do so for short periods of time (24–72 hours), require that sensors be replaced every 24 hours (e.g., Biolog), or their validity has not been widely established (e.g., FlashMarkPro41). It should also be noted that any objective monitoring technology may be limited for evaluation of vasomotor symptoms during PA. As most rely on measurements of sweating responses or humidity detection, they are by design unsuitable for use during exercise or periods of PA that result in profuse sweating. Self-report measures thus remain the best option for PA researchers but should be conducted in real time (e.g., using electronic diaries) to minimize recall bias. Self-reported HFs have the additional advantage of face validity; they reflect symptoms that matter to women. In fact, just as in other studies,42 objective and subjective HFs were highly discordant in our study, with significant number of objective HFs not reported by women or reported by women but not objectively detected. Potential explanations for this discordance may include individual differences in somatosensory sensitivity or affect 43, but more studies are needed to help disentangle the reasons behind under-reporting as well as to improve sensitivity of objective measures of HFs.44
Our sample was well-characterized in terms of health, PA, vasomotor symptoms, and menopausal status, however the women were mostly non-Latina White, educated, and of above average socioeconomic status. Future studies should incorporate more ethnically diverse samples to allow for broader generalizability, especially given ethnic and racial differences in symptom experiences and reporting 45 and in rates of PA.46 Although this is the first study to evaluate changes in objectively and subjectively measured symptoms as a function of acute exercise, our baseline monitoring session did not immediately precede our acute exercise bout. Given the high variability in symptoms, it would be useful to monitor HFs objectively for longer than 24 hours and as close to an acute exercise bout as possible. It should also be noted that the within-person association between moderate intensity activity and HFs may be dependent on the operational definition of moderate intensity activity used. We utilized previously established criteria,47 however other criteria can be used to classify intensity levels. For example, the criteria established by Matthews48 specify moderate intensity as activity level involving accelerometer counts between (760–5724 counts per minute), a wider range than proposed by Freedson (1953–5724 counts per minute considered as moderate). The within-person association was no longer significant when moderate intensity PA was defined using the Matthews criteria. Thus, it is important to interpret our findings as specifically reflecting intensity corresponding to 1953–5724 accelerometer counts per minute.
CONCLUSIONS
This study demonstrated that an acute bout of moderate intensity exercise decreases both objectively and subjectively assessed HFs, but overweight and late perimenopausal women may see smaller reductions in subjective HFs as compared to normal weight women and women in the early perimenopausal and postmenopausal stages. The reasons for this potentially differential response could not be discerned in this study, warranting further investigation. Objectively assessed overall physical activity was not associated with self-reported HFs across a two-week period, however, performing more moderate intensity physical activity on a given day was associated with higher reporting of HFs, especially among lower fit women. Cardiorespiratory fitness may represent an important individual difference variable that is modifiable, underscoring the importance of sustained physical activity participation in women undergoing the menopausal transition.
Acknowledgments
The project described was supported by Grant Number K 12HD055882, “Career Development Program in Women’s Health Research at Penn State,” from the National Institute of Child Health and Human Development (PI: Weisman) and by pilot funds from the Social Sciences Research Institute and Center on Population Health and Aging at Pennsylvania State University. The data were collected with the assistance of the General Clinical Research Center at Pennsylvania State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. S. Elavsky is the recipient of the 2009–2011 NAMS Mentorship Award 2009–2011.
The authors would like to acknowledge the assistance of Justin Swartzwelder and Dominica Bernardo during data collection for this study.
Footnotes
There are no conflicts of interest associated with the journal, this submission, and the authors.
