Abstract
A rapid progress has been made in the field of lymphatic research during the last 15 years. This includes better understanding of the cellular events and molecular players involved in the lymphatic vessel formation and remodeling in development. The key players identified in developmental lymphangiogenesis, including vascular endothelial cell growth factor-C (VEGF-C) / VEGFR-3 and angiopoietins (ANGPTs)/ TIE pathways, are also crucial for pathological lymphatic vessel growth. In solid tumor, tumor cells as well as tumor-associated stromal cells, such as tumor-infiltrating leukocytes, contribute to intra- and peri-tumoral lymphangiogenesis via secreting lymphangiogenic growth factors. Tumor-associated lymphatic endothelial cells also interact actively with tumor cells and leukocytes via secreting various chemokines. It has been well established that tumor lymphangiogenesis promotes tumor cell dissemination to regional lymph nodes. Thus manipulation of lymphangiogenic microenvironment could become another valuable approach in the combat of tumor progression.
Keywords: Lymphatic endothelial cell, Lymphangiogenesis, Tumor microenvironment, Tumor metastasis
Lymphatic system plays important roles in the maintenance of tissue homeostasis by regulating fluid balance, cell metabolism and immune function. To fulfill their functions, initial lymphatics have to acquire some specific structural characteristics, including overlapping lymphatic endothelial junctions and discontinuous basement membrane as well as lack of mural cells, and collecting lymphatics contain valves and smooth muscle cells (SMC) for propelling lymph (Wang and Oliver 2010; Norrmen et al. 2011). These features facilitate the transportation of fluid and macromolecules, and also the leukocyte trafficking between tissues and blood circulation during immune-surveillance (Kim et al. 2012), and may also be employed by tumor cells during their metastatic spread to regional lymph nodes and distant organs (Alitalo 2011).
Mechanism of Lymphatic Vessel Formation and Remodeling
Initiation of Lymphatic Structure
During embryogenesis in mammals, lymphatic vessel development initiates with the specification of lymphatic endothelial cells (LEC) from a population of venous endothelial cells in the lateral parts of the anterior cardinal veins. This is followed by the formation of the primary lymphatic structure, lymph sac. It then undergoes expansion into the lymphatic plexus by the process of lymphangiogenesis. The primary lymphatic network is further remodeled into a mature lymphatic system composed of lymphatic capillaries and collecting lymphatics (Fig. 1). The molecular and cellular mechanisms are emerging, resulting from the great effort invested in the field over the last two decades. A number of key factors have been identified to participate in the process, including lymphangiogenic growth factors and receptors, extracellular matrix proteins and cell junction molecules, intracellular signal mediators and transcription factors.
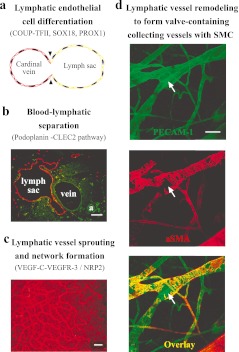
Fig. 1.
Key events and molecular regulators during lymphatic network formation and remodeling. a. A schematic illustration to show the differentiation of LECs from the cardinal vein and the formation of lymph sac. b. Sections from mouse embryos (E13.5) were immunostained with LYVE-1 (red, lymph sac and sprouting lymphatics) and PECAM-1 (green, blood vessels; a, artery), to show the separation of lymphatic-blood vascular circulation. c. Whole-mount immunostaining of mouse skin (E15.5) with LYVE-1 (red) to show the primary lymphatic network. Lymphatic vessels at this stage are still immature and do not have valves. d. Primary lymphatic network undergoes further remodeling to form collecting lymphatic vessels containing valves (white arrows) and SMCs (red), and factors indentified to participate in this process have been described in the text. Images shown in the figure are from mouse ear skin immunostained with PECAM-1 (green) and α-smooth muscle actin (αSMA, red) to visualize a collecting lymphatic vessel (blood vessels also stained green). Scale bar, 100 μm in b, 200 μm in c, 50 μm in d
The key transcription factors involved in the regulation of LEC differentiation include PROX1 (Prospero homeobox protein 1) (Wigle et al. 1999), SOX18 (sex determining region Y, SRY-box 18) (Francois et al. 2008) and COUP-TFII (Chicken ovalbumin upstream promoter transcription factor II) (Lin et al. 2010). Mice deficient of Prox1 failed to develop lymph sac due to the disruption of the LEC differentiation (Wigle and Oliver 1999; Wigle et al. 2002). Endothelial cell-specific overexpression of PROX1 in mice resulted in the embryonic lethality at E13.5 (embryonic day 13.5) due to the reprogramming of gene expression in blood vascular endothelial cells (BEC) to a LEC signature (Kim et al. 2010a). This phenomenae has also been observed with in vitro cultured endothelial cells, where overexpression of PROX1 delivered by an adenoviral vector suppressed the expression of a number of BEC-specific genes with the simultaneous induction of LEC-specific genes (Hong et al. 2002; Petrova et al. 2002). Intriguingly, it was found recently that exposure of lymphatic vessels to blood flow down-regulated LEC expression of PROX1 and converted lympatics to blood vessels (Chen et al. 2012). PROX1 expression is controlled by SOX18 (Francois et al. 2008). SOX18 is a member of SOX family of transcription factors defined by the presence of the highly conserved HMG box (a protein domain with a signature sequence RPMNAFMVW) responsible for DNA binding to the heptameric motif (A/T)(A/T)CAA(A/T)G (Wegner 1999; Bowles et al. 2000; Schepers et al. 2002). In vascular system, SOX18 can be detected in endothelial cells lining the dorso-lateral sector of the cardinal vein prior to PROX1 expression and the emergence of lymphatic vessels (Pennisi et al. 2000; Francois et al. 2008). Mice null for Sox18 or with the Ragged mutation displayed subcutaneous oedema and embryonic lethality due to the lack of lymphatic vessel development (Francois et al. 2008). Furthermore, COUP-TFII has also been shown to interact with PROX1 to maintain LEC phenotype (Srinivasan et al. 2010). COUP-TFII is an orphan nuclear receptor encoded by Nr2f2, and is necessary for the activation of PROX1 in embryonic veins by directly binding to a conserved DNA sequence in the regulatory region of PROX1. Deletion of Couptf2 at the early stage of embryonic development disrupts lymphatic vessel formation due to the failure of LEC differentiation (Lin, Chen et al. 2010).
LEC differentiation is followed by the formation of lymph sacs, which need to be separated from blood vessels. Platelets, small cell fragments without nuclei derived from megakaryocytes, play crucial roles in hemostasis through blood clot formation. In mice deficient of Meis1 (myeloid ecotropic viral integration site 1), the lymphatic-blood vessel separation was disrupted due to the lack of megakaryocytes (Carramolino et al. 2010). It has been revealed recently that the lymphatic-blood vessel separation involves a distinct pathway, Podoplanin-CLEC-2-mediated signaling, for platelet activation (Bertozzi et al. 2010; Suzuki-Inoue et al. 2010; Uhrin et al. 2010). CLEC-2, the c-type lectin receptor, is expressed by platelets. It has been demonstrated that deletion of Clec2 leads to embryonic or neonatal lethality, resulting from misconnections between blood and lymphatic vessels and also impairs thrombus formation (Suzuki-Inoue et al. 2010). Podoplanin, