Abstract
The origin and propagation of normal and leukemic hematopoietic cells critically depend on their interplays with the hematopoietic microenvironment (or so-called niche), which represent important biological models for understanding organogenesis and tumorigenesis. Nevertheless, the anatomic and functional characterizations of the niche cells for normal hematopoietic stem cells (HSCs) have proved a formidable task. It is uncertain whether the combinational effects of a few sets of molecular niche elements, behind the long-sought cellular architectures with preferred anatomic locations, actually meets the functional definition of HSC niche. Moreover, even much less is known about the niche components for numerous types of leukemia-stem cells (LSCs) that originate via discrete cellular and molecular transforming mechanisms. However, one interesting scenario is emerging, i.e., the leukemia cells can positively remodel the hematopoietic microenvironment favorable for their competition over the normal hematopoiesis that co-exists within the same eco-system. This property probably represents a previously unappreciated essential trait of a functional LSC. Obviously, the further exploration into how the hematopoietic microenvironment interplay with normal or malignant hematopoiesis will shed light onto the designing of novel types of niche-targeting therapies for leukemia.
Keywords: Niche, HSCs, LSCs, Interplay
Introduction
The non-random transition pattern of major hematopoietic sites along with ontogenic development indicates that certain specific hematopoietic microenvironments, supplied by a few particular tissues or organs, must exist to induce and also confine the colonization and proliferation of normal hematopoietic stem cells (HSCs) and progenitors. It is believed that a collaborative development of hematopoietic microenvironment and hematopoietic cells themselves underlies the formation of nascent hematopoietic tissues or organs, such as bone marrow (BM) and spleen.
Schofield proposed the term—hematopoietic niche more than 30 years ago, assuming the existence of putative hematopoietic stromal cells that anatomically associate with HSCs and thus instruct HSCs to undergo self-renewal expansion [1]. Indeed, the self-renewal expansion or/and long-term maintenance of apex HSCs, out of a variety of distinctive developmental processes of hematopoiesis, seems critically dependent on the supporting activity from a niche structure that is not easy to simulate in vitro. A single freshly isolated HSC from BM, once inoculated into a proper recipient, has demonstrated a robust capability of re-initiating and subsequently sustaining a hematopoietic hierarchy encompassing whole hematopoietic lineages throughout life, while in striking contrast, even a lot of HSCs, once put in the ordinary in vitro culture, will inevitably bias to differentiate, usually toward myeloid directions, ending up with only a transient hematopoietic activity. Nevertheless, despite the highly invested research activities over last decade, the accurate cellular identities and molecular profile of the HSC niche still remain elusive.
Although even much less is known about the putative niches for leukemic hematopoiesis, they, in most instances, definitely play an essential role in the sustained survival and proliferation of the malignant hematopoietic cells. Analogue to the case of HSCs, in most instances, no leukemia cells, once removed from patients’ body and cultivated in vitro, can proliferate and/or survive more than 3 weeks. Only occasionally a subset of rare leukemia cells (for example the original LSCs or the non-LICs that have experienced substantial reprogramming) contained within the freshly isolated hematopoietic samples from the most malignant cases, do have obtained the unusual ability to lead an almost complete autonomous growth in a niche-absent in vitro culture conditions [2].
A Variety of Niche Cells are Found for HSCs
It is becoming obvious that even for the apex HSC subset alone, numerous types of niche cells may exist. Moreover, frequently the observations and claims from the different reports are conflicting. The total candidate niche cell types for HSCs or/and progenitors residing in BM may add up to >10 (Table 1). Except for the BM stromal cells that have been documented in many studies, it is worth of mentioning that many HSC progenies, such as macrophage, osteoclasts and T cells, have also been shown to play an active niche-like role, indirectly or even directly, especially in the conditions of inflammation [3–6]. For the sake of maintaining homeostasis of ongoing hematopoiesis, it is understandable that the proliferation and differentiation of HSCs and progenitors towards a specific direction should be regulated via a feedback mechanism, directly or indirectly, to monitor the accumulation of discrete progenies [7]. Adding to this complexity, numerous insoluble or soluble molecular factors such as the extracellular matrix fibers, nerve transmitters, hormones, nutrients, and even metabolites, besides these local live regulators, not surprisingly, will also exert crucial influence on HSC and progenitor behaviors.
Table 1.
The key niche-related molecules that regulate HSCs or/and LSCs
| Molecule | Relevant stromal cells | Regulation on HSCs | Regulation on non-HSC hematopoietic cells | Regulation on acute leukemia cells | References |
|---|---|---|---|---|---|
| N-cadherin | Endosteal osteoblasts | Homophilic tethering | ? | Homophilic tethering | [13, 14, 18, 36, 42, 43, 70–72] |
| Fibronectin | Integrin αv β3 | CD11b/NK cells | Vla-4/AML | [55, 57, 73, 74] | |
| Jagged-1/2, DLL1 | MSCs, osteoblasts, endothelium | Notch1,2 | Notch/T cells, Megakaryocyte | Notch/B-ALL | [12, 29, 61, 75–78] |
| SCF | Endothelium, peri-vascular stromal cells, osteoblast, leukemia cells | c-Kit | c-Kit/mast cells, B cells, T cells | c-Kit/AML | [15, 23, 66, 79–82] |
| SDF-1α | CAR cells, osteogenic progenitors, osteoblasts | CXCR4 | CXCR4/leukocytes | CXCR4/ALs | [3, 4, 22, 33, 48–54, 60, 65, 83] |
| Angiopoietin-1 | Endothelial cells, mesenchymal progenitors, hematopoietic cells | Tie2 | Tie2/AML | [16, 84, 85] | |
| TPO | MPL | MPL/thrombopoiesis | MPL/AML | [74, 83, 86–88] | |
| Wnt/DKK1 | Osteoblast, mesenchymal progenitors | Fz/LRP5/6 co-receptor | Fz/LRP5/6 co-receptor/Myeloid progenitors, T cells | Fz/LRP5/6 co-receptor/AML | [47, 71, 77, 89–93] |
| VEGFA | HSC/leukemia cells | Flk1 | Flk1/AML? | [41, 94] | |
| Hedgehog | ? | Ptch-Smo | Ptch-Smo/T cell, B cell, erythroid progenitors | Ptch-Smo/ALL, BCR-ABL myeloid leukemias | [95–101] |
| TGFβ | GFAP+ Nonmyelinating Schwann cells | TGFBR | TGFBR/T cells, DCs | [31, 102–106] | |
| CD44-ligands | CD44/AML | [56, 58, 59] | |||
| Prostaglandin E2(PGE 2) | Osteoblasts,osteoclasts,endothelial cells | COX-1 and COX-2 | WBC and platelets | [107],[108–110] |
As the HSC niche basically bears an anatomic implication, the putative HSC niches have been arbitrarily classified by their locations. Up to now, at least two major kinds of niches—endosteal osteoblastic niches and peri-vascular niches have been described within the adult BM.