References
- 1.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. 2008. ODPHP Publication No. U0036. [Google Scholar]
- 2.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Affect Disord. 2007;103(1–3):267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromberger JT, Meyer PM, Kravitz HM, et al. Psychologic distress and natural menopause: a multiethnic community study. Am J Public Health. 2001;91(9):1435–42. doi: 10.2105/ajph.91.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elavsky S. Physical activity, menopause, and quality of life: the role of affect and self-worth across time. Menopause. 2009;16(2):265–271. doi: 10.1097/gme.0b013e31818c0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods NF, Mitchell ES. Symptom interference with work and relationships during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2011;18(6):654–61. doi: 10.1097/gme.0b013e318205bd76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Col NF, Guthrie JR, Politi M, Dennerstein L. Duration of vasomotor symptoms in middle-aged women: a longitudinal study. Menopause. 2009;16(3):453–7. doi: 10.1097/gme.0b013e31818d414e. [DOI] [PubMed] [Google Scholar]
- 7.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med. 2008;23(9):1507–13. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coope J. Hormonal and Non-Hormonal Interventions for Menopausal Symptoms. Maturitas. 1996;23(2):159–168. doi: 10.1016/0378-5122(95)00971-x. [DOI] [PubMed] [Google Scholar]
- 9.Daley A, Macarthur C, Mutrie N, Stokes-Lampard H. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007;(4):CD006108. doi: 10.1002/14651858.CD006108.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Daley AJ, Stokes-Lampard HJ, Macarthur C. Exercise to reduce vasomotor and other menopausal symptoms: a review. Maturitas. 2009;63(3):176–80. doi: 10.1016/j.maturitas.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Daley A, Stokes-Lampard H, Macarthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2011;5:CD006108. doi: 10.1002/14651858.CD006108.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Aiello EJ, Yasui Y, Tworoger SS, et al. Effect of a yearlong, moderate-intensity exercise intervention on the occurrence and severity of menopause symptoms in postmenopausal women. Menopause. 2004;11(4):382–8. doi: 10.1097/01.gme.0000113932.56832.27. [DOI] [PubMed] [Google Scholar]
- 13.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181(1):66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 14.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosomatic Medicine. 2005;67:137–146. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 15.Freedman RR. Hot flashes revisited. Menopause. 2000;7(1):3–4. [PubMed] [Google Scholar]
- 16.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360(9348):1851–61. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 17.Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiol Scand. 1989;137:1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- 18.Chaouloff F. Effects of acute physical exercise on central serotoninergic systems. Medicine & Science in Sports & Exercise. 1997;29:58–62. doi: 10.1097/00005768-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Weicker H, Struder HK. Influence of exercise on serotonergic neuromodulation in the brain. Amino Acids. 2001;20:35–47. doi: 10.1007/s007260170064. [DOI] [PubMed] [Google Scholar]
- 20.Dennerstein L. Well-being, symptoms and the menopausal transition. Maturitas. 1996;23(2):147–57. doi: 10.1016/0378-5122(95)00970-1. [DOI] [PubMed] [Google Scholar]
- 21.Dennerstein L, Lehert P, Guthrie JR, Burger HG. Modeling women’s health during the menopausal transition: a longitudinal analysis. Menopause. 2007;14(1):53–62. doi: 10.1097/01.gme.0000229574.67376.ba. [DOI] [PubMed] [Google Scholar]
- 22.Sternfeld B, Marcus R. Exercise. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 495–504. [Google Scholar]
- 23.Elavsky S, McAuley E. Physical activity and mental health outcomes during menopause: A Randomized controlled trial. Annals of Behavioral Medicine. 2007;33(2):132–142. doi: 10.1007/BF02879894. [DOI] [PubMed] [Google Scholar]
