Abstract
Spinal and bulbar muscular atrophy is unique among the polyglutamine diseases in that the toxicity of the mutant protein, the androgen receptor, is ligand-dependent. In cell culture and animal models the mutant androgen receptor causes protein aggregation and alterations in transcriptional regulation, axonal transport, and mitochondrial function. Various therapeutic approaches have shown efficacy in mouse models, including androgen reduction and agents that alter the processing and degradation of the mutant androgen receptor protein, including HSP90 inhibitors, IGF-1, and ASC-J9. Clinical trials of androgen-reducing agents have shown indications of efficacy but not proof of clinically meaningful benefit to date. This trial experience has set the stage for future clinical studies of other agents that have been found to be beneficial in transgenic animal models.
Keywords: Spinal and bulbar muscular atrophy, Kennedy’s disease, motor neuron disease, androgen receptor, polyglutamine disease, IGF-1
1. Introduction
Spinal and bulbar muscular atrophy (SBMA, Kennedy’s disease) is an X-linked, adult-onset disease with slowly progressive weakness of the bulbar and extremity muscles due to degeneration of motor neurons in the brainstem and spinal cord. Affected males may show signs of androgen insensitivity, such as breast enlargement and reduced fertility. The causative mutation is a CAG repeat expansion in the androgen receptor gene that leads to an expanded polyglutamine tract in the N-terminal domain of the receptor protein (La Spada et al., 1991). SBMA is thus one of about 30 diseases known to be caused by repeat expansion and one of at least nine that are caused by expanded polyglutamine tracts. This review summarizes what has been learned since the disease gene discovery about the pathophysiology of SBMA and the prospects for treatment. Other reviews have recently appeared elsewhere (Parodi and Pennuto, 2011; Banno et al., 2012)
The androgen receptor is a nuclear receptor, and the N-terminal domain is in a part of the protein separate from the hormone and DNA binding domains, a part of the protein that is involved in interaction with other nuclear proteins. Thus the mutant receptor has normal ligand biding, and in the presence of ligand it dissociates from cytoplasmic heat shock proteins and is normally taken up into the nucleus, but target gene activation is altered due to aberrant binding to other nuclear factors. This causes some loss of normal receptor function, as evidenced by functional studies and by the signs of androgen insensitivity that occur in patients with SBMA, but the primary effect is to cause a toxic gain of function in the receptor protein, i.e., it alters the protein so that it becomes toxic to motor neurons. This is based on the finding that animals and humans with other mutations that cause a loss of androgen receptor function have a different phenotype, with feminization but not the progressive weakness and motor neuron loss of SBMA. Further support for a gain of function comes from the finding that the mutant protein is toxic and reproduces features of the disease phenotype when introduced into cultured cells and transgenic animals. In these model systems, the toxicity is associated with aggregation of the mutant protein, and it is ligand (androgen) dependent.
2. SBMA disease mechanism
As with other polyglutamine diseases, the mutant androgen receptor is prone to aggregation and forms inclusions in patient tissues and in cell culture and animal models. In cell culture models, there is repeat-dependent cell death that is greater with nuclear localization. The toxicity is associated with transcriptional dysregulation, aberrant interaction with other nuclear factors, and changes in histone acetylation (Shimohata et al., 2000; McCampbell et al., 2000, 2001; Lieberman et al., 2002; Taylor & Fischbeck, 2002).
Importantly, the toxicity of the mutant protein is ligand-dependent in transgenic flies and mice. In flies, which do not have endogenous androgens, the mutant gene has no effect unless they are exposed to androgens in their food (Takeyama et al., 2002; Pandey et al., 2007). And in transgenic mice, where the mutant human gene is no longer on the X chromosome, the disease manifestations are still only seen in males. Castration of the males rescues the phenotype, and females given exogenous androgens develop disease manifestations (Katsuno et al., 2002; Chevalier-Larsen et al., 2004). Thus it appears that SBMA occurs in males rather than females because they have higher androgen levels, and the disease is male-limited rather than simply X-linked recessive. A report of one family with homozygous females who are more mildly affected than their male relatives indicates that the disease is androgen dependent in humans, as it is in transgenic animals (Schmidt et al., 2002). Cell culture models of ligand-dependent toxicity are also available (Palazzolo et al., 2007; Montie et al., 2011), and these can be used to indentify potential therapeutic targets.
