Abstract
Lysyl oxidase (LOX) family oxidases, LOX and LOXL1-4, oxidize lysine residues in collagens and elastin, resulting in the covalent crosslinking and stabilization of these extracellular matrix (ECM) structural components, thus provide collagen and elastic fibers much of their tensile strength and structural integrity. Abnormality in LOX expression and/or activity results in connective tissue disorders and fibrotic diseases. Despite LOX family oxidases have been reported to function as tumor suppressors, recent studies have highlighted the roles of LOX family oxidases in promoting cancer metastasis. LOX family oxidases are highly expressed in invasive tumors, and are closely associated with metastasis and poor patient outcome. Consistent to their roles in connective tissue homeostasis, LOX family oxidases expedite tumorigenesis and metastasis through active remodeling of tumor microenvironment. LOX family oxidases are also actively involved in the process of epithelial-mesenchymal transition (EMT), an event critical in cancer cell invasion and metastasis. In this review, we will summarize the recent progress on LOX family oxidases, with much of the focus on the roles and mechanism of LOX in tumor progression and metastasis.
Keywords: Lysyl oxidase (LOX), Metastasis, Desmoplasia, Hypoxia-inducible factor (HIF), Epithelial-Mesenchymal Transition (EMT)
Introduction
In the past decades, majority efforts of cancer research have focused on the functional consequences of oncogene and tumor suppressor gene mutations. However, cancer is heterogeneous entity dependent on reciprocal interactions between cancer cells and their dynamic microenvironment, provided by fibroblasts, endothelial cells, pericytes, inflammatory cells, and extracellular matrix [1]. The temporal-spatial changes of microenvironment and the interplay between cancer cells and their microenvironment are critical in all different aspects of cancer development, including maintenance of cancer cell dormancy, cancer progression and metastasis, as well as drug resistance [1]. Microenvironment of cells, via cell-cell contact, cell-extracellular matrix (ECM) interaction and growth factor, retains the characteristics of cells, as well as their response to stimuli. The importance of microenvironment to pathogenesis is becoming much more recognized, from the role of ECM and matrix rigidity in determining polarity and growth potential of tissues, to the extracellular metabolism of growth factors and matrix molecules during cancer progression and metastasis. ECM remodeling is a common feature of diverse pathological processes, including tissue fibrosis and cancer [2, 3]. ECM components, closely associated with cancer prognosis and therapy response, are promising therapeutic targets under extensive investigation [4–6]. Lysyl oxidase (LOX) and its family members LOXL1-4, the copper-dependent amine oxidases playing critical roles in ECM crosslinking and remodeling, are implicated in cancer progression and metastasis [7, 8]. In this review, we will summarize the recent progress on aberrant expression and activity of LOX family oxidases in cancers, with much of the focus on the roles and mechanism of LOX in tumor progression and metastasis.
LOX Family Oxidases, Regulation and Functions
LOX Family Oxidases
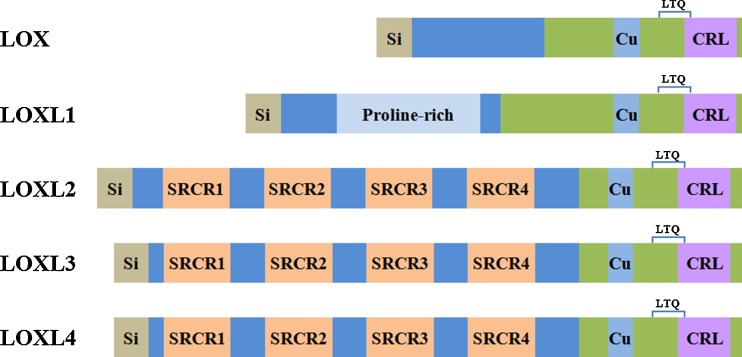
LOX and its family members LOX-like proteins (LOXL) 1–4 are copper-dependent amine oxidases that oxidize ε-amino group of peptidyl lysine to reactive peptidyl aldehydes, followed by formation of the dehydrolysinonorleucine and aldol condensation products from peptidyl aldehydes and lysine residues [9, 10]. LOX family members are highly conserved at their C- terminal mature catalytic domains, including the copper binding site, the lysyl tyrosyl quinine (LTQ) cofactor residues, and the cytokine receptor like (CRL) domain. Both copper and LTQ cofactor are required for the oxidase activity. Copper, which may not be directly involved in LOX catalytic activity, is believed to be essential for the maintenance of protein conformation and LTQ cofactor. The LTQ cofactor, formed by covalently linked Lys314 and Tyr349 residues, functions in electron transmission [9, 10]. LOX family oxidases differ significantly at their N- terminal pro-peptides that LOXL1 pro-peptide contains a proline-rich domain, and LOXL2, LOXL3 and LOXL4 each contain four scavenger receptor cysteine-rich (SRCR) domains [9, 10] (Fig. 1). The SRCR domains, frequently found in cell surface proteins associated with the immune system, are suggested to be involved in protein-protein interaction [10]. Based on sequence similarity and domain structure, LOX and LOXL1 form a subfamily, while LOXL2, 3, and 4 exist as a separate subfamily. LOX family oxidases are synthesized as zymogens or proenzymes. Removal of the NH2-terminal pro-peptide of LOX by bone morphogenetic protein-1 (BMP-1)/Tolloid metalloproteinases in the extracellular space is necessary for enzyme activation and the exhibition of its oxidase activity [11, 12]. It remains somewhat unclear whether LOX-like oxidases undergo similar cleavage/activation events in vivo, as LOXL1 is present predominantly in its full length form in vivo [13]. Collagens and elastin are well characterized physiological substrates of LOX family oxidases. The resultant crosslinked collagen and elastic fibers provide the connective tissues much of their tensile strength and structural integrity. It was also reported that LOX can utilize histone H1, PDGFR-β, as well as bFGF as substrates in regulating transcription and cell migration [14–16]. LOX utilizes multiple lysine residues in collagens and elastin as substrates [10]. However, the sequences surrounding these lysine residues lack obvious consensus. Purified LOX readily oxidizes basic globular proteins, e.g. histone H1 [14], and non-peptidyl amine substrates, e.g. 1,5-diaminopentane [17], but not acidic proteins. The electrostatic potential between LOX and its substrates, rather than a consensus sequence, might be essential to its catalytic activity. LOX and LOXL1 are tethered to the sites of elastogenesis via binding to fibulin-4 and −5 respectively [18, 19]. The temporal-spatial localization and the vicinity to its substrates, together with the electrostatic potential, may determine the substrate specificity of LOX.
Fig. 1.
Schematics of LOX family oxidases. LOX and LOX-like proteins (LOXL) 1–4 are synthesized as proenzymes. LOX family oxidases are highly conserved at their C- terminal mature domains, including the copper binding site, the lysyl tyrosyl quinine (LTQ) cofactor residues, and the cytokine receptor like (CRL) domain, but differ significantly at their N- terminal pro-peptides. The pro-peptides, especially the proline-rich domain in LOXL1 and the scavenger-receptor cysteine-rich (SRCR) domains in LOXL2-4, are possibly involved in protein-protein interactions. Si: Signal peptide
Regulation of LOX Family Oxidases
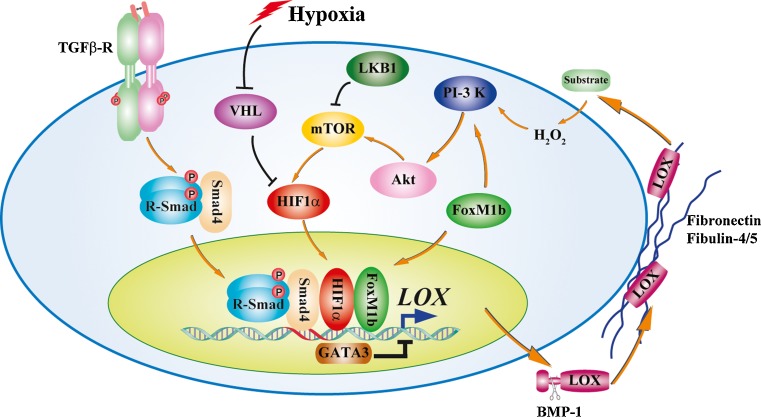
Precisely regulated expression and activity of LOX family oxidases are prerequisite to their critical functions in connective tissue homeostasis. This is achieved not only by the transcriptional regulation, but also by temporal-spatial distribution and the modulation of oxidase activity of LOX family oxidases (Fig. 2). Aberrant expression of LOX family oxidases and/or deregulated oxidase activity is responsible to the pathogenesis of many types of diseases, e.g. tissue fibrosis and cancer. TGF-β is one of the key cytokines in regulating ECM, not only by regulating expression of ECM structural proteins, but also by affecting enzymes involved in ECM biosynthesis and degradation. TGF-β increased steady state LOX mRNA level in a dose- and time-dependent manner, through integrated Smad3, PI-3 kinase, and MAPK signaling [20]. Chronic inflammation plays pivotal roles in the pathogenesis of fibrotic diseases. High dose proinflammatory factor TNF-α stimulated LOX expression, which may play an important role in progressive cardiac fibrosis [21]. LOX is highly expressed in invasive basal breast cancer, but not in non-invasive ductal breast cancer. GATA-3, a transcriptional factor essential for normal mammary gland development and luminal cell differentiation, negatively regulates the expression of LOX through methylation of the LOX promoter [22]. The forkhead box M1b (FoxM1b) transcription factor, overexpressed in human cancers and correlated with poor prognosis, directly binds to the promoters of LOX and LOXL2 genes. FoxM1b, by inducing LOX and LOXL2 expression and activating the Akt-Snail pathway, drives epithelial-mesenchymal transition (EMT), hepatic fibrosis and metastasis of hepatocellular carcinoma [23]. Hypoxia has been proposed as an important microenvironmental factor in the development of many types of diseases, including tissue fibrosis and cancer. Hypoxia-inducible factor-1 (HIF-1) is the key regulator of the cellular response to hypoxia. LOX mRNA level is highly up-regulated under hypoxic conditions, mediated by HIF-1 at transcriptional level [7]. Notch and tumor suppressor LKB1 also regulate LOX expression through HIF-1 [8, 24].