- 24.Elavsky S, McAuley E. Personality, menopausal symptoms, and physical activity outcomes in middle-aged women. Personality and Individual Differences. 2009;46:123–128. doi: 10.1016/j.paid.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daley A, Macarthur C, Stokes-Lampard H, McManus R, Wilson S, Mutrie N. Exercise participation, body mass index, and health-related quality of life in women of menopausal age. Br J Gen Pract. 2007;57(535):130–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Borg GA. Borg’s Perceived Exertion and Pain Scales Book. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 27.Whaley MH, Brubaker PH, Otto RM, Armstrong LE. American College of Sports Medicine [ACSM] ACSM’s guidelines for exercise testing and prescription. 7. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 28.Harlow SD, Crawford S, Dennerstein L, et al. Recommendations from a multi-study evaluation of proposed criteria for staging reproductive aging. Climacteric. 2007;10(2):112–9. doi: 10.1080/13697130701258838. [DOI] [PubMed] [Google Scholar]
- 29.Weiss HM, Beal DJ, Lucy SL, MacDermid SM. Constructing EMA studies with PMAT: The Purdue Momentary Assessment Tool user’s manual. Purdue University: Military Family Research Institute; 2004. [Google Scholar]
- 30.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6:209–215. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 31.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 32.Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. SAGE Publications; 1999. [Google Scholar]
- 33.Bolger N, Davis A, Rafaeli E. Diary methods: Capturing life as it is lived. Annual Review of Psychology. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz JE, Stone AA. Strategies for analyzing ecological momentary assessment data. Health Psychology. 1998;17:6–16. doi: 10.1037//0278-6133.17.1.6. [DOI] [PubMed] [Google Scholar]
- 35.Littell RC, Miliken GA, Stoup WW, Wolfinger RD. SAS system for mixed models. 1996. [Google Scholar]
- 36.Guidance for Industry: Estrogen and Estrogen/Progestin Drug Products to Treat Vasomotor Symptoms and Vulvar and Vaginal Atrophy Symptoms — Recommendations for Clinical Evaluation. 2003 Retrieved from http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM135338.pdf.
- 37.Kenney WL. Thermoregulation at Rest and During Exercise in Healthy Older Adults. Exerc Sport Sci Rev. 1997;25:41–75. [PubMed] [Google Scholar]
- 38.O’Sullivan SE, Bell C. Training reduces autonomic cardiovascular responses to both exercise-dependent and -independent stimuli in humans. Auton Neurosci. 2001;91(1–2):76–84. doi: 10.1016/S1566-0702(01)00288-0. [DOI] [PubMed] [Google Scholar]
- 39.Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control: a link to cardiovascular risk? Menopause. 2010;17(3):456–461. doi: 10.1097/gme.0b013e3181c7dea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Meersman RE. Heart rate variability and aerobic fitness. Am Heart J. 1993;125(3):726–731. doi: 10.1016/0002-8703(93)90164-5. [DOI] [PubMed] [Google Scholar]
- 41.Freedman RR, Wasson S. Miniature hygrometric hot flash recorder. Fertil Steril. 2007;88(2):494–6. doi: 10.1016/j.fertnstert.2006.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79:777–781. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
- 43.Hunter MS, Haqqani JR. An investigation of discordance between subjective and physiological measures of vasomotor symptoms. Climacteric. 2011 Feb;14(1):146–51. doi: 10.3109/13697131003735585. [DOI] [PubMed] [Google Scholar]
- 44.Thurston RC, Hernandez J, Del Rio JM, De La Torre F. Support Vector Machines to improve physiologic hot flash measures: application to the ambulatory setting. Psychophysiology. 2011;48(7):1015–1021. doi: 10.1111/j.1469-8986.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford SL. The roles of biologic and nonbiologic factors in cultural differences in vasomotor symptoms measured by surveys. Menopause. 2007;14(4):725–733. doi: 10.1097/GME.0b013e31802efbb2. [DOI] [PubMed] [Google Scholar]
- 46.Yancey AK, Ory MG, Davis SM. Dissemination of physical activity promotion interventions in underserved populations. American Journal of Preventive Medicine. 2006;31(4):S82–S91. doi: 10.1016/j.amepre.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Freedson PS, Melason EL, Jr, Sirard J. Calibration of the Computer Science and Applications, Inc. Accelerometer Medicine and Science in Sports and Exercise. 1998;30(5):777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 48.Matthews CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–22. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]