Recent evidence indicates that native functions and interactions of the receptor protein are important to its toxicity. When events downstream of ligand-dependent androgen receptor activation were systematically evaluated in mouse and fly models of SBMA, it was found that nuclear translocation of the mutant protein is necessary but not sufficient for its toxicity and that DNA binding may be necessary (Montie et al., 2009; Nedelsky et al., 2010). Mutagenesis studies demonstrated that a functional AF-2 domain is essential for toxicity, a finding corroborated by a genetic screen that identified AF-2 interactors as dominant modifiers of degeneration. These findings indicate that SBMA pathogenesis is mediated by misappropriation of native protein function, a mechanism that may be applicable to other polyglutamine diseases.
3. Clinical trials of androgen reduction
Anti-androgen therapy is very effective at blocking the disease onset and preventing the motor deficit in the transgenic mice (Katsuno et al, 2003). This raises the question of whether or not such treatment is effective in SBMA patients.
3.1. Leuprorelin
Randomized, placebo-controlled clinical trials in Japan with the centrally acting androgen-reducing agent leuprorelin in SBMA did not show significant effects on overall muscle strength and function, although there were indications of beneficial effects on swallow function. First, a phase 2 study in 50 subjects showed decreased nuclear accumulation of the mutant androgen receptor in scrotal skin cells and improved swallowing time on cine-swallow studies after 48 weeks (Banno et al., 2009). Later, a phase 3 study of the same duration in nearly 200 subjects showed no effect on swallow function overall, but there was a significant beneficial effect in a subgroup with disease duration less than 10 years (Katsuno et al., 2010).
3.2. Dutasteride
A randomized, controlled trial was also recently performed at the National Institutes of Health. This study used a different androgen-reducing agent, dutasteride, which blocks the conversion of testosterone to the more potent dihydrotestosterone by 5-alpha reductase. The rationale was that this agent would block toxic effects of dihydrotestosterone in motor neurons, where 5-alpha reductase is highly expressed (Pozzi et al., 2003), while preserving the anabolic effects of testosterone in muscle. A baseline cross-sectional analysis of clinical history, laboratory findings, and muscle strength and function was done in 57 patients with genetically confirmed disease who were recruited for the trial (Rhodes et al., 2009). There was an average delay of over 5 years from onset of weakness to diagnosis in this cohort. Muscle strength and function correlated directly with serum testosterone levels and inversely with CAG repeat length, age, and duration of weakness. Motor unit number estimation was decreased by about half compared to healthy controls (Lehky et al., 2009). Sensory nerve action potentials were reduced in nearly all subjects. The direct correlation of testosterone levels with muscle strength indicates that androgens may have a positive effect on muscle function in SBMA patients, in addition to the toxic effects described in animal models (Rhodes et al., 2009).
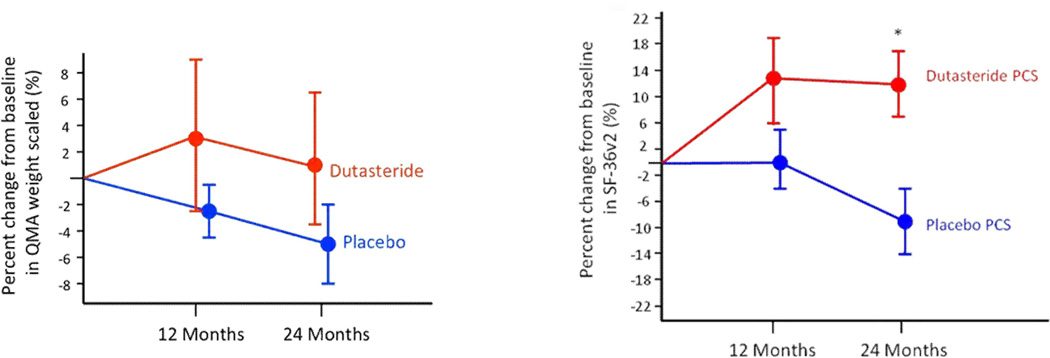
A randomized, double-blind, placebo-controlled, single-site clinical trial was then done (Fernández-Rhodes et al., 2011). Participants were randomly assigned to receive dutasteride or placebo orally for 24 months. The subjects and investigators were masked to treatment allocation. The primary outcome measure was quantitative muscle assessment (QMA). At 24 months, the placebo group showed a decrease of 4.5% from baseline in weight-scaled muscle strength as indicated by QMA, and the dutasteride group had an increase in strength of 1.3%, but this difference between the groups was not significant. Quality of life, as measured by the physical component summary of the SF-36v2 questionnaire, favored dutasteride (p=0.01), whereas the mental component summary favored placebo (Figure 1). With dutasteride, the subjects felt physically better but mentally worse, perhaps due to decreased libido with androgen reduction. Individual sub-scales within these component summaries did not show significant change, however. The dutasteride group had fewer patients reporting falls than did the placebo group; there were no other significant differences in reported adverse events. Although this study did not show a significant effect of dutasteride on the progression of muscle weakness in SBMA, there were secondary indications of positive effects compared with placebo.
Figure 1.
Effects of dutasteride on SBMA in a 24 month trial. The change in QMA is not significantly different from placebo, although there was a significant difference in physical quality of life (Fernández-Rhodes et al, 2011).
Translating a therapeutic intervention that is effective in pre-clinical mouse studies into effective treatment in patients is challenging, in part because the patient population is more diverse and the clinical manifestations are more variable and more slowly progressive. A longer trial duration or larger number of patients and better outcome measures may be needed to show an effect on disease progression. This study identified performance testing and quality of life measures as potentially useful endpoints for future therapeutic trials, but more needs to be done to promote early diagnosis and to standardize currently available care, such as exercise and physical therapy.
4. Other approaches to treatment
Current understanding of the SBMA disease mechanism is that the mutant protein is activated by ligand binding and actively taken up into the nucleus, where it aggregates and has aberrant interactions with toxic effects on transcription, leading to defects in axonal transport, signal transduction, and mitochondrial function, and resulting in neuronal dysfunction and death (Ranganathan and Fischbeck, 2010). Cellular mechanisms that protect against these effects include molecular chaperones (heat shock proteins), the ubiquitin-proteasome system, and autophagy. Approaches to treatment include blocking the pathogenic pathway and enhancing the protective mechanisms, for example, suppressing disease gene expression, blocking the processing and intracellular transport of the mutant protein, enhancing the degradation of the mutant protein, and mitigating its downstream toxic effects. Cell culture and animal models are readily available to screen for and test potential therapeutic agents.
Treatments that have been reported to be effective in mouse models of SBMA include histone deacetylase (HDAC) inhibition, upregulation of heat shock proteins with geldanamycin derivatives 17-AAG and 17-DMAG, and increasing degradation of the mutant protein with the synthetic curcumin-related compound ASC-J9 (Minamiyama et al, 2004; Waza et al, 2005; Yang et al, 2007). Potent HDAC inhibitors and geldanamycin derivatives and related compounds have been developed for cancer chemotherapy, but they have toxic effects that make them unsuitable for treatment of a chronic, slowly progressive neurological disorder like SBMA. The current need in developing treatment for SBMA is to optimize the safety and efficacy of these compounds in preclinical animal studies, to identify new targets for therapeutic intervention, and to prepare for new clinical trials by establishing standard care with existing treatment, such as exercise, and by developing and validating clinically meaningful outcome measures.