Fig. 2.
Transcriptional and post-transcriptional regulation of LOX family oxidases. LOX family oxidases are temporal-spatially regulated at both transcriptional and post-transcriptional levels. TGF-β upregulates LOX mRNA level through Smad3 transcription factor. Hypoxia-inducible factor-1 (HIF-1), in response to hypoxic stress or deregulated mammalian target of rapamycin (mTOR) kinase downstream of tumor suppressor LKB1, directly binds to LOX and LOXL2 promoters, and initiates LOX and LOXL2 transcription. The forkhead box M1b (FoxM1b) transcription factor, in addition to direct binding to the promoters of LOX and LOXL2 genes, activates LOX and LOXL2 transcription through enhanced PI3-kinase-Akt-mTOR signaling. LOX enhances HIF1α expression by activating the PI3-kinase-Akt signaling, thus providing a fast-forward regulatory circuit of LOX expression. GATA-3 transcriptional factor, on the other hand, negatively regulates LOX expression through LOX promoter methylation. At post-transcriptional level, the proteolytic removal of LOX pro-peptide by BMP-1/Tolloid metalloproteinases is essential to the exhibition of its oxidase activity, faciliated by the colocalization of LOX and BMP-1 to fibronectin matrix. LOX and LOXL1 interact with fibulin-4 and fibulin-5 respectively through cognate pro-peptides. The interaction of LOX family oxidases with ECM components determines their spatial distribution, substrate specificity, oxidase activity and their physiological and pathological functions
The observation that increased LOX enzyme activity upon TGF-β stimulation was delayed and was of lower magnitude than the increase in its mRNA level suggested rate-limiting post-transcriptional regulation of LOX. Indeed, LOX family oxidases are synthesized as zymogens or proenzymes. The proteolytic removal of LOX pro-peptide by BMP-1/Tolloid metalloproteinases after secretion is essential to the exhibition of its oxidase activity [11, 12]. The activation of LOX, however, should be a tightly regulated process. Both LOX and BMP-1 bind to cellular fibronectin, an abundant component of the ECM that regulates manifold cellular functions through its interaction with various ECM and cell surface proteins [25, 26]. Impaired LOX processing and oxidase activity were evident in FN-null MEFs, compared to wild-type MEFs [25]. Despite several studies suggested LOXL1 undergoes similar BMP-1-mediated proteolytic activation in vitro, this has to be further confirmed, as almost exclusive full-length LOXL1 protein was detected in vivo [13]. Nevertheless, LOXL1 is tethered to the sites of elastogenesis via interaction between its pro-peptide and fibulin-5 to exert its oxidase activity [19]. LOXL1 binding to fibulin-5 may also have a regulatory role on its oxidase activity. Interaction between fibulin-4 and the pro-peptide of LOX efficiently promotes assembly of LOX onto tropoelastin [18]. Thus, the pro-peptides of LOX family oxidases and possibly their mature domains, via interaction with ECM components, exert regulatory roles in determining the spatial distribution, substrate specificity, oxidase activity and their physiological and pathological functions.
Physiological Functions of LOX Family Oxidases
LOX family oxidases crosslink collagen and elastin, and are essential to the biogenesis of connective tissues [11]. Lox-null mice are perinatal lethal with cardiovascular fragility, burst arterial aneurysms, ruptured diaphragm, and fragmented elastic fibers, suggesting that LOX has an essential role in the development and function of the cardiovascular system [27, 28]. The Lox null mice also display impaired development of the distal and proximal airways [29]. Elastic and collagen fibers were markedly more disperse in the mesenchyme surrounding the distal airways, bronchioles, bronchi, and trachea, and in pulmonary arterial walls in Lox null mice than in the wild type mice [29]. Although viable, Loxl1-null mice are featured with enlarged pulmonary alveoli, redundant skin, prolapse of pelvic viscera, and vascular abnormalities [19], similar to that observed in the fibulin-5 knockout mice [30]. The LOXL2-4 knockout mice are yet not available to study their physiological functions.
Despite lack of substrate specificity in vitro, the largely non-overlapping phenotypes, and the inability of LOX and LOXL1 to compensate for each other in the knockout mice have shed light on potentially distinct substrate spectrum and physiological functions of LOX family oxidases in vivo. The phenotypic difference could only be partially attributed to the tissue expression pattern of LOX family oxidases. In contrast to the restricted and low level expression of LOXL2-4, LOX and LOXL1 are broadly expressed with overlapping expression domains [31]. Immunohistochemical staining suggested LOXL1 to be solely associated with elastic lamina, whereas LOX was broadly distributed [19]. This is consistent to the observation that reduced crosslinks in both collagen and elastic fibers were detected in Lox-null mice, whereas aberrant elastic, but not collagen, fibers were evident in Loxl1 mice, suggesting spatially defined roles of LOXL1 in guiding elastin deposition. Unlike proteolytically processed LOX, LOXL1 is present largely as full-length protein in vivo [13]. LOXL1 interacts with fibulin-5 through its pro-peptide, and is targeted to elastic fibers [19]. Therefore, besides the roles of retaining the oxidase in the latent format, the divergent pro-peptides, along with their cognate mature domains, may determine the spatial distribution, substrate specificity and function of LOX family oxidases via distinct interacting proteins. Indeed, LOXL2 inhibits keratinocyte differentiation via the function of its fourth SRCR domain, independent of its oxidase activity [32]. In addition to well documented roles in connective tissue homeostasis, LOX family oxidases are participated in many other critical biological functions, including cell migration [16, 33–37], cell polarity and epithelial-mesenchymal transition (EMT) [24, 38–42], as well as angiogenesis [43], possibly mediated by less well characterized substrates/binding proteins of LOX family oxidases.
LOX Family Oxidases in Cancer and Other Diseases
X-linked fatal disorder Menkes’ disease and autosomal recessive disorder Wilson’s disease are two widely studied genetic diseases of copper metabolism in humans [44]. Remarkably low LOX activity, as the pathophysiological consequence of copper deficiency, accounts in part for the connective tissue disorders observed in Menkes’ disease and Wilson’s disease [44–46]. LOX is involved in a variety of pathological process related to connective tissue. In contrast to reduced LOX activity in cutis laxa [47], Menkes’ disease [46], and spontaneous coronary artery dissection [48], which show abnormalities and deficiency of elastic fibers, LOX expression is markedly elevated in atherosclerosis, scleroderma, and liver cirrhosis, featured with prominent symptom of fibrosis [6, 49, 50]. Other family members, e.g. LOXL1 and LOXL2 also play critical roles in elastic fiber homeostasis and maintenance at adult age [19, 51, 52].
The role of LOX family oxidases in cancer emerges from the up-regulated LOX expression in spontaneous revertants of H-ras-transformed rat fibroblasts [53]. Reduction of LOX expression in head and neck squamous cell carcinomas [54] and gastric cancers [55], of LOXL2 expression in head and neck squamous cell carcinomas [54], and of LOXL1 and LOXL4 expression in bladder cancer via epigenetic silencing have been reported [56] (Table 1). The pro-peptide of LOX is responsible for its anti-tumor effect through interacting with Hsp70 and c-Raf to reduce ERK activation [57], and to repress Bcl-2 [58]. Despite LOX has been implicated as a tumor suppressor, LOX is now more widely accepted as a poor prognosis factor (Table 1), especially in promoting cancer metastasis in breast [7, 59, 60], head and neck squamous cell [7, 61], lung [8], prostatic [62], and bronchogenic [63] carcinomas. LOX is highly expressed in invasive basal breast cancer, but not in non-invasive ductal breast cancer [7]. Tumor suppressor LKB1 contributes to ~30 % lung cancer [64]. LOX is highly expressed in LKB1-deficient lung adenocarcinomas, and is responsible to the enhanced cancer cell proliferation and invasiveness [8]. Elevated LOXL4 expression has been reported in head and neck cancer [65], and colorectal adenocarcinomas [66]. Breast cancer, lung cancer or head and neck cancer patients with high LOX-expressing tumors have poor distant metastasis-free and overall survival [7, 8], while LOXL2 is a novel poor prognosis marker of lung squamous cell carcinomas, lung adenocarcinomas and pancreatic carcinoma [5, 40, 67–70]. LOX family oxidases are necessary and sufficient to repress E-cadherin, and are required for EMT and metastatic dissemination of basal-like breast carcinomas [41]. Pharmacological inhibitors or therapeutic antibodies of LOX family oxidases satisfactorily impede the disease progression and metastasis in breast cancer and lung cancer mouse models [5, 7, 8, 71].
Table 1.
Expression of LOX Family Members in Tumor Tissues and/or Cell Lines
| Cancer Type | Expression | System | Function | References |
|---|---|---|---|---|
| LOX | ||||
| Basal and squamous cell carcinomas | Pa: ↓ | Tb; Cc | [103] | |
| Breast cancer | R, Pd: ↑ | T; C | Poor distant metastasis-free and overall survivals; activate HIF1-Akt pathway; mediate hypoxic control of metastasis; regulate actin filament formation; contribute to mechanotransduction- mediated regulation of TGF-β signaling; recruit bone marrow cell to form the pre-metastatic niche | [7, 74, 85, 104] |
| Breast cancer | R, P: ↓ | C | Repress ERK activation and Bcl-2 expression | [57, 105–109] |
| Bronchogenic carcinoma | R, P: ↓ | T | [63] | |
| Choriocarcinoma | R: ↓ | C | [110] | |
| Colorectal cancer | R, P: ↑ | T; C | Correlated with absence of lymphovascular invasion; activate the PI3K-Akt pathway to upregulate HIF-1α protein synthesis | [66, 75, 111] |
| Gastric cancers | R: ↓ | T; C | Loss of heterozygosity and promoter methylation | [55] |
| Head and neck squamous cell carcinoma | P, P: ↑ | T; | Strongly associated with increased metastasis, progression, and death | [7, 61] |
| Head and neck squamous cell carcinomas | R: ↓ | T; C | [54] | |
| Lung adenocarcinoma | R, P: ↑ | T; C | ECM remodling; associated with advanced stage and metastasis | [8, 112] |
| Melanoma | R: ↑ | C | Correlate to invasive/metastatic potential | [74] |
| Oral and oropharyngeal squamous cell carcinoma | R, P: ↑ | T | Independent prognostic biomarker and predictor of lymph node metastasis | [113] |
| Prostate adenocarcinoma | R: ↑ | T | Marker of hypoxia in prostate cancer | [62, 74] |
| Prostate cancer | R, P: ↓ | C | Inhibit FGF2 signaling | [114] |
| Renal cell carcinoma | R: ↑ | T | [115] | |
| LOXL1 | ||||
| Bladder cancer | R: ↓ | C | Silenced predominantly by promoter methylation; inhibit Ras/ERK signaling pathway | [56] |
| Lung adenocarcinoma | R: ↑ | C | [116] | |
| Renal cell carcinoma | R: ↓ | C | [117] | |
| Salivary gland adenoid cystic carcinoma | R: ↑ | T | Hypomethylated CpG islands | [118] |
| LOXL2 | ||||
| Breast cancer | R, P: ↑ | T; C | Promote deposition onto elastic fibers; highly expressed in the basal/myoepithelial mammary cell lineage; increase in disease-associated stroma; maintains the mesenchymal phenotype of basal-like carcinoma cells | [5, 33, 40, 74, 82, 83, 119] |
| Colorectal adenocarcinomas | R, P: ↑ | T | Correlate with absence of lymphovascular invasion; increase in disease-associated stroma | [5, 66] |
| Gastric cancer | R, P: ↑ | T; C | Associate with tumor invasion, lymph node metastasis and poor overall survival | [69] |
| Head and neck squamous cell carcinomas | R: ↓ | T; C | [54] | |
| Hepatocellular carcinoma | R: ↑ | T; | Increase in disease-associated stroma | [5] |
| Lung Squamous Cell Carcinoma | R, P: ↑ | T; | Increase in disease-associated stroma; decreased overall and disease-free survival | [5, 68] |
| Melanoma | R: ↑ | C | Correlate to invasive/metastatic potential | [74] |
| Pancreatic carcinoma | R, P: ↑ | T; C | Increase in disease-associated stroma; epithelial– mesenchymal transition | [5, 70] |
| Prostate adenocarcinoma | R: ↑ | T | Correlate to invasive/metastatic potential | [74] |
| Renal cell carcinoma | R: ↑ | T | Increase in disease-associated stroma | [5] |
| LOXL3 | ||||
| Breast cancer | R: ↑ | C | Interact and cooperate with Snail to downregulate E-cadherin expression | [38] |
| Melanoma | R: ↑ | C | Correlate to invasive/metastatic potential | [38, 74] |
| LOXL4 | ||||
| Bladder cancer | R: ↓ | C | Epigenetically silenced by promoter methylation; somatic mutations | [56] |
| Colorectal adenocarcinomas | R: ↑ | T | Correlate with absence of lymphovascular invasion | [66] |
| Head and neck squamous cell carcinoma | R, P: ↑ | T; C | Correlate to local lymph node metastases and higher tumour stages; suitability as a vaccination strategy | [65, 120, 121] |
| Mesothelioma | R: ↑ | T | Alternatively spliced in an anatomic site-specific manner | [122] |
| Ovarian carcinoma | R: ↑ | T | Alternatively spliced in an anatomic site-specific manner | [122] |
| Serosal cavities-breast carcinoma | R: ↑ | T | Alternatively spliced in an anatomic site-specific manner | [122] |
Abbreviations: aP protein; bT tumor tissues; cC cell lines; dR RNA
Role of LOX Family Oxidases in Cancer
Hypoxia
Tumors often encounter hypoxic, hypoglycemic and acidic microenvironment. Hypoxia, present in solid tumors larger than 1 cm3 due to inadequate blood supply, has received considerable attention as an inducer of tumor metastasis, and is strongly correlated to poor patient outcome. Hypoxia promotes tumor angiogenesis, EMT, invasion, metastasis, and de-differentiation, largely mediated by the targets of hypoxia-inducible factors (HIFs) [72]. LOX, among the hypoxia gene signature [73], has been shown to be directly regulated by HIF1α transcription factor, and is essential for hypoxia-induced metastasis in breast cancer and head and neck cancer [7]. Hypoxia-induced cancer cell invasion was severely impaired by inhibiting LOX expression or oxidase activity [74]. The decreased invasive ability could be rescued by over-expression of mature LOX or treatment of conditioned medium from cancer cells, suggesting secreted LOX plays key roles in this process [74]. Besides hypoxia, we have previously reported that LOX expression is elevated in tumor suppressor LKB1-deficient lung cancers [8]. The deregulated LOX expression, however, is mediated by HIF1α as well [8]. Under normoxic conditions, HIF1α expression is promoted by disrupted regulation of mammalian target of rapamycin (mTOR) kinase and subsequent increased HIF1α translation. Indeed, gain-of-function of oncogenes, e.g. H-Ras, or loss-of-function of tumor suppressors, e.g. LKB1, leads to the accumulation of HIF1α in both normoxic and hypoxic conditions [8, 72]. Converge of hypoxia, oncogene gain-of-function, and tumor suppressor loss-of-function at HIF1α transcription factor has placed HIF1α at the central position in LOX expression regulation. Interestingly, LOX induction in human colorectal carcinoma cell lines enhanced HIF1α expression, by activating the PI3-kinase-Akt signaling pathway and upregulating HIF1α protein synthesis, in which LOX-mediated hydrogen peroxide production is necessary [75]. Cancer cell proliferation was stimulated by LOX in an HIF1α-dependent manner both in vitro and in vivo. Thus, the regulatory circuit between LOX and HIF1α act in synergy to foster tumor formation in the adaptation of tumor cells to hypoxia. Hypoxia is one of the key drivers of angiogenesis [76]. Under hypoxic conditions, HIF1α induces expression of pro-angiogenic factors and endothelial cell mitogens, e.g. vascular endothelial growth factor A (VEGF-A), thus induces proliferation, sprouting and tube formation of endothelial cells and sustained angiogenesis [77]. LOXL2 is accumulated in the endothelial ECM and regulates sprouting angiogenesis through assembling type IV collagen in the endothelial basement membrane in zebrafish [43]. LOX family oxidases therefore play manifold roles in cancer progression and metastasis, promoting not only cancer cell migration and invasion, but also angiogenesis in concert with proangiogenic factors under hypoxia (Fig. 3).
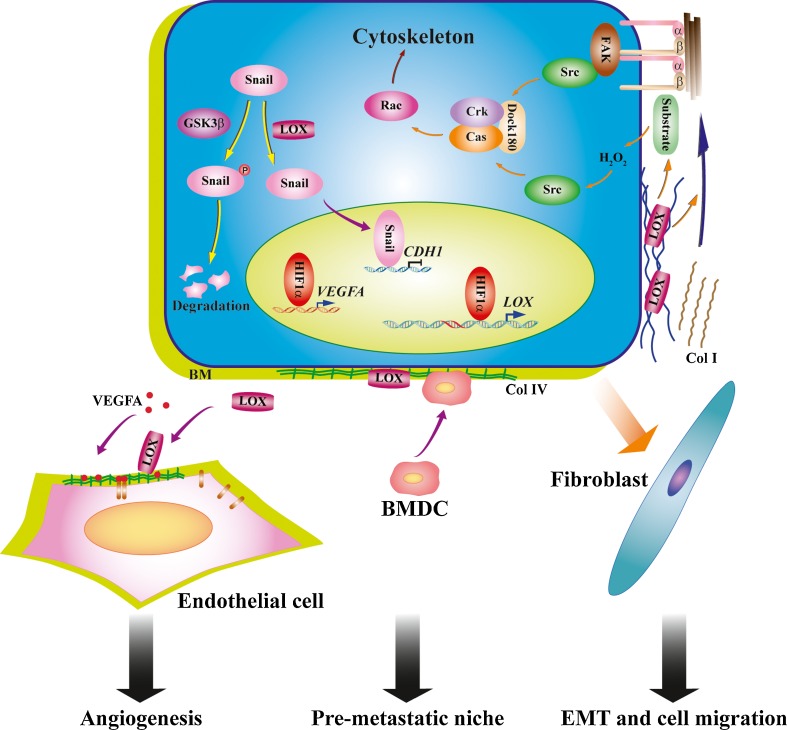
Fig. 3.
Pathological functions of LOX family oxidases during cancer progression and metastasis. LOX, as a potent chemokine, orchestrates FAK/Src, Rho GTPases and p130Cas/Crk/DOCK180 signaling pathway and cytoskeleton rearrangement to regulate cell-matrix adhesion and cell motility. Cells sense the mechanical force from stiff extracellular matrix, resultant from increased LOX expression and collagen crosslinking, through cell surface integrin receptors and downstream activation of FAK and Src kinases and cytoskeleton rearrangement to acquire the invasion ability. LOX family oxidases are actively involved in the regulation of cell polarity and epithelial-mesenchymal transition, by interacting with Snail transcription factor and protecting Snail from GSK3β-dependent phosphorylation and degradation to induce mesenchymal-like morphological changes and enhanced cell motility. LOX and LOXL2 secreted by primary tumors accumulate at the premetastatic sites, crosslink basement membrane type IV collagen and generate chemotactic cue for bone marrow-derived cells (BMDCs) to form the premetastatic niche to facilitate tumor cell metastasis. LOXL2 regulates angiogenesis through assembling type IV collagen in the endothelial basement membrane, in concert with other proangiogenic factors, e.g. VEGF-A under hypoxia
Extracellular Matrix Remodeling
LOX family oxidases have been implicated to be associated with cancer metastasis and shorter overall survival. Over-expression of LOX family oxidases potentiates tumor progression and metastasis in breast cancer and lung cancer [7, 8, 71]. Inhibition of LOX family oxidase activity by pharmacological inhibitors, therapeutic antibodies or reduced LOX expression impeded tumor progression [5, 7, 8, 71]. Consistent to their roles in connective tissue homeostasis, LOX family oxidases modulate tumor behavior in part through ECM remodeling and stiff microenvironment (Fig. 3). In solid tumors, there is evident pervasive growth of dense fibrous tissues, featured with accumulation of fibroblasts and excess and/or disordered ECM deposition, a phenomenon named desmoplasia. In clinical practice, the correlation between tissue fibrosis and cancer has attracted much attention. Indeed, high mammographic density, characterized by dense type I collagen accumulation, increases breast cancer risk [78].
Collagens, the most abundant ECM proteins in the stroma, contribute most significantly to the tensile strength and rigidity of tissues [79]. Increased expression and excess deposition of collagens, as well as disordered ECM organization and turnover have been implicated in tumor progression [80]. An extracellular matrix gene cluster is closely associated with breast cancer prognosis and endocrine therapy response [60]. Despite that breakdown of surrounding matrix is believed to be prerequisite for tumor metastasis, histopathological analyses had clearly shown correlation of poor outcome in patients with fibrotic lesions in a variety of cancers, underscoring the essential roles of ECM remodeling during tumor progression and metastasis. Cancer invasion is facilitated by stromal collagen re-organization and this behavior is significantly increased in collagen-dense tissues [81]. Many ECM modifying enzymes, including matrix metalloproteinases (MMPs) and LOX family oxidases are aberrantly expressed during malignant transformation, progression and metastasis of cancers. Over-expression of active LOX could increase tissue tension and ECM rigidity by crosslinking collagens and elastin [8, 71]. LOXL2 expression in non-invasive breast cancer cells promotes tumor fibrosis and tumor invasiveness simultaneously in a xenograft model, indicating intrinsic connection between these two processes [82]. Reduced collagen crosslinking by downregulation of LOX impeded tumor progression [8, 71]. Genetic, chemical or antibody-mediated inhibition of LOXL2 resulted in marked reduction in activated fibroblasts, desmoplasia, and metastasis [5, 83]. The matrix stiffness, as well as the ECM composition and architecture, play fundamental roles in cell fate determination. Normal breast epithelial cells in stiff 3D microenvironment share characteristics with transformed breast cancer cells in disrupted cell adherens junction, enhanced cell proliferation, and failure in lumen formation [84]. Cancer cells in stiff microenvironment are more proliferative and invade into surrounding matrix [8, 71, 84]. Cells sense the mechanical force from stiff ECM through cell surface integrin receptors [8, 71, 84]. The dense collagen matrix microenvironment provokes the increase of cancer cell invasion ability through activation of FAK and Src kinases and cytoskeleton rearrangement downstream of β1 integrin, whereas β1 integrin blocking antibody or depletion of FAK significantly decreased cancer cell proliferation and invasiveness in the stiff microenvironment [8, 71, 84].
On the other hand, LOX family oxidases affect tumor progression and metastasis beyond simply stiffening surrounding tissues by impacting other aspects of microenvironment (Fig. 3). Inhibition of LOXL2 resulted in a marked reduction in activated fibroblasts, endothelial cells, and decreased production of growth factors and cytokines [5]. Increasing extracellular matrix rigidity promoted the proliferation of malignant MECs, which was abrogated by inhibiting the activities of TGF-β1 or LOX [39]. Inactivating LOX activity impaired TGF-β1-mediated EMT and invasion in breast cancer cells, suggesting LOX may serve as a potential mediator that couples mechanotransduction to TGF-β signaling [39]. Tumor cell metastasis is facilitated by premetastatic niches formed in the destination organs by invading bone marrow-derived cells (BMDCs). LOX and LOXL2 secreted by primary tumors accumulate at the premetastatic sites, crosslink basement membrane type IV collagen, and recruit CD11b+myeloid cells to form the premetastatic niche. LOX inhibition prevents CD11b+cell recruitment and metastatic tumor growth [23, 85, 86]. More broad microenvironmental changes triggered by LOX family oxidases, in synergy with stiff ECM, promote cancer cell proliferation and invasion.
Epithelial-Mesenchymal Transition (EMT), Cell Migration and Invasion
Accumulating evidences indicate the importance of the LOX family oxidases in promoting epithelial neoplasms towards their more aggressive forms. LOX expression is up-regulated in distant metastatic tumors compared with primary tumors in breast cancer [36]. LOX family oxidases are only expressed in the invasive and/or metastatic breast cancer cells, but not in the non-invasive cells [87]. Expression of LOXL2 in non-invasive MCF-7 cells promoted mesenchymal morphological change and migratory ability [33]. On the one hand, LOX is a potent chemokine inducing directional migration of varied cell types. LOX, secreted by vascular smooth muscle cells (VSMCs), strongly induces directional migration of VSMCs. LOX-dependent chemotaxis was abolished by β-aminopropionitrile (BAPN), an active site inhibitor of LOX, or by catalase, indicating that the hydrogen peroxide product of amine oxidation by LOX is critical to its chemotactic activity [88]. In invasive breast cancer cells [36] and malignant astrocytoma cells [34], hydrogen peroxide-mediated FAK/Src signaling is required to facilitate cell-matrix adhesion formation and cell migration. LOX, by orchestrating the activities of Rho GTPases and p130Cas/Crk/DOCK180 signaling, regulates cell motility through changes in actin filament polymerization [89]. In addition, LOX oxidizes cell surface PDGFR-β receptor, and is essential to generate optimal chemotactic sensitivity of rat aortic smooth muscle cells to the chemoattractant PDGF [16]. Moreover, LOX crosslinks basement membrane type IV collagen and generates chemotactic cue for CD11b+myeloid cells to form the premetastatic niche [85, 86].
On the other hand, LOX family oxidases are actively involved in the regulation of cell polarity, and in the process of EMT (Fig. 3). EMT, initially discovered as a critical event during embryogenesis and gastrulation, is believed to be a critical step in cancer cell dissemination and metastasis [90–92]. EMT is characterized by decreased expression of epithelial markers, e.g. E-cadherin, loss of cell-cell adhesion and cell polarity, as well as increased expression of mesenchymal markers, e.g. vimentin and N-cadherin, reorganization of cytoskeleton and gain of cell motility [91]. Numerous intracellular signaling pathways trigger the EMT process, including TGF-β [92], Wnt [93], Notch [94, 95], receptor tyrosine kinases (RTKs) [96] pathways and hypoxia [97]. This is mediated by the transcriptional factors including Snail, Slug, Twist, and ZEB1/2, which repress the expression of adherens junction component E-cadherin [91]. Snail protein stability and cellular localization is finely controlled by GSK3β-dependent phosphorylation and subsequent ubiquitination [93, 98]. LOXL2 and LOXL3 were found to interact with Snail and attenuate GSK3β-dependent Snail degradation. LOXL2 and Snail cooperate to downregulate E-cadherin expression and to induce mesenchymal-like morphological changes [38, 99]. Hypoxia represses E-cadherin expression, and promotes EMT [24, 42, 97]. HIF-1α enhanced EMT in vitro and induced epithelial cell migration through upregulation of LOX [24, 41, 42, 100]. The upregulated expression of LOX and LOXL2 under hypoxia is required and sufficient for hypoxic repression of E-cadherin, possibly through stabilization of snail transcription factor [24, 41]. The FoxM1b transcription factor simultaneously induces LOX and LOXL2 expression and activates the Akt-Snail pathway, and drives EMT [23]. Whether FoxM1b initiates the EMT process via LOX/LOXL2-mediated Snail stabilization needs further investigation. Conversely, LOXL2 silencing in basal-like carcinoma cells induces a mesenchymal-epithelial transition (MET). However, LOXL2 maintains the mesenchymal phenotype of basal-like breast cancer cells by transcriptional downregulation of Lgl2 and claudin1 and disorganization of cell polarity and tight junction complexes, independent of its catalytic activity, Snail stability, and alteration in E-cadherin expression, suggesting multi-faceted mechanisms of LOX family oxidases in regulating EMT [40]. TGF-β, one of the major triggers of EMT, induces the expression and secretion of LOX family oxidases. LOX family oxidases were reported to positively [39] or negatively [101, 102] regulate TGF-β signaling. Nevertheless, the fact that inactivating LOX impaired stiff matrix, TGF-β-mediated EMT and cell invasiveness in breast cancer cells suggests LOX as a potential mediator that couples mechanotransduction to TGFβ signaling [39]. Further studies are warranted to investigate the contribution of individual LOX family members to the induction of EMT in the context of dynamic microenvironment during cancer cell invasion and metastasis.
Future Study
LOX family oxidases trigger desmoplastic reaction and active ECM remodeling. The transduction of resultant matrix mechanical property changes into cellular signaling promotes disruption of cell polarity, dynamic cytoskeleton rearrangement, cell migration and invasion. The acquisition of invasive behavior of cells expressing LOX family oxidases are partially attributed to the EMT in transcription factor snail dependent- and independent- manners. The LOX-mediated recruitment of myeloid cells and establishment of premetastatic niche facilitate the distant organ colonization and metastasis of cancer cells. These insightful studies have provided us the first knowledge how LOX family oxidases modulate tumor microenvironment and promote cancer progression and metastasis. However, as important extracellular oxidative enzymes, LOX family oxidases may interact with and/or oxidize other proteins besides collagens and elastin, thereby affecting diversified signaling pathways and cellular functions. These largely uncharacterized substrates and/or interacting proteins may reside in the extracellular space, on the cell surface, or even inside of the cells. Despite lack of substrate specificity in vitro, the LOX family oxidases may well have preference towards distinct spectrum of substrates and/or interacting partners. This arises not only from the divergent pro-peptides of each family member, but also from overlapping, but not identical distribution and physiological functions of LOX family oxidases. The temporal-spatial distribution and sequence specificity of LOX family oxidases may determine the spectrum of their substrates and/or interacting proteins and possible diversified functions in cancer progression and metastasis. More comprehensive studies, with no doubt, will lead to further understanding of the mechanisms how LOX family oxidases modulate the cancer microenvironment and exert their promoting roles in cancer progression and metastasis, and to the development of novel anti-cancer therapeutics.
Acknowledgments
This work was supported by the National Basic Research Program of China (2010CB912102) and National Natural Science Foundation of China (30971495). G.G. is a scholar of the Hundred Talents Program of the Chinese Academy of Sciences.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev. 2008;9(8):628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 3.Araya J, Nishimura SL. Fibrogenic reactions in lung disease. Annu Rev Pathol. 2010;5:77–98. doi: 10.1146/annurev.pathol.4.110807.092217. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez C, Rodriguez-Sinovas A, Martinez-Gonzalez J. Lysyl oxidase as a potential therapeutic target. Drug News Perspect. 2008;21(4):218–224. doi: 10.1358/dnp.2008.21.4.1213351. [DOI] [PubMed] [Google Scholar]
- 5.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Vlasselaer P, Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 6.Kagan HM. Intra- and extracellular enzymes of collagen biosynthesis as biological and chemical targets in the control of fibrosis. Acta Trop. 2000;77(1):147–152. doi: 10.1016/s0001-706x(00)00128-5. [DOI] [PubMed] [Google Scholar]
- 7.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Xiao Q, Ma H, Li L, Liu J, Feng Y, Fang Z, Wu J, Han X, Zhang J, Sun Y, Wu G, Padera R, Chen H, Wong KK, Ge G, Ji H. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc Natl Acad Sci USA. 2010;107(44):18892–18897. doi: 10.1073/pnas.1004952107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer–a prospect. J Cell Biochem. 2007;101(6):1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- 10.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19–20):2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge G, Greenspan DS. Developmental roles of the BMP1/TLD metalloproteinases. Birth Defects Res C Embryo Today. 2006;78(1):47–68. doi: 10.1002/bdrc.20060. [DOI] [PubMed] [Google Scholar]
- 12.Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, Hong HH, Greenspan DS, Trackman PC. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem. 2001;276(25):22537–22543. doi: 10.1074/jbc.M102352200. [DOI] [PubMed] [Google Scholar]
- 13.Borel A, Eichenberger D, Farjanel J, Kessler E, Gleyzal C, Hulmes DJ, Sommer P, Font B. Lysyl oxidase-like protein from bovine aorta. Isolation and maturation to an active form by bone morphogenetic protein-1. J Biol Chem. 2001;276(52):48944–48949. doi: 10.1074/jbc.M109499200. [DOI] [PubMed] [Google Scholar]
- 14.Kagan HM, Williams MA, Calaman SD, Berkowitz EM. Histone H1 is a substrate for lysyl oxidase and contains endogenous sodium borotritide-reducible residues. Biochem Biophys Res Commun. 1983;115(1):186–192. doi: 10.1016/0006-291x(83)90987-7. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Nugent MA, Zhao Y, Chau AN, Li SJ, Chou IN, Liu G, Kagan HM. Lysyl oxidase oxidizes basic fibroblast growth factor and inactivates its mitogenic potential. J Cell Biochem. 2003;88(1):152–164. doi: 10.1002/jcb.10304. [DOI] [PubMed] [Google Scholar]
- 16.Lucero HA, Ravid K, Grimsby JL, Rich CB, DiCamillo SJ, Maki JM, Myllyharju J, Kagan HM. Lysyl oxidase oxidizes cell membrane proteins and enhances the chemotactic response of vascular smooth muscle cells. J Biol Chem. 2008;283(35):24103–24117. doi: 10.1074/jbc.M709897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palamakumbura AH, Trackman PC. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal Biochem. 2002;300(2):245–251. doi: 10.1006/abio.2001.5464. [DOI] [PubMed] [Google Scholar]
- 18.Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci USA. 2009;106(45):19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36(2):178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 20.Voloshenyuk TG, Landesman ES, Khoutorova E, Hart AD, Gardner JD. Induction of cardiac fibroblast lysyl oxidase by TGF-beta1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine. 2011;55(1):90–97. doi: 10.1016/j.cyto.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Voloshenyuk TG, Hart AD, Khoutorova E, Gardner JD. TNF-alpha increases cardiac fibroblast lysyl oxidase expression through TGF-beta and PI3Kinase signaling pathways. Biochem Biophys Res Commun. 2011;413(2):370–375. doi: 10.1016/j.bbrc.2011.08.109. [DOI] [PubMed] [Google Scholar]
- 22.Chu IM, Michalowski AM, Hoenerhoff M, Szauter KM, Luger D, Sato M, Flanders K, Oshima A, Csiszar K, Green JE (2011) GATA3 inhibits lysyl oxidase-mediated metastases of human basal triple-negative breast cancer cells. Oncogene. doi:10.1038/onc.2011.382 [DOI] [PMC free article] [PubMed]
- 23.Park HJ, Gusarova G, Wang Z, Carr JR, Li J, Kim KH, Qiu J, Park YD, Williamson PR, Hay N, Tyner AL, Lau LF, Costa RH, Raychaudhuri P. Deregulation of FoxM1b leads to tumour metastasis. EMBO Mol Med. 2011;3(1):21–34. doi: 10.1002/emmm.201000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105(17):6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fogelgren B, Polgar N, Szauter KM, Ujfaludi Z, Laczko R, Fong KS, Csiszar K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem. 2005;280(26):24690–24697. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- 26.Huang G, Zhang Y, Kim B, Ge G, Annis DS, Mosher DF, Greenspan DS. Fibronectin binds and enhances the activity of bone morphogenetic protein 1. J Biol Chem. 2009;284(38):25879–25888. doi: 10.1074/jbc.M109.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278(16):14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- 28.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106(19):2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 29.Maki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167(4):927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, Keller P, Word RA. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol. 2007;170(2):578–589. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molnar J, Fong KS, He QP, Hayashi K, Kim Y, Fong SF, Fogelgren B, Szauter KM, Mink M, Csiszar K. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta. 2003;1647(1–2):220–224. doi: 10.1016/s1570-9639(03)00053-0. [DOI] [PubMed] [Google Scholar]
- 32.Lugassy J, Zaffryar-Eilot S, Soueid S, Mordoviz A, Smith V, Kessler O, Neufeld G (2011) The enzymatic activity of lysyl oxidase like-2 (LOXL2) is not required for LOXL2 induced inhibition of keratinocyte differentiation. J Biol Chem. doi:10.1074/jbc.M111.261016 [DOI] [PMC free article] [PubMed]
- 33.Hollosi P, Yakushiji JK, Fong KS, Csiszar K, Fong SF. Lysyl oxidase-like 2 promotes migration in noninvasive breast cancer cells but not in normal breast epithelial cells. Int J Cancer. 2009;125(2):318–327. doi: 10.1002/ijc.24308. [DOI] [PubMed] [Google Scholar]
- 34.Laczko R, Szauter KM, Jansen MK, Hollosi P, Muranyi M, Molnar J, Fong KS, Hinek A, Csiszar K. Active lysyl oxidase (LOX) correlates with focal adhesion kinase (FAK)/paxillin activation and migration in invasive astrocytes. Neuropathol Appl Neurobiol. 2007;33(6):631–643. doi: 10.1111/j.1365-2990.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 35.Polgar N, Fogelgren B, Shipley JM, Csiszar K. Lysyl oxidase interacts with hormone placental lactogen and synergistically promotes breast epithelial cell proliferation and migration. J Biol Chem. 2007;282(5):3262–3272. doi: 10.1074/jbc.M609407200. [DOI] [PubMed] [Google Scholar]
- 36.Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SF, Csiszar K, Hendrix MJ, Kirschmann DA. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res. 2005;65(24):11429–11436. doi: 10.1158/0008-5472.CAN-05-1274. [DOI] [PubMed] [Google Scholar]
- 37.Nelson JM, Diegelmann RF, Cohen IK. Effect of beta-aminopropionitrile and ascorbate on fibroblast migration. Proc Soc Exp Biol Med. 1988;188(3):346–352. doi: 10.3181/00379727-188-42745. [DOI] [PubMed] [Google Scholar]
- 38.Peinado H, Iglesias-de DC, Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24(19):3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor MA, Amin JD, Kirschmann DA, Schiemann WP. Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-beta signaling in breast cancer cells. Neoplasia. 2011;13(5):406–418. doi: 10.1593/neo.101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno-Bueno G, Salvador F, Martin A, Floristan A, Cuevas EP, Santos V, Montes A, Morales S, Castilla MA, Rojo-Sebastian A, Martinez A, Hardisson D, Csiszar K, Portillo F, Peinado H, Palacios J, Cano A (2011) Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med [DOI] [PMC free article] [PubMed]
- 41.Schietke R, Warnecke C, Wacker I, Schodel J, Mole DR, Campean V, Amann K, Goppelt-Struebe M, Behrens J, Eckardt KU, Wiesener MS. The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: insights into cellular transformation processes mediated by HIF-1. J Biol Chem. 2010;285(9):6658–6669. doi: 10.1074/jbc.M109.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bignon M, Pichol-Thievend C, Hardouin J, Malbouyres M, Brechot N, Nasciutti L, Barret A, Teillon J, Guillon E, Etienne E, Caron M, Joubert-Caron R, Monnot C, Ruggiero F, Muller L, Germain S (2011) Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood. doi:10.1182/blood-2010-10-313296 [DOI] [PubMed]
- 44.Prohaska JR. Genetic diseases of copper metabolism. Clin Physiol Biochem. 1986;4(1):87–93. [PubMed] [Google Scholar]
- 45.Kemppainen R, Palatsi R, Kallioinen M, Oikarinen A. A homozygous nonsense mutation and a combination of two mutations of the Wilson disease gene in patients with different lysyl oxidase activities in cultured fibroblasts. J Invest Dermatol. 1997;108(1):35–39. doi: 10.1111/1523-1747.ep12285622. [DOI] [PubMed] [Google Scholar]
- 46.Gacheru S, McGee C, Uriu-Hare JY, Kosonen T, Packman S, Tinker D, Krawetz SA, Reiser K, Keen CL, Rucker RB. Expression and accumulation of lysyl oxidase, elastin, and type I procollagen in human Menkes and mottled mouse fibroblasts. Arch Biochem Biophys. 1993;301(2):325–329. doi: 10.1006/abbi.1993.1151. [DOI] [PubMed] [Google Scholar]
- 47.Khakoo A, Thomas R, Trompeter R, Duffy P, Price R, Pope FM. Congenital cutis laxa and lysyl oxidase deficiency. Clin Genet. 1997;51(2):109–114. doi: 10.1111/j.1399-0004.1997.tb02430.x. [DOI] [PubMed] [Google Scholar]
- 48.Sibon I, Sommer P, Lamaziere JM, Bonnet J. Lysyl oxidase deficiency: a new cause of human arterial dissection. Heart. 2005;91(5):e33. doi: 10.1136/hrt.2004.053074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chanoki M, Ishii M, Kobayashi H, Fushida H, Yashiro N, Hamada T, Ooshima A. Increased expression of lysyl oxidase in skin with scleroderma. Br J Dermatol. 1995;133(5):710–715. doi: 10.1111/j.1365-2133.1995.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 50.Murawaki Y, Kusakabe Y, Hirayama C. Serum lysyl oxidase activity in chronic liver disease in comparison with serum levels of prolyl hydroxylase and laminin. Hepatology. 1991;14(6):1167–1173. [PubMed] [Google Scholar]
- 51.Akagawa H, Narita A, Yamada H, Tajima A, Krischek B, Kasuya H, Hori T, Kubota M, Saeki N, Hata A, Mizutani T, Inoue I. Systematic screening of lysyl oxidase-like (LOXL) family genes demonstrates that LOXL2 is a susceptibility gene to intracranial aneurysms. Hum Genet. 2007;121(3–4):377–387. doi: 10.1007/s00439-007-0333-3. [DOI] [PubMed] [Google Scholar]
- 52.Cenizo V, Andre V, Reymermier C, Sommer P, Damour O, Perrier E. LOXL as a target to increase the elastin content in adult skin: a dill extract induces the LOXL gene expression. Exp Dermatol. 2006;15(8):574–581. doi: 10.1111/j.1600-0625.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 53.Hajnal A, Klemenz R, Schafer R. Up-regulation of lysyl oxidase in spontaneous revertants of H-ras-transformed rat fibroblasts. Cancer Res. 1993;53(19):4670–4675. [PubMed] [Google Scholar]
- 54.Rost T, Pyritz V, Rathcke IO, Gorogh T, Dunne AA, Werner JA. Reduction of LOX- and LOXL2-mRNA expression in head and neck squamous cell carcinomas. Anticancer Res. 2003;23(2B):1565–1573. [PubMed] [Google Scholar]
- 55.Kaneda A, Wakazono K, Tsukamoto T, Watanabe N, Yagi Y, Tatematsu M, Kaminishi M, Sugimura T, Ushijima T. Lysyl oxidase is a tumor suppressor gene inactivated by methylation and loss of heterozygosity in human gastric cancers. Cancer Res. 2004;64(18):6410–6415. doi: 10.1158/0008-5472.CAN-04-1543. [DOI] [PubMed] [Google Scholar]
- 56.Wu G, Guo Z, Chang X, Kim MS, Nagpal JK, Liu J, Maki JM, Kivirikko KI, Ethier SP, Trink B, Sidransky D. LOXL1 and LOXL4 are epigenetically silenced and can inhibit ras/extracellular signal-regulated kinase signaling pathway in human bladder cancer. Cancer Res. 2007;67(9):4123–4129. doi: 10.1158/0008-5472.CAN-07-0012. [DOI] [PubMed] [Google Scholar]
- 57.Sato S, Trackman PC, Maki JM, Myllyharju J, Kirsch KH, Sonenshein GE. The Ras signaling inhibitor LOX-PP interacts with Hsp70 and c-Raf to reduce Erk activation and transformed phenotype of breast cancer cells. Mol Cell Biol. 2011;31(13):2683–2695. doi: 10.1128/MCB.01148-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu M, Min C, Wang X, Yu Z, Kirsch KH, Trackman PC, Sonenshein GE. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67(13):6278–6285. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
- 59.Mbeunkui F, Metge BJ, Shevde LA, Pannell LK. Identification of differentially secreted biomarkers using LC-MS/MS in isogenic cell lines representing a progression of breast cancer. J Proteome Res. 2007;6(8):2993–3002. doi: 10.1021/pr060629m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helleman J, Jansen MP, Ruigrok-Ritstier K, Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn JG, Sleijfer S, Foekens JA, Berns EM. Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response. Clin Cancer Res. 2008;14(17):5555–5564. doi: 10.1158/1078-0432.CCR-08-0555. [DOI] [PubMed] [Google Scholar]
- 61.Le QT, Harris J, Magliocco AM, Kong CS, Diaz R, Shin B, Cao H, Trotti A, Erler JT, Chung CH, Dicker A, Pajak TF, Giaccia AJ, Ang KK. Validation of lysyl oxidase as a prognostic marker for metastasis and survival in head and neck squamous cell carcinoma: Radiation Therapy Oncology Group trial 90–03. J Clin Oncol. 2009;27(26):4281–4286. doi: 10.1200/JCO.2008.20.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart GD, Gray K, Pennington CJ, Edwards DR, Riddick AC, Ross JA, Habib FK. Analysis of hypoxia-associated gene expression in prostate cancer: lysyl oxidase and glucose transporter-1 expression correlate with Gleason score. Oncol Rep. 2008;20(6):1561–1567. [PubMed] [Google Scholar]
- 63.Woznick AR, Braddock AL, Dulai M, Seymour ML, Callahan RE, Welsh RJ, Chmielewski GW, Zelenock GB, Shanley CJ. Lysyl oxidase expression in bronchogenic carcinoma. Am J Surg. 2005;189(3):297–301. doi: 10.1016/j.amjsurg.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 64.Gao Y, Ge G, Ji H. LKB1 in lung cancerigenesis: a serine/threonine kinase as tumor suppressor. Protein Cell. 2011;2(2):99–107. doi: 10.1007/s13238-011-1021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scola N, Gorogh T. LOXL4 as a selective molecular marker in primary and metastatic head/neck carcinoma. Anticancer Res. 2010;30(11):4567–4571. [PubMed] [Google Scholar]
- 66.Kim Y, Roh S, Park JY, Kim Y, Cho DH, Kim JC. Differential expression of the LOX family genes in human colorectal adenocarcinomas. Oncol Rep. 2009;22(4):799–804. doi: 10.3892/or_00000502. [DOI] [PubMed] [Google Scholar]
- 67.Fong SF, Dietzsch E, Fong KS, Hollosi P, Asuncion L, He Q, Parker MI, Csiszar K. Lysyl oxidase-like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosomes Cancer. 2007;46(7):644–655. doi: 10.1002/gcc.20444. [DOI] [PubMed] [Google Scholar]
- 68.Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, Diego JI, Nistal M, Quintanilla M, Portillo F, Cano A. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68(12):4541–4550. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- 69.Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z, Sun YM, Sun LC, Pan J, Sun LX, Zhao P, Yang ZH. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30(10):1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- 70.Ruckert F, Joensson P, Saeger HD, Grutzmann R, Pilarsky C. Functional analysis of LOXL2 in pancreatic carcinoma. Int J Colorectal Dis. 2010;25(3):303–311. doi: 10.1007/s00384-009-0853-5. [DOI] [PubMed] [Google Scholar]
- 71.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17(1):71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, Wang Y, Kristensen GB, Helland A, Borresen-Dale AL, Giaccia A, Longaker MT, Hastie T, Yang GP, Vijver MJ, Brown PO. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3(3):e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJ. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62(15):4478–4483. [PubMed] [Google Scholar]
- 75.Pez F, Dayan F, Durivault J, Kaniewski B, Aimond G, Provost GS, Deux B, Clezardin P, Sommer P, Pouyssegur J, Reynaud C. The HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway in a positive regulation loop and synergizes with HIF-1 in promoting tumor cell growth. Cancer Res. 2011;71(5):1647–1657. doi: 10.1158/0008-5472.CAN-10-1516. [DOI] [PubMed] [Google Scholar]
- 76.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10(7):505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 77.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6(5):485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 78.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10(1):201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolacna L, Bakesova J, Varga F, Kostakova E, Planka L, Necas A, Lukas D, Amler E, Pelouch V. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol Res. 2007;56(Suppl 1):S51–S60. doi: 10.33549/physiolres.931302. [DOI] [PubMed] [Google Scholar]
- 80.Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25(1):35–43. doi: 10.1007/s10555-006-7887-8. [DOI] [PubMed] [Google Scholar]
- 81.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, Neufeld G. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63(7):1657–1666. [PubMed] [Google Scholar]
- 83.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, Evans HR, Gartland A, Erler JT. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71(5):1561–1572. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO, Wirtz D, Semenza GL (2011) Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. doi:10.1073/pnas.1113483108 [DOI] [PMC free article] [PubMed]
- 87.Kirschmann DA, Seftor EA, Nieva DR, Mariano EA, Hendrix MJ. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Breast Cancer Res Treat. 1999;55(2):127–136. doi: 10.1023/a:1006188129423. [DOI] [PubMed] [Google Scholar]
- 88.Li W, Liu G, Chou IN, Kagan HM. Hydrogen peroxide-mediated, lysyl oxidase-dependent chemotaxis of vascular smooth muscle cells. J Cell Biochem. 2000;78(4):550–557. [PubMed] [Google Scholar]
- 89.Payne SL, Hendrix MJ, Kirschmann DA. Lysyl oxidase regulates actin filament formation through the p130(Cas)/Crk/DOCK180 signaling complex. J Cell Biochem. 2006;98(4):827–837. doi: 10.1002/jcb.20792. [DOI] [PubMed] [Google Scholar]
- 90.Levayer R, Lecuit T. Breaking down EMT. Nat Cell Biol. 2008;10(7):757–759. doi: 10.1038/ncb0708-757. [DOI] [PubMed] [Google Scholar]
- 91.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 92.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280(12):11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 94.Grego-Bessa J, Diez J, Timmerman L, Pompa JL. Notch and epithelial-mesenchyme transition in development and tumor progression: another turn of the screw. Cell Cycle. 2004;3(6):718–721. [PubMed] [Google Scholar]
- 95.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 97.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 98.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6(10):931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 99.Peinado H, Portillo F, Cano A. Switching on-off Snail: LOXL2 versus GSK3beta. Cell Cycle. 2005;4(12):1749–1752. doi: 10.4161/cc.4.12.2224. [DOI] [PubMed] [Google Scholar]
- 100.Sion AM, Figg WD. Lysyl oxidase (LOX) and hypoxia-induced metastases. Cancer Biol Ther. 2006;5(8):909–911. doi: 10.4161/cbt.5.8.3230. [DOI] [PubMed] [Google Scholar]
- 101.Atsawasuwan P, Mochida Y, Katafuchi M, Kaku M, Fong KS, Csiszar K, Yamauchi M. Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J Biol Chem. 2008;283(49):34229–34240. doi: 10.1074/jbc.M803142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim DJ, Lee DC, Yang SJ, Lee JJ, Bae EM, Kim DM, Min SH, Kim SJ, Kang DC, Sang BC, Myung PK, Park KC, Yeom YI. Lysyl oxidase like 4, a novel target gene of TGF-beta1 signaling, can negatively regulate TGF-beta1-induced cell motility in PLC/PRF/5 hepatoma cells. Biochem Biophys Res Commun. 2008;373(4):521–527. doi: 10.1016/j.bbrc.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 103.Bouez C, Reynaud C, Noblesse E, Thepot A, Gleyzal C, Kanitakis J, Perrier E, Damour O, Sommer P. The lysyl oxidase LOX is absent in basal and squamous cell carcinomas and its knockdown induces an invading phenotype in a skin equivalent model. Clin Cancer Res. 2006;12(5):1463–1469. doi: 10.1158/1078-0432.CCR-05-1456. [DOI] [PubMed] [Google Scholar]
- 104.Postovit LM, Abbott DE, Payne SL, Wheaton WW, Margaryan NV, Sullivan R, Jansen MK, Csiszar K, Hendrix MJ, Kirschmann DA. Hypoxia/reoxygenation: a dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem. 2008;103(5):1369–1378. doi: 10.1002/jcb.21517. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Y, Min C, Vora SR, Trackman PC, Sonenshein GE, Kirsch KH. The lysyl oxidase pro-peptide attenuates fibronectin-mediated activation of focal adhesion kinase and p130Cas in breast cancer cells. J Biol Chem. 2009;284(3):1385–1393. doi: 10.1074/jbc.M802612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Min C, Zhao Y, Romagnoli M, Trackman PC, Sonenshein GE, Kirsch KH. Lysyl oxidase propeptide sensitizes pancreatic and breast cancer cells to doxorubicin-induced apoptosis. J Cell Biochem. 2010;111(5):1160–1168. doi: 10.1002/jcb.22828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patani N, Jiang W, Newbold R, Mokbel K. Prognostic implications of carboxyl-terminus of Hsc70 interacting protein and lysyl-oxidase expression in human breast cancer. J Carcinog. 2010;9:9. doi: 10.4103/1477-3163.72505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Min C, Yu Z, Kirsch KH, Zhao Y, Vora SR, Trackman PC, Spicer DB, Rosenberg L, Palmer JR, Sonenshein GE. A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res. 2009;69(16):6685–6693. doi: 10.1158/0008-5472.CAN-08-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, Sonenshein GE. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res. 2007;67(3):1105–1112. doi: 10.1158/0008-5472.CAN-06-3867. [DOI] [PubMed] [Google Scholar]
- 110.Hamalainen ER, Kemppainen R, Kuivaniemi H, Tromp G, Vaheri A, Pihlajaniemi T, Kivirikko KI. Quantitative polymerase chain reaction of lysyl oxidase mRNA in malignantly transformed human cell lines demonstrates that their low lysyl oxidase activity is due to low quantities of its mRNA and low levels of transcription of the respective gene. J Biol Chem. 1995;270(37):21590–21593. doi: 10.1074/jbc.270.37.21590. [DOI] [PubMed] [Google Scholar]
- 111.Baker AM, Cox TR, Bird D, Lang G, Murray GI, Sun XF, Southall SM, Wilson JR, Erler JT. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J Natl Cancer Inst. 2011;103(5):407–424. doi: 10.1093/jnci/djq569. [DOI] [PubMed] [Google Scholar]
- 112.Wilgus ML, Borczuk AC, Stoopler M, Ginsburg M, Gorenstein L, Sonett JR, Powell CA (2010) Lysyl oxidase: A lung adenocarcinoma biomarker of invasion and survival. Cancer [DOI] [PubMed]
- 113.Albinger-Hegyi A, Stoeckli SJ, Schmid S, Storz M, Iotzova G, Probst-Hensch NM, Rehrauer H, Tinguely M, Moch H, Hegyi I. Lysyl oxidase expression is an independent marker of prognosis and a predictor of lymph node metastasis in oral and oropharyngeal squamous cell carcinoma (OSCC) Int J Cancer. 2010;126(11):2653–2662. doi: 10.1002/ijc.24948. [DOI] [PubMed] [Google Scholar]
- 114.Palamakumbura AH, Vora SR, Nugent MA, Kirsch KH, Sonenshein GE, Trackman PC. Lysyl oxidase propeptide inhibits prostate cancer cell growth by mechanisms that target FGF-2-cell binding and signaling. Oncogene. 2009;28(38):3390–3400. doi: 10.1038/onc.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stassar MJ, Devitt G, Brosius M, Rinnab L, Prang J, Schradin T, Simon J, Petersen S, Kopp-Schneider A, Zoller M. Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br J Cancer. 2001;85(9):1372–1382. doi: 10.1054/bjoc.2001.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, Padera RF, Bronson RT, Lindeman NI, Christiani DC, Lin X, Shapiro GI, Janne PA, Johnson BE, Meyerson M, Kwiatkowski DJ, Castrillon DH, Bardeesy N, Sharpless NE, Wong KK. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 117.Tsuchiya MI, Okuda H, Takaki Y, Baba M, Hirai S, Ohno S, Shuin T. Renal cell carcinoma- and pheochromocytoma-specific altered gene expression profiles in VHL mutant clones. Oncol Rep. 2005;13(6):1033–1041. [PubMed] [Google Scholar]
- 118.Bell A, Bell D, Weber RS, El-Naggar AK. CpG island methylation profiling in human salivary gland adenoid cystic carcinoma. Cancer. 2011;117(13):2898–2909. doi: 10.1002/cncr.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brekhman V, Lugassie J, Zaffryar-Eilot S, Sabo E, Kessler O, Smith V, Golding H, Neufeld G. Receptor activity modifying protein-3 mediates the protumorigenic activity of lysyl oxidase-like protein-2. FASEB J. 2010;25(1):55–65. doi: 10.1096/fj.10-162677. [DOI] [PubMed] [Google Scholar]
- 120.Weise JB, Csiszar K, Gottschlich S, Hoffmann M, Schmidt A, Weingartz U, Adamzik I, Heiser A, Kabelitz D, Ambrosch P, Gorogh T. Vaccination strategy to target lysyl oxidase-like 4 in dendritic cell based immunotherapy for head and neck cancer. Int J Oncol. 2008;32(2):317–322. [PubMed] [Google Scholar]
- 121.Holtmeier C, Gorogh T, Beier U, Meyer J, Hoffmann M, Gottschlich S, Heidorn K, Ambrosch P, Maune S. Overexpression of a novel lysyl oxidase-like gene in human head and neck squamous cell carcinomas. Anticancer Res. 2003;23(3B):2585–2591. [PubMed] [Google Scholar]
- 122.Sebban S, Davidson B, Reich R. Lysyl oxidase-like 4 is alternatively spliced in an anatomic site-specific manner in tumors involving the serosal cavities. Virchows Arch. 2009;454(1):71–79. doi: 10.1007/s00428-008-0694-6. [DOI] [PubMed] [Google Scholar]