A hematopoietic niche role of endosteal osteogenic cells other than synthesizing bone matrix was postulated by reasoning why a supposedly non-random migration of the definitive hematopoietic site finally settles within the endosteal osteoblasts-lined bone marrow cavity [8, 9]. And this notion was also supported by the early observation that the hematopoietic progenitors were most enriched within the subendosteal region [10], and by the in vitro co-culture experiments showing that osteoblasts are able to secret hematopoietic cytokines to facilitate the proliferation and survival of hematopoietic progenitors [11]. Later, the in vivo experimental evidence supporting osteoblasts as a putative HSC niche element was provided by Zhang, et al. and Calvi, et al. works that were based on the analyses of two genetically modified mouse models [12–14].
Although the subsequent attempts to further characterize the niche-like role of endosteal osteogenic cells (including not only the mature osteoblasts but also a portion of osteoblastic progenitors), especially on HSCs, have made the conflicting observations (see next paragraph), there is indeed experimental evidence supporting the existence of an endosteal niche. The prominent ones include: 1) it is repeatedly shown by different groups that at least a portion of putative HSCs, especially those being intravenously transplanted, reside close to the endosteal region [6, 13, 15, 16]; 2) endosteal osteogenic cells express or secret many HSC-regulating factors such as SDF-1, c-Kit and angiopoitin-1 (ang-1) (see Table 1); 3) the osteogenic progenitor-specific (by osterix promoter-coupled Cre) Dicer- or Sbds-targeting manipulation that impaired osteoblastic differentiation coincidently perturbed the proliferation, survival and differentiation of HSCs and the hematopoietic progenitors [17]. Moreover, there are also reports indicating the existence of the endosteal niches that co-localize with the sinusoidal endothelial cells that constitute a major component of the perivascular niche (see following paragraphs) [18].
On the other hand, in the biglycan-deficient or osterix promoter-guided Rac-knockout mice wherein reductions in osteoprogenitor and osteoblast numbers were documented, no quantitatively correlated alterations to HSCs and their progenies were detected [14, 19]. So it seems still an open question as to whether endosteal osteogenic cells play an essential niche role in regulating HSC behaviors. If they do, probably it is osteogeneic progenitors rather than the mature osteoblasts that exert meaningful influence on a small portion of HSCs [17]. It is difficult to reconcile these contradictory observations. Nevertheless, perhaps only a particular property of osteogenic cells rather than the whole osteogenic cells, or only a portion of redundant osteogenic cells or relevant properties, is vital for providing a sufficient HSC niche function, while these subtle attributes are differentially altered or can be compensated in the different gene-targeting models.
Later, it was realized that the primitive self-renewing osteogenic progenitors that seed the formation of bone and BM might also account for why the final location of major hematopoietic site goes to the bone [20]. In line with this, an early observation showed that co-transplantation of MSCs facilitated the hematopoietic recovery by infused HSCs [21]. The general picture is that the mesenchymal stem cells (MSCs) or other MSCs-like cells, as a kind of peri-vascular cells reside in close neighborhood with the sinusoidal endothelial cells, constitute an essential element of so-called perivascular (sinusoid) niche. The location of the peri-vascular niche is not necessarily close to the endosteal region. Several reports indicate that probably more HSCs locate close to this perivascular niche than to the endosteal osteoblastic niche mentioned above [15, 20, 22–25].
Several types of mesenchymal stromal cells with overlapping phenotypic or functional attributes have been reported to participate in the formation of the peri-vascular niche. The one first described was human CD146+ adventitial reticular cells (ARCs) that reside as a kind of mural cell expressing Ang-1 [20]. Interestingly, ARCs belongs to a subset of self-renewing osteogenic progenitor, seeding the formation of BM-containing heterotopic ossicle in the immunocompromised mice by sequentially recruiting host-derived vasculature and hematopoiesis, wherein ARCs account for 3 % of human derived cells. Theoretically, ARCs are able to directly signal to not only HSCs but also endothelial cells that ARCs surround with their bodies, which in turn relays signals to HSCs. Similar to ARCs, a portion of mouse CXCL-12/SDF-1 abundant reticular (CAR) cells that account for 0.27 % of BM nucleated cells, as determined by flow cytometry assay, were also reported of attaching to the sinusoid with their body or long processes [22, 23]. CAR cells were envisioned to provide a basement facilitating HSC division and accumulation. A marked decrease of total HSC pool (to about 30 %) or the competitive repopulating units (to about 45 %) was observed after CAR cell depletion. Interestingly, like ARCs, they also harbor the differentiation potentials of maturing into the endosteal osteoblast or adipocytes. Moreover, the detectable expressions of SCF, PDGFRα/β, Runx2, PPARγ, and osterix together indicate an identity of mesenchymal progenitor. The third one is the sympathetic fiber-innervated Nestin+ MSCs that account for about 0.08 % of total murine BM nucleated cells. Apparently being distinct from osterix-expressing pre-osteoblasts and N-cadherin-expressing osteoblasts, they also show a preferred peri-vascular distribution [24]. As its name suggests, like ARCs and CARs, they contribute to the physiological bone remodeling in vivo by differentiating into the mature chondrocyte, and osteoblasts. Interestingly, Nestin+ MSCs express multiple hematopoietic regulatory factors such as CXCL12/SDF-1, SCF, IL-7, Ang-1 and Vcam-1, and contribute to HSC pool size maintenance seemingly by preventing HSCs and other primitive progenitors from egressing into the extramedullary sites such as spleen. The depletion of Nestin+ MSCs results in about 50 % loss of HSC pool size. Moreover, the niche role of Nestin+ MSCs is subject to G-CSF or β-3AR stimulation. The fourth MSC-like cells were a distinctive subset of SCF-expressing LepR+ perivascular stromal cells (although SCF-expressing cells also include a portion of endothelium and osteoblasts) that account for about 0.027 % of total mouse BM cells, as measured by flow cytometry [15]. The LepR promoter-Cre induced SCF deletion results in a significant reduction in HSC pool (up to 80 %). Interestingly, like three other types of perivascular cells mentioned above, these cells also express numerous MSC-associated markers such as PDGFRα/β, CXCL12, ALP, Vcam-1. Interestingly they do not express Nestin.
As all these HSC niche-related MSC-like cells are distributed as perivascular cells, probably the vascular endothelium represents the most basic component of the so-called peri-vascular HSC niche [25]. Actually, ontogenic studies of hematopoiesis have already shown that definitive HSCs bud as cellular clusters from the hematogenic endothelium in situ [26], such as the surrounding vascular endothelium is implicated in supporting the early HSC expansion. Moreover, during ectopic bone formation initiated by donor osteogenic progenitors the establishment of functional HSCs-containing BM tissue is preceded by a prior VEGF secretion and vasculature formation [27]. In accordance, the hematopoietic recovery following a myelo-suppression was preceded by a vasculature restoration [28]. In an anatomic viewpoint, sinusoidal endothelial cells are the portals for the egress or ingress of traveling HSCs out of or into BM, therefore, a specific interaction between HSCs and sinusoidal endothelial cells is not out of expectation. Moreover, many other supporting evidence exist. Firstly, some HSCs and/or progenitors, either in the steady state of BM or after being transplanted, locate close or attach to sinusoidal endothelial cells, although it is not clear whether this preferred anatomic association is actually due to a possibility that a lot of HSCs/progenitors are just passing endothelial portal. Secondly, endothelial cells express certain HSCs-regulating factors such as Angptl3 and SCF, and blockage of these factors produced by endothelial cells resulted in a reduction in HSC pool size (see Table 1). Thirdly, in vitro co-culture experiments showed that the endothelial cells facilitate the HSC self-renewal expansion in a contact-dependent manner, at least partially by supplying Notch-ligands [29, 30].
Intriguingly, apart from the Nestin+ peri-vascular MSCs that probably belong to the derivatives of the neuro-crest cells, the peri-vascular niche architecture has also found another member of neuro-crest derived cell lineages: GFAP+ Nonmyelinating Schwann cells [31]. GFAP+ cells carry the phenotypes of Nestin+Itgb8+PDGFRα−, express CXCL12, SCF, Anginpoitin-1, and Tpo, and account for about 0.005 % of BM cells (as compared to PDGFRα+ cells, 0.05 %; nestin+ cells, 0.026 %). They act by providing the active TGF-β to hibernate HSCs.
As mentioned at the beginning, it has long been suspected that hematopoietic cells, in a reverse manner, regulate the development and function of osteogenic cells, which in turn influence their niche-related roles. As such, recent works suggest that HSC progenies such as macrophages within the BM or endosteal trophic macrophages facilitate the growth and activity of osteoblasts, which in turn influences BM retention of HSCs. The well-known agonist of HSC mobilization, G-CSF, promotes the egress of HSCs out of BM by directly decreasing the activity and number of these supporting macrophages, resulting in a reduced production of SDF-1α by osteoblasts [3, 4]. Likewise, BM CD169+ macrophages were shown to signal peri-vascular Nestin+ cells to secret SDF-1α, thus preventing HSCs from egressing out of BM [3]. Most recently, it was shown that SDF-1α-secreting niche cells recruit Treg cells to cluster to the endosteal region, thus co-localizing with the transplanted allogenic HSCs/progenitors and shielding them from immuno-rejection [6].
Finally, a much less explored while otherwise an important biological setting for understanding HSC niche is how HSCs and progenitors interact with microenvironmental factors to initiate and constitute an extramedullary hematopoiesis outside of BM, which occurs during adulthood when the primary BM hematopoietic microenvironment is disrupted by pathological processes. The establishment of extramedullary hematopoiesis seems not an accidental process, rather it needs the reactivation of niche-like roles of certain extrameduallary stromal cells back to an embryonic hematopoietic status [32]. Very little is known about the nature of extramedullary HSC niche cells. Nevertheless, in both spleen and liver [25, 33], it was observed that the putative HSCs were found attached to a portion of sinusoidal endothelial cells, although numerous mature hematopoietic cells also distributed into this region. This localization was at least partly determined by SDF-1α secretion from the extramedullary sinusoidal endothelium.
HSC Niches are Hypoxic
In line with a widely accepted observation that at least a portion of HSCs are staying at cell-cycle dormant status, the evidence is accumulating that HSCs are located within a blood circulation low-perfused area [34], thus undergoing a relatively anaerobic metabolism [35], and harboring low levels of ROS [36, 37]. Moreover, it is suspected that this distal location of HSCs from circulation also helps prevent the loss of certain niche cells-produced local soluble factors. Notably, results that indicate a basically hypoxic status for both endosteal osteoblastic cells and sinusoidal endothelial cells (but not capillary endothelial cells) within the BM have been reported [38, 39]. In accordance, hypoxia inducible HIF-1α was shown of essential for maintaining the long-term self-renewal ability of HSCs at several biological settings [35, 40, 41].
The Molecular Niche Elements
The niche cells interplay with HSCs via adhesion molecules and soluble factors, and increasing numbers of such niche molecules or the relevant molecular pathways are being discovered. As mentioned above, in many studies the preferential expression of certain HSCs-regulatory molecules has been used as the identity label for the putative niche cells. Worthy of mentioning, the expressions of many regulatory molecules are not restricted to one or two types of niche cells, and rarely the regulatory effects of a given niche molecule is only restricted onto HSCs. Although the current data that characterize the expression pattern and function of many niche-related molecules are not totally in harmony, dozens of molecules as the highly relevant molecular niche elements are documented in Table 1.
At least for certain molecules or pathways listed in Table 1, a niche role for HSCs remains undetermined. This situation is well illustrated in the case of N-cadherin that expresses on the endosteal osteoblasts. While a homophilic-tethering of N-cadherin between osteogenic cells and hematopoietic cells seems not essential for maintaining a physiological hematopoiesis, as indicated by osteogenic or hematopoietic-specific N-cadherin targeting experiments [42, 43], the siRNA or dominant negative mutant-expression experiments demonstrate a potential hematopoiesis-regulatory effect by manipulating N-cadherin expression [44]. For SCF, although the Lepr-Cre mediated targeting of SCF in perivascular stromal cells affects HSC pool size [15], the ectopic BM formed by SL/SL donor cells contains the same amount of HSC as that produced by WT donor [27]. Actually, for many signaling pathways including Wnt/β-catenin, Notch and hedgehog, inconsistent conclusions were drawn from the different “gain of function” and “loss of function” studies (see Table 1 for references). It seems that the dosage-effect of gene alterations has to be taken into account. Moreover, the fact that depletion of a single pathway results in no significant influence on HSC maintenance or recovery probably can not preclude an involvement of this pathway. It is possible that the redundant HSCs-maintaining signaling pathways are available, while only a combinational effect of a portion of them is sufficient to maintain the homeostasis or recovery of a functional HSC pool.
The Niche for Malignant Hematopoietic Cells
It is gradually accepted recently that perhaps the final transformation steps for a majority of various types of LSCs do not occur to the HSCs, but rather to different types of immature hematopoietic progenitors being distributed over a long range of differentiation path down an imaginary hierarchy. So it is not surprising to find that different types of LSCs as well as their progeny each propagates by depending on the particular supports from discrete microenvironmental factors. In a striking case, the same LSC clone may interplay with the different microenvironments to produce either acute myeloid leukemia (AML) or acute lymphoid leukemia (ALL) phenotype [45]. We will highlight a few cases about the putative niche cells or molecules for AML and ALL in the following paragraphs.
AMLs: an earlier report showed evidence that LSCs-enriched CD34+ cells of human AML (M4) preferentially localized to the metaphyseal endosteal region of NOD/SCID/IL2γnull mice after transplantation [46]. However, after being intravenously injected to the lethally-irradiated GFP osteoblast report mice, the mouse AML LSCs, originated from the viral vector-mediated transduction of oncogenic MLL-AF9, were found of homing to the BM places away from the endosteal osteoblasts, where instead a preferred distribution by normal HSCs or pre-LSCs was observed [47]. In line with the expectation that an increasingly autonomous status will be gained by LSCs, the activation of a key self-renewing pathway, Wnt-β catenin axis, in MLL-AF9 AML, was no longer dependent on the BM niche-derived Wnt signals [47]. At molecular mechanism level, as a serial studies have continued to highlight, the SDF-1α-CXCR4 axis not only maintains the retention of AML cells within BM, but also provides survival-promoting signal, which in turn protects AML cells from chemotherapy or tyrosine kinase inhibitor-induced apoptosis [48–54]. On the other hand, the role for leukemic expression of Vla-4 in protecting LSCs seems still controversial [55–57]. And interestingly, CD44 signaling (its ligands in microenvironment include hyaluronan, osteopontin, fibronectin and selectin) on AML LICs might otherwise promote their differentiation rather than maintaining leukemogenic stemness, and also inhibit LICs’ homing ability into both BM and spleen [58, 59].
ALL: a human pre-B ALL cell line Nalm-6 cells were observed of homing to certain specific BM vasculature domain that express SDF-1 and E-selectin soon after being intravenously inoculated, and then they diapedased and proliferated around the peri-vascular area [60]. The in parallel experiments showed that this specific vasculature domain was exactly the portal that allowed entry of normal HSCs and progenitors into BM from circulation. On the other hand, quite surprisingly, unlike what has been documented for Notch signaling in favoring T cell development versus B lymphopoiesis, a recent study suggest that Notch signaling activation within B-ALL cells by BM mesenchymal stromal cells protected these malignant B cells from apoptosis [61].
Acute Leukemia Cells Prepare a Favorable Niche to Strive
Intriguingly, experimental evidence accumulates that the primary alterations in hematopoietic microenvironment may contribute to the initiation of leukemogenesis [62]. In corroboration of this notion, one recent report documented that up to 16 % of patients with AML/MDS harbored chromosomal abnormalities in BM mesenchymal stromal cells, and these abnormalities were different from the genetic abnormalities harbored by leukemic cells themselves [63]. However, in most cases, the primary genetic defects intrinsic to the hematopoietic cells probably are already sufficient to create LSC within hematopoietic microenvironment otherwise with a normal genetic background. Nevertheless, the “interplay theory” predicts that even in the latter, the critical epigenetic alterations occurring to the hematopoietic stromal cells, as induced by a small population of starting LSCs at the initial of stage of malignancy, pave the avenue for the further leukemic propagation that ultimately results in clinical manifestation of overt leukemia.
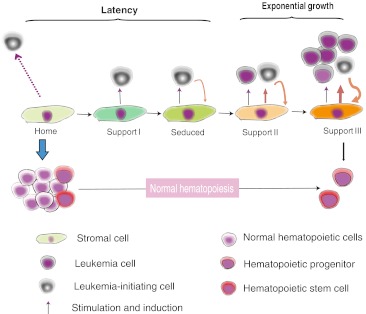
Leukemia belongs to a clonal disease that originates from the expansion, refractory differentiation and probably also an enhanced survival of a single LSC. It is possible that not all transformed hematopoietic cells, once created in situ or inoculated from outside, will immediately embark on a seemingly unlimited leukemic propagation, which will lead to an overt leukemic phenotype. For example, certain newly generated LSCs may be guided by the microenvironment to stay at a dormant status. Moreover, it is reasonable to take into account of the competitive occupancy and usage of hematopoietic niches by normal HSCs and progenitors that greatly outnumber LSCs at the initial stage of leukemogenesis. However, for all the cases that lead to an overt leukemia, a mini malignant hematopoiesis starting from one single LSC will surpass a giant normal hematopoietic activity within the same eco-system. The resultant depression of normal hematopoiesis virtually represents the direct and major pathological alterations leading to clinical mortality [64]. The observation that many solid malignant growths such as multiple myeloma and solid tumor metastasis within BM usually result in a much less severe depression of normal hematopoiesis indicates that the gross physical compression on the hematopoietic microenvironment is not the sole vital mechanism. It seems reasonable to hypothesize that a niche-related delicate alteration may have to be implicated in this lead-overtake process (Fig. 1). This scenario was well illustrated by a recent observation that chronic myeloid leukemia cells secret G-CSF to reshape the cytokine-expression profiles of BM stromal cells, skewing the supporting activity towards LSCs [65]. As also shown in xenograft model of human B-ALL cells [60], the established proliferative peri-vascular foci of B-ALL cells would reshape the original SDF-1-expressing vasculature domain into SDF-1−domain afterwards. This newly created leukemic cell bed then relocated the normal HSCs and progenitors from their physiological niches by secreting higher levels of SCF, inhibited mobilization of, and also reduced the proliferation of HSCs and progenitors [66]. In addition, it was shown that in the in vitro co-culture system, human FAK+ CD34+CD38−/loCD123+ AML LSCs-enriched cells would upregulate the expressions of SDF-1, IL-6, IL-8, Ang-1 and Wnt5a from the mesenchymal stromal cells [67]. In a mouse AML model, leukemia cells displaying an inhibiting ability on osteoblast production and function [68]. Thus, rather than passively receiving the supports from a basically normal microenvironment, leukemia cells (not necessarily restricted to LICs) might be able to positively reprogram the normal microenvironment, so that the limited microenvironmental resources will be greatly biased to the malignant hematopoiesis at the expense of normal hematopoiesis (supposedly the malignant hematopoiesis may exploit a niche-providing mechanism different from that used by normal hematopoiesis).
Fig. 1.
A proposed dynamic interplays between the hematopoietic microenvironment factors with the normal hematopoietic cells or leukemic cells. A seduced or/and enhanced micro-environmental support exploited by leukemic cells underlies the establishment of leukemia predominance over normal hematopoiesis
Final Remarks and Questions
Obviously, these new developments in niche-related research have brought out more questions than ones they may have solved. We are tempted to conclude this review by elaborating three following issues.
The Functional Attributes of HSC Niche
The most conduced hematopoietic microenvironment studies have focused on characterizing a putatively specialized niche for HSCs at cellular and molecular levels. Nevertheless, it is temporarily unclear what a HSC is doing right upon its occupying a specialized niche or being away from the niche? It is generally assumed that one HSC could conduct a self-renewal expansion or an asymmetric division that reserves a progeny as HSC, or simply staying at dormant status to survive or/and to avoid differentiation. As such, in complementary to these distinct HSC behaviors, a niche may supply an inductive platform for HSCs to conduct symmetric or asymmetric division, or alternatively serve as an architectural sanctuary hosting and preventing HSC from differentiation or apoptosis. Unfortunately these fundamental questions remain to be clarified. It is even not determined whether all these stemness-preserving processes of HSCs absolutely need the supports from a specialized architectural complex. And if it is, do these different HSC behaviors need to elaborate with different kinds of niches, or with a single type of niche with versatile attributes?
Adding to this complexity, the putatively specific interactions existing between HSCs and the microenvironment are dynamic and reciprocal, as frequently in both physiological and pathological conditions, HSCs egress out and home back to the potential niches distributed within a few different hematopoietic tissues or organs. In this respect, these different kinds of niches may be regarded as the portals, apartment/hotel or even a temporary sanctuary where these hematopoietic tenants or travelers are staying over a transient or longer time. From this viewpoint, it is interesting to ask whether these niches are also available for other hematopoietic cells when HSCs are emptied out.
After all, the cell fate of a HSC or its immediate post-division progeny finally depends on the folding and unfolding of certain intrinsic programs that decide developmental fate of one cell. So understanding how intrinsic mechanisms regulate the behaviors of HSC will provide crucial clues onto what niches are able or need to do to preserve HSC stemness. That is to say, what exterior regulation mechanisms can interplay with the interior machinery? Actually the studies on the intrinsic mechanisms governing HSC stemness have represented a hot focus of recent studies, although basically only a corner of the whole picture has been unveiled. Nevertheless, one emerging theme is that keeping a HSC at quiescence seems an effective way to prevent it from exhaustion, especially at biological settings where repopulation ability of HSCs are exposed to stress conditions such as being transplanted into a hematopoietic ablated recipient.
HSC Niche Components are Shared by Non-HSCs
As indicated in the extended discussion above, against a simple assumption that a few specialized niche elements are exclusively reserved for the privileged HSCs, the majority of HSC niche cell types and almost all the molecules or signaling pathways are also implicated in regulating the proliferation and differentiation of hematopoietic progenitors. For example, a specific niche role of osteogenic progenitors or osteoblasts on regulating early B lymphopoiesis has been indicated [17, 69]. The studies demonstrate that BM sinusoidal endothelial cells are a kind of supporting niche cells for the differentiation of megakaryocytic progenitors. At the molecular level, the proliferation or/and survival of B cell and erythroid progenitors, to an extent more than that in HSCs, depends on the existence of CXCL12-expressing cells (not necessarily CAR cells). During the in vitro co-culture, endothelium simultaneously balances the expansion differentiation of HSCs. These observations raise a critical question as to by which means a specialized HSC niche function is achieved. One possibility is that the niche is basically a hub comprising numerous elements. The specific and architectural combinations of several elements from the total pool specialize the formation of individual niches (like a molecular barcode). So a particular niche is alternatively used by different HSCs or/and even progenitors in an economical way. Actually we don’t know whether there are enough anatomic niche units for simultaneously hosting every HSC and progenitors.
The Leukemic Niche Elements as the Therapeutic Targets
There are accumulating evidences that the microenvironmental factors confer the leukemia cells the resistance to the clinical treatments. Therefore, mobilization of LSCs from these sanctuary niches represents a potential strategy to sensitize LSCs to differentiation or clearance by therapeutic reagents, such as by interfering SDF-1-CXCR4 axis. Nevertheless, as AML LSCs may distribute differently within BM from normal HSCs, they might also respond distinctly to a given environmental regulatory cue. It is important to explore into these unknown differences and using them to design safe LSCs-targeting therapies.
Acknowledgments
This work was sponsored by National Natural Scientific Foundation of China (81090412) to Jiang Zhu.
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Roobrouck VD, Vanuytsel K, Verfaillie CM. Concise review: culture mediated changes in fate and/or potency of stem cells. Stem Cells. 2011;29:583–589. doi: 10.1002/stem.603. [DOI] [PubMed] [Google Scholar]
- 3.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, Rooijen N, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208:251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, Rooijen N, Alexander KA, Raggatt LJ, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 6.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, Emerson SG. A new bone to pick: osteoblasts and the haematopoietic stem-cell niche. Bioessays. 2004;26:595–599. doi: 10.1002/bies.20052. [DOI] [PubMed] [Google Scholar]
- 10.Gong JK. Endosteal marrow: a rich source of hematopoietic stem cells. Science. 1978;199:1443–1445. doi: 10.1126/science.75570. [DOI] [PubMed] [Google Scholar]
- 11.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87:518–524. [PubMed] [Google Scholar]
- 12.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 14.Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 19.Lane SW, Vita S, Alexander KA, Karaman R, Milsom MD, Dorrance AM, Purdon A, Louis L, Bouxsein ML, Williams DA. Rac signaling in osteoblastic cells is required for normal bone development but is dispensable for hematopoietic development. Blood. 2012;119:736–744. doi: 10.1182/blood-2011-07-368753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacchetti B, Funari A, Michienzi S, Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Bertrand JY, Traver D. Hematopoietic cell development in the zebrafish embryo. Curr Opin Hematol. 2009;16:243–248. doi: 10.1097/MOH.0b013e32832c05e4. [DOI] [PubMed] [Google Scholar]
- 27.Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi H, Butler JM, O'Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 32.Johns JL & Christopher MM (2012) Extramedullary Hematopoiesis: a new Look at the Underlying Stem Cell Niche, Theories of Development, and Occurrence in Animals. Vet Pathol [DOI] [PubMed]
- 33.Mendt M & Cardier JE (2012) Stromal-Derived Factor-1 and Its Receptor, CXCR4, Are Constitutively Expressed by Mouse Liver Sinusoidal Endothelial Cells: Implications for the Regulation of Hematopoietic Cell Migration to the Liver During Extramedullary Hematopoiesis. Stem Cells Dev [DOI] [PMC free article] [PubMed]
- 34.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116:375–385. doi: 10.1182/blood-2009-07-233437. [DOI] [PubMed] [Google Scholar]
- 38.Levesque JP, Winkler IG, Hendy J, Williams B, Helwani F, Barbier V, Nowlan B, Nilsson SK. Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor A in bone marrow. Stem Cells. 2007;25:1954–1965. doi: 10.1634/stemcells.2006-0688. [DOI] [PubMed] [Google Scholar]
- 39.Kubota Y, Takubo K, Suda T. Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun. 2008;366:335–339. doi: 10.1016/j.bbrc.2007.11.086. [DOI] [PubMed] [Google Scholar]
- 40.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Rehn M, Olsson A, Reckzeh K, Diffner E, Carmeliet P, Landberg G, Cammenga J. Hypoxic induction of vascular endothelial growth factor regulates murine hematopoietic stem cell function in the low-oxygenic niche. Blood. 2011;118:1534–1543. doi: 10.1182/blood-2011-01-332890. [DOI] [PubMed] [Google Scholar]
- 42.Greenbaum AM, Revollo LD, Woloszynek JR, Civitelli R & Link DC (2012) N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood [DOI] [PMC free article] [PubMed]
- 43.Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell. 2009;4:170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Hembree M, Yin T, Nakamura Y, Gomei Y, Takubo K, Shiama H, et al. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell. 2010;6:194–198. doi: 10.1016/j.stem.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, Zheng Y, Cancelas JA, Gu Y, Jansen M, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 47.Lane SW, Wang YJ, Lo Celso C, Ragu C, Bullinger L, Sykes SM, Ferraro F, Shterental S, Lin CP, Gilliland DG, et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011;118:2849–2856. doi: 10.1182/blood-2011-03-345165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kojima K, McQueen T, Chen Y, Jacamo R, Konopleva M, Shinojima N, Shpall E, Huang X, Andreeff M. p53 activation of mesenchymal stromal cells partially abrogates microenvironment-mediated resistance to FLT3 inhibition in AML through HIF-1alpha-mediated down-regulation of CXCL12. Blood. 2011;118:4431–4439. doi: 10.1182/blood-2011-02-334136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsnes AM, Hatfield KJ, Bruserud O. The chemokine system and its contribution to leukemogenesis and treatment responsiveness in patients with acute myeloid leukemia. J Buon. 2009;14(Suppl 1):S131–140. [PubMed] [Google Scholar]
- 51.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tavor S, Eisenbach M, Jacob-Hirsch J, Golan T, Petit I, Benzion K, Kay S, Baron S, Amariglio N, Deutsch V, et al. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia. 2008;22:2151–5158. doi: 10.1038/leu.2008.238. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Guo L, Jie S, Liu W, Zhu J, Du W, Fan L, Wang X, Fu B, Huang S. Berberine inhibits SDF-1-induced AML cells and leukemic stem cells migration via regulation of SDF-1 level in bone marrow stromal cells. Biomed Pharmacother. 2008;62:573–578. doi: 10.1016/j.biopha.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Spoo AC, Lubbert M, Wierda WG, Burger JA. CXCR4 is a prognostic marker in acute myelogenous leukemia. Blood. 2007;109:786–791. doi: 10.1182/blood-2006-05-024844. [DOI] [PubMed] [Google Scholar]
- 55.Becker PS, Kopecky KJ, Wilks AN, Chien S, Harlan JM, Willman CL, Petersdorf SH, Stirewalt DL, Papayannopoulou T, Appelbaum FR. Very late antigen-4 function of myeloblasts correlates with improved overall survival for patients with acute myeloid leukemia. Blood. 2009;113:866–874. doi: 10.1182/blood-2007-12-124818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradstock KF, Gottlieb DJ. Interaction of acute leukemia cells with the bone marrow microenvironment: implications for control of minimal residual disease. Leuk Lymphoma. 1995;18:1–16. doi: 10.3109/10428199509064917. [DOI] [PubMed] [Google Scholar]
- 57.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, Akiyama T, Kuroda H, Kawano Y, Kobune M, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 58.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 59.Charrad RS, Li Y, Delpech B, Balitrand N, Clay D, Jasmin C, Chomienne C, Smadja-Joffe F. Ligation of the CD44 adhesion molecule reverses blockage of differentiation in human acute myeloid leukemia. Nat Med. 1999;5:669–676. doi: 10.1038/9518. [DOI] [PubMed] [Google Scholar]
- 60.Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nwabo Kamdje AH, Mosna F, Bifari F, Lisi V, Bassi G, Malpeli G, Ricciardi M, Perbellini O, Scupoli MT, Pizzolo G, et al. Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood. 2011;118:380–389. doi: 10.1182/blood-2010-12-326694. [DOI] [PubMed] [Google Scholar]
- 62.Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23:2233–2241. doi: 10.1038/leu.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blau O, Baldus CD, Hofmann WK, Thiel G, Nolte F, Burmeister T, Turkmen S, Benlasfer O, Schumann E, Sindram A, et al. Mesenchymal stromal cells of myelodysplastic syndrome and acute myeloid leukemia patients have distinct genetic abnormalities compared with leukemic blasts. Blood. 2011;118:5583–5592. doi: 10.1182/blood-2011-03-343467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu X, Shen H, Tian C, Yu H, Zheng G, XuFeng R, Ju Z, Xu J, Wang J, Cheng T. Kinetics of normal hematopoietic stem and progenitor cells in a Notch1-induced leukemia model. Blood. 2009;114:3783–3792. doi: 10.1182/blood-2009-06-227843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang B, Ho YW, Huang Q, Maeda T, Lin A, Lee SU, Hair A, Holyoake TL, Huettner C, Bhatia R. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell. 2012;21:577–592. doi: 10.1016/j.ccr.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 67.Despeaux M, Labat E, Gadelorge M, Prade N, Bertrand J, Demur C, Recher C, Bonnevialle P, Payrastre B, Bourin P, et al. Critical features of FAK-expressing AML bone marrow microenvironment through leukemia stem cell hijacking of mesenchymal stromal cells. Leukemia. 2011;25:1789–1793. doi: 10.1038/leu.2011.145. [DOI] [PubMed] [Google Scholar]
- 68.Frisch BJ, Ashton JM, Xing L, Becker MW, Jordan CT, Calvi LM. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood. 2012;119:540–550. doi: 10.1182/blood-2011-04-348151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 70.Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Nakamura Y, Gomei Y, Suda T. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood. 2010;116:554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 71.Nygren MK, Dosen-Dahl G, Stubberud H, Walchli S, Munthe E, Rian E. beta-catenin is involved in N-cadherin-dependent adhesion, but not in canonical Wnt signaling in E2A-PBX1-positive B acute lymphoblastic leukemia cells. Exp Hematol. 2009;37:225–233. doi: 10.1016/j.exphem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 72.Zhang B, Groffen J, Heisterkamp N. Increased resistance to a farnesyltransferase inhibitor by N-cadherin expression in Bcr/Abl-P190 lymphoblastic leukemia cells. Leukemia. 2007;21:1189–1197. doi: 10.1038/sj.leu.2404667. [DOI] [PubMed] [Google Scholar]
- 73.Zhang T, Liu S, Yang P, Han C, Wang J, Liu J, Han Y, Yu Y, Cao X. Fibronectin maintains survival of mouse natural killer (NK) cells via CD11b/Src/beta-catenin pathway. Blood. 2009;114:4081–4088. doi: 10.1182/blood-2009-05-219881. [DOI] [PubMed] [Google Scholar]
- 74.Umemoto T, Yamato M, Ishihara J, Shiratsuchi Y, Utsumi M, Morita Y, Tsukui H, Terasawa M, Shibata T, Nishida K, et al. Integrin-alphavbeta3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells. Blood. 2012;119:83–94. doi: 10.1182/blood-2011-02-335430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mercher T, Cornejo MG, Sears C, Kindler T, Moore SA, Maillard I, Pear WS, Aster JC, Gilliland DG. Notch signaling specifies megakaryocyte development from hematopoietic stem cells. Cell Stem Cell. 2008;3:314–326. doi: 10.1016/j.stem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 78.Varnum-Finney B, Halasz LM, Sun M, Gridley T, Radtke F, Bernstein ID. Notch2 governs the rate of generation of mouse long—and short-term repopulating stem cells. J Clin Invest. 2011;121:1207–1216. doi: 10.1172/JCI43868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aerbajinai W, Zhu J, Kumkhaek C, Chin K, Rodgers GP. SCF induces gamma-globin gene expression by regulating downstream transcription factor COUP-TFII. Blood. 2009;114:187–194. doi: 10.1182/blood-2008-07-170712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simon C, Dondi E, Chaix A, Sepulveda P, Kubiseski TJ, Varin-Blank N, Velazquez L. Lnk adaptor protein down-regulates specific Kit-induced signaling pathways in primary mast cells. Blood. 2008;112:4039–4047. doi: 10.1182/blood-2008-05-154849. [DOI] [PubMed] [Google Scholar]
- 81.Calderon L, Boehm T. Synergistic, context-dependent, and hierarchical functions of epithelial components in thymic microenvironments. Cell. 2012;149:159–172. doi: 10.1016/j.cell.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 82.Wunderlich M, Chou FS, Link KA, Mizukawa B, Perry RL, Carroll M, Mulloy JC. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia. 2011;24:1785–1788. doi: 10.1038/leu.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 84.Hatfield K, Oyan AM, Ersvaer E, Kalland KH, Lassalle P, Gjertsen BT, Bruserud O. Primary human acute myeloid leukaemia cells increase the proliferation of microvascular endothelial cells through the release of soluble mediators. Br J Haematol. 2009;144:53–68. doi: 10.1111/j.1365-2141.2008.07411.x. [DOI] [PubMed] [Google Scholar]
- 85.Nakajima H, Ito M, Smookler DS, Shibata F, Fukuchi Y, Morikawa Y, Ikeda Y, Arai F, Suda T, Khokha R, et al. TIMP-3 recruits quiescent hematopoietic stem cells into active cell cycle and expands multipotent progenitor pool. Blood. 2010;116:4474–4482. doi: 10.1182/blood-2010-01-266528. [DOI] [PubMed] [Google Scholar]
- 86.Corazza F, Hermans C, D'Hondt S, Ferster A, Kentos A, Benoit Y, Sariban E. Circulating thrombopoietin as an in vivo growth factor for blast cells in acute myeloid leukemia. Blood. 2006;107:2525–2530. doi: 10.1182/blood-2005-06-2552. [DOI] [PubMed] [Google Scholar]
- 87.Kirito K, Fox N, Komatsu N, Kaushansky K. Thrombopoietin enhances expression of vascular endothelial growth factor (VEGF) in primitive hematopoietic cells through induction of HIF-1alpha. Blood. 2005;105:4258–4263. doi: 10.1182/blood-2004-07-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kirito K, Fox N, Kaushansky K. Thrombopoietin stimulates Hoxb4 expression: an explanation for the favorable effects of TPO on hematopoietic stem cells. Blood. 2003;102:3172–3178. doi: 10.1182/blood-2003-03-0944. [DOI] [PubMed] [Google Scholar]
- 89.Luis TC, Naber BA, Roozen PP, Brugman MH, Haas EF, Ghazvini M, Fibbe WE, Dongen JJ, Fodde R, Staal FJ. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 90.Malhotra S, Kincade PW. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell. 2009;4:27–36. doi: 10.1016/j.stem.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 94.Kim DH, Lee NY, Lee MH, Sohn SK, Do YR, Park JY. Vascular endothelial growth factor (VEGF) gene (VEGFA) polymorphism can predict the prognosis in acute myeloid leukaemia patients. Br J Haematol. 2008;140:71–79. doi: 10.1111/j.1365-2141.2007.06893.x. [DOI] [PubMed] [Google Scholar]
- 95.Gao J, Graves S, Koch U, Liu S, Jankovic V, Buonamici S, El Andaloussi A, Nimer SD, Kee BL, Taichman R, et al. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell Stem Cell. 2009;4:548–558. doi: 10.1016/j.stem.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Merchant A, Joseph G, Wang Q, Brennan S, Matsui W. Gli1 regulates the proliferation and differentiation of HSCs and myeloid progenitors. Blood. 2010;115:2391–2396. doi: 10.1182/blood-2009-09-241703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trowbridge JJ, Scott MP, Bhatia M. Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc Natl Acad Sci U S A. 2006;103:14134–14139. doi: 10.1073/pnas.0604568103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hofmann I, Stover EH, Cullen DE, Mao J, Morgan KJ, Lee BH, Kharas MG, Miller PG, Cornejo MG, Okabe R, et al. Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Cell Stem Cell. 2009;4:559–567. doi: 10.1016/j.stem.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin TL, Wang QH, Brown P, Peacock C, Merchant AA, Brennan S, Jones E, McGovern K, Watkins DN, Sakamoto KM, et al. Self-renewal of acute lymphocytic leukemia cells is limited by the Hedgehog pathway inhibitors cyclopamine and IPI-926. PLoS One. 2012;5:e15262. doi: 10.1371/journal.pone.0015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell. 2008;2:484–496. doi: 10.1016/j.stem.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Larsson J, Blank U, Helgadottir H, Bjornsson JM, Ehinger M, Goumans MJ, Fan X, Leveen P, Karlsson S. TGF-beta signaling-deficient hematopoietic stem cells have normal self-renewal and regenerative ability in vivo despite increased proliferative capacity in vitro. Blood. 2003;102:3129–3135. doi: 10.1182/blood-2003-04-1300. [DOI] [PubMed] [Google Scholar]
- 105.Ledran MH, Krassowska A, Armstrong L, Dimmick I, Renstrom J, Lang R, Yung S, Santibanez-Coref M, Dzierzak E, Stojkovic M, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 106.Yamazaki S, Iwama A, Takayanagi S, Eto K, Ema H, Nakauchi H. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2009;113:1250–1256. doi: 10.1182/blood-2008-04-146480. [DOI] [PubMed] [Google Scholar]
- 107.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frisch BJ, Porter RL, Gigliotti BJ, Olm-Shipman AJ, Weber JM, O'Keefe RJ, Jordan CT, Calvi LM. In vivo prostaglandin E2 treatment alters the bone marrow microenvironment and preferentially expands short-term hematopoietic stem cells. Blood. 2009;114:4054–4063. doi: 10.1182/blood-2009-03-205823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rocca B, Morosetti R, Habib A, Maggiano N, Zassadowski F, Ciabattoni G, Chomienne C, Papp B, Ranelletti FO. Cyclooxygenase-1, but not-2, is upregulated in NB4 leukemic cells and human primary promyelocytic blasts during differentiation. Leukemia. 2004;18:1373–1379. doi: 10.1038/sj.leu.2403407. [DOI] [PubMed] [Google Scholar]