Mitochondrial abnormalities have been implicated in the pathogenesis of other, related neurodegenerative diseases such as Huntington’s disease and familial amyotrophic lateral sclerosis. A recent study investigated whether the mutant androgen receptor protein in SBMA alters mitochondrial function (Ranganathan et al, 2009). It was found that expression of the mutant androgen receptor in stably transfected neuronal cells is associated with depolarization of the mitochondrial membrane. This was mitigated by cyclosporine A, which inhibits opening of the mitochondrial permeability transition pore. It was also found that the expression of the mutant protein in the presence of ligand results in an elevated level of reactive oxygen species, which is blocked by the treatment with the antioxidants co-enzyme Q10 and idebenone. The mutant protein also causes activation of Bax, caspase 9 and caspase 3 (Young et al, 2009; Ranganathan et al, 2009). The effects of mutant protein on the transcription of mitochondrial proteins were assessed, and altered expression of the peroxisome proliferator-activated receptor gamma coactivator 1 and the mitochondrial specific antioxidant superoxide dismutase-2 was found in affected tissues of SBMA knock-in mice. In addition, it was found that the protein associates with mitochondria in cultured cells with or without androgen. This study thus provides evidence for mitochondrial dysfunction in SBMA cell and animal models, either through indirect effects on the transcription of nuclear-encoded mitochondrial genes, or through direct effects of the mutant protein on mitochondria, or both. These findings indicate the possibility of benefit with mitochondrial therapy for SBMA.
Nuclear inclusions of mutant protein, a pathological hallmark of SBMA and other polyglutamine diseases, were initially thought to be toxic but are now generally believed to represent a cellular protective response. The compound B2 (5-[4-(4-chlorobenzoyl)-1-piperazinyl]-8-nitroquinoline) was identified as increasing inclusion formation and decreasing the toxicity of polyglutamine-expanded huntingtin in cultured cells (Bodner et al, 2006). The mechanism of action is not known. B2 was studied in SBMA cell models, and it was found that B2 increases the deposition of mutant androgen receptor into nuclear inclusions, without altering the ligand-induced aggregation, expression, or subcellular distribution of the mutant protein (Palazzolo et al, 2010). The effect of B2 on inclusions was associated with a decrease in the receptor’s transactivation function. It was shown that B2 reduces mutant androgen receptor toxicity in cell and fly models of SBMA, further supporting the conclusion that accumulation of polyglutamine-expanded protein into inclusions may be protective and suggesting B2 as an approach to therapy for SBMA.
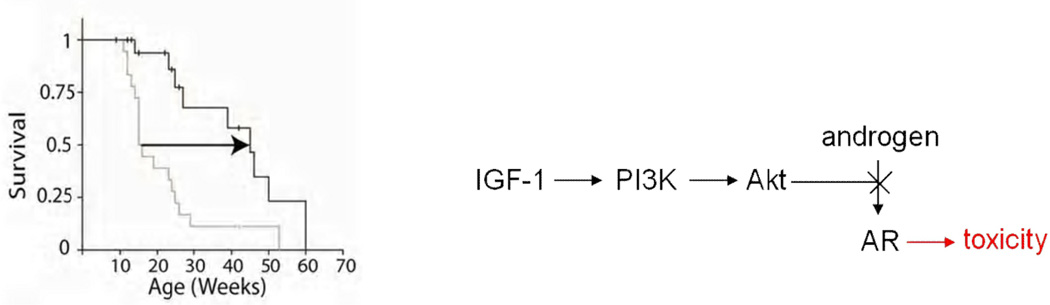
Post-translational modification of the mutant androgen receptor protein has also been investigated, with the goal of identifying potential sites for therapeutic intervention (Palazzolo et al, 2007, 2009). The androgen receptor is phosphorylated by Akt at two consensus sites, S215 and S792 (Lin et al, 2001). Substitution of the serines at these sites with aspartate, which mimics phosphorylation, reduced ligand binding, ligand-dependent nuclear translocation, transcriptional activation and toxicity of the mutant protein (Palazzolo et al, 2007). Insulin-like growth factor-1 (IGF-1), which activates Akt through phosphatidylinositol-3-kinase (PI3K), also reduces toxicity of the polyglutamine-expanded androgen receptor in motor neuron-derived MN-1 cells. It was further shown that IGF-1 reduces aggregation of the mutant protein and increases its clearance via the ubiquitin-proteasome system through phosphorylation by Akt in cell culture (Palazzolo et al, 2009). SBMA transgenic mice overexpressing a muscle-specific isoform of IGF-1 in skeletal muscle showed increased Akt activation, and increased phosphorylation and decreased aggregation of the androgen receptor protein. IGF-1/Akt activation rescued behavioral and histopathological abnormalities, extended the life span (Figure 2), and reduced both muscle and spinal cord pathology of SBMA mice. This study established IGF-1/Akt-mediated inhibition of mutant androgen receptor toxicity as a strategy to treat the disease in vivo and demonstrated that therapy aimed at skeletal muscle may be effective in SBMA.
Figure 2.
Improved survival of SBMA mice overexpressing IGF-1, which blocks the ligand-dependant toxicity of the mutant androgen receptor (AR) through phosphatidylinositol-3-kinase (PI3K) and Akt (Palazzolo et al, 2009).
We have recently investigated the effects of chronic administration of IGF-1 complexed with IGF-1 binding protein (Rinaldi et al, submitted). This treatment mitigates the muscle pathology in SBMA transgenic mice and increases muscle fiber size. Levels of phosphorylated (activated) Akt are increased, and both soluble and aggregated androgen receptor protein are decreased. SBMA patients in the dutasteride trial had reduced levels of serum IGF-1, about two standard deviations below age-matched controls, indicating that there is room for therapeutic benefit with IGF-1 administration in this disease (Grunseich et al, unpublished). Thus, IGF-1 mimetics are worth studying as potential therapeutics for SBMA.
4. Conclusion
In summary, several different approaches to treatment for SBMA have shown benefits in pre-clinical efficacy studies and are prospects for future clinical trials. Androgen reduction is very effective at blocking the disease manifestations in mouse models, but clinical trials to date have given mixed results, in part due to the slow progression of muscle weakness and perhaps also due to mixed beneficial and deleterious effects of androgens in the disease. Future trials with more subjects, longer duration, and more sensitive outcome measures may show clearer evidence of efficacy. Also, other drugs with different mechanisms of action, such as selective androgen receptor modulators, may be beneficial.
HSP-90 inhibitors show benefit in mice, but they are generally too toxic for long term use. However, an inhibitor with a better safety profile, particularly one that is CNS-penetrant, would warrant further investigation in patients. ASC-J9 and its derivatives may be better tolerated, particularly if formulated for oral use.
Finally, IGF-1 mimetics may detoxify the mutant protein through PI3K and Akt, as well as having neuroprotective effects and promoting muscle regeneration. If an agent of this class could be developed with a suitable therapeutic index, then further study in patients would be warranted. Exercise, which increases IGF-1 levels and improves muscle conditioning, is also worth investigating in patients with SBMA.
Highlights.
SBMA is caused by polyglutamine expansion in the androgen receptor
The mutant receptor has ligand-dependent toxicity
The disease can be mitigated in mice with androgen reduction and other treatments
Clinical trials have shown indications of efficacy but not proof of benefit to date
Acknowledgements
Supported in part by intramural research funds from the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Abbreviations
- AR
androgen receptor
- HDAC
histone deacetylase
- IGF-1
insulin-like growth factor 1
- PI3K
phosphatidylinositol 3 kinase
- SBMA
spinal and bulbar muscular atrophy
- QMA
quantitative muscle assessment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banno H, Katsuno M, Suzuki K, Takeuchi Y, Kawashima M, Suga N, Takamori M, Ito M, Nakamura T, Matsuo K, Yamada S, Oki Y, Adachi H, Minamiyama M, Waza M, Atsuta N, Watanabe H, Fujimoto Y, Nakashima T, Tanaka F, Doyu M, Sobue G. Phase 2 trial of leuprorelin in patients with spinal and bulbar muscular atrophy. Ann. Neurol. 2009;65:140–150. doi: 10.1002/ana.21540. [DOI] [PubMed] [Google Scholar]
- Banno H, Katsuno M, Suzuki K, Tanaka F, Sobue G. Pathogenesis and molecular targeted therapy of spinal and bulbar muscular atrophy (SBMA) Cell Tissue Res. 2012 doi: 10.1007/s00441-012-1377-9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bodner RA, Outeiro TF, Altmann S, Maxwell MM, Cho SH, Hyman BT, McLean PJ, Young AB, Housman DE, Kazantsev AG. Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington's and Parkinson's diseases. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4246–4251. doi: 10.1073/pnas.0511256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Larsen ES, O'Brien CJ, Wang H, Jenkins SC, Holder L, Lieberman AP, Merry DE. Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci. 2004;24:4778–4786. doi: 10.1523/JNEUROSCI.0808-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Rhodes LE, Kokkinis AD, White MJ, Watts CA, Auh S, Jeffries NO, Shrader JA, Lehky TJ, Li L, Ryder JE, Levy EW, Solomon BI, Harris-Love MO, La Pean A, Schindler AB, Chen CJ, Di Prospero NA, Fischbeck KH. Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: a randomised placebo-controlled trial. Lancet Neurol. 2011;10:140–147. doi: 10.1016/S1474-4422(10)70321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Kume A, Li M, Nakagomi Y, Niwa H, Sang C, Kobayashi Y, Doyu M, Sobue G. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35:843–854. doi: 10.1016/s0896-6273(02)00834-6. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Doyu M, Minamiyama M, Sang C, Kobayashi Y, Inukai A, Sobue G. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat. Med. 2003;9:768–773. doi: 10.1038/nm878. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Banno H, Suzuki K, Takeuchi Y, Kawashima M, Yabe I, Sasaki H, Aoki M, Morita M, Nakano I, Kanai K, Ito S, Ishikawa K, Mizusawa H, Yamamoto T, Tsuji S, Hasegawa K, Shimohata T, Nishizawa M, Miyajima H, Kanda F, Watanabe Y, Nakashima K, Tsujino A, Yamashita T, Uchino M, Fujimoto Y, Tanaka F, Sobue G. Efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:875–884. doi: 10.1016/S1474-4422(10)70182-4. [DOI] [PubMed] [Google Scholar]
- La Spada A, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Lehky TJ, Chen CJ, Di Prospero NA, Rhodes LE, Fischbeck K, Floeter MK. Standard and modified statistical MUNE evaluations in spinal-bulbar muscular atrophy. Muscle Nerve. 2009;40:809–814. doi: 10.1002/mus.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AP, Harmison G, Strand AD, Olson JM, Fischbeck KH. Altered transcriptional regulation in cells expressing the expanded polyglutamine androgen receptor. Hum. Mol. Genet. 2002;11:1967–1976. doi: 10.1093/hmg/11.17.1967. [DOI] [PubMed] [Google Scholar]
- Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCampbell A, Taylor JP, Taye AA, Robitschek J, Li M, Walcott J, Merry D, Sobue G, Fischbeck KH. CREB-binding protein sequestration by expanded polyglutamine. Hum. Mol. Genet. 2000;9:2197–2202. doi: 10.1093/hmg/9.14.2197. [DOI] [PubMed] [Google Scholar]
- McCampbell A, Taye AA, Whitty L, Penney E, Steffan JS, Fischbeck KH. Histone deacetylase inhibitors reduce polyglutamine toxicity. Proc. Natl. Acad. Sci. U.S. A. 2001;98:15179–15184. doi: 10.1073/pnas.261400698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamiyama M, Katsuno M, Adachi H, Waza M, Sang C, Kobayashi Y, Tanaka F, Doyu M, Inukai A, Sobue G. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 2004;13:1183–1192. doi: 10.1093/hmg/ddh131. [DOI] [PubMed] [Google Scholar]
- Montie HL, Cho MS, Holder L, Liu Y, Tsvetkov AS, Finkbeiner S, Merry DE. Cytoplasmic retention of polyglutamine-expanded androgen receptor ameliorates disease via autophagy in a mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 2009;18:1937–1950. doi: 10.1093/hmg/ddp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montie HL, Pestell RG, Merry DE. SIRT1 modulates aggregation and toxicity through deacetylation of the androgen receptor in cell models of SBMA. J. Neurosci. 2011;31:17425–17436. doi: 10.1523/JNEUROSCI.3958-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelsky NB, Pennuto M, Smith RB, Palazzolo I, Moore J, Nie Z, Taylor JP. Native functions of the androgen receptor are essential pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron. 2010;67:936–952. doi: 10.1016/j.neuron.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo I, Burnett BG, Young JE, Brenne PL, La Spada AR, Fischbeck KH, Howell BW, Pennuto M. Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum. Mol. Genet. 2007;16:1593–1603. doi: 10.1093/hmg/ddm109. [DOI] [PubMed] [Google Scholar]
- Palazzolo I, Stack C, Kong L, Musaro A, Adachi H, Katsuno M, Sobue G, Taylor JP, Sumner CJ, Fischbeck KH, Pennuto M. Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron. 2009;63:316–328. doi: 10.1016/j.neuron.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo I, Nedelsky NB, Askew CE, Harmison GG, Kazantsev AG, Taylor JP, Fischbeck KH, Pennuto M. B2 attenuates polyglutamine-expanded androgen receptor toxicity in cell and fly models of spinal and bulbar muscular atrophy. J. Neurosci. Res. 2010;88:2207–2216. doi: 10.1002/jnr.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the ubiquitin-proteasome system. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- Parodi S, Pennuto M. Neurotoxic effects of androgens in spinal and bulbar muscular atrophy. Front. Neuroendocrinol. 2011;32:416–425. doi: 10.1016/j.yfrne.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Pozzi P, Bendotti C, Simeoni S, Piccioni F, Guerini V, Marron TU, Martini L, Poletti A. Androgen 5-alpha-reductase type 2 is highly expressed and active in rat spinal cord motor neurones. J. Neuroendocrinol. 2003;15:882–887. doi: 10.1046/j.1365-2826.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- Ranganathan S, Harmison GG, Meyertholen K, Pennuto M, Burnett BG, Fischbeck KH. Mitochondrial abnormalities in spinal and bulbar muscular atrophy. Hum. Mol. Genet. 2009;18:27–42. doi: 10.1093/hmg/ddn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan S, Fischbeck KH. Therapeutic approaches to spinal and bulbar muscular atrophy. Trends Pharmacol. Sci. 2010;31:523–527. doi: 10.1016/j.tips.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes LE, Freeman BK, Auh S, Kokkinis AD, La Pean A, Chen C, Lehky TJ, Schrader JA, Levy EW, Harris-Love M, Di Prospero NA, Fischbeck KH. Clinical features of spinal and bulbar muscular atrophy. BrainM. 2009;132:3242–3251. doi: 10.1093/brain/awp258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Greenberg CR, Allingham-Hawkins DJ, Spriggs EL. Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology. 2002;59:770–772. doi: 10.1212/wnl.59.5.770. [DOI] [PubMed] [Google Scholar]
- Shimohata T, Nakajima T, Yamada M, Uchida C, Onodera O, Naruse S, Kimura T, Koide R, Nozaki K, Sano Y, Ishiguro H, Sakoe K, Ooshima T, Sato A, Ikeuchi T, Oyake M, Sato T, Aoyagi Y, Hozumi I, Nagatsu T, Takiyama Y, Nishizawa M, Goto J, Kanazawa I, Davidson I, Tanese N, Takahashi H, Tsuji S. Expanded polyglutamine stretches interact with TAFII130, interfering with CREB-dependent transcription. Nat. Genet. 2000;26:29–36. doi: 10.1038/79139. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Ito S, Yamamoto A, Tanimoto H, Furutani T, Kanuka H, Miura M, Tabata T, Kato S. Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron. 2002;35:855–864. doi: 10.1016/s0896-6273(02)00875-9. [DOI] [PubMed] [Google Scholar]
- Taylor JP, Fischbeck KH. Altered acetylation in polyglutamine disease: an opportunity for therapeutic intervention? Trends Molec. Med. 2002;8:195–197. doi: 10.1016/s1471-4914(02)02332-8. [DOI] [PubMed] [Google Scholar]
- Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat. Med. 2005;11:1088–1095. doi: 10.1038/nm1298. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, Merry DE, Sobue G, Chen LM, Chang SS, Chang C. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat. Med. 2007;13:348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- Young JE, Garden GA, Martinez RA, Tanaka F, Sandoval CM, Smith AC, Sopher BL, Lin A, Fischbeck KH, Ellerby LM, Morrison RS, Taylor JP, La Spada AR. Polyglutamine-expanded androgen receptor truncation fragments activate a Bax-dependent apoptotic cascade mediated by DP5/Hrk. J. Neurosci. 2009;29:1987–1997. doi: 10.1523/JNEUROSCI.4072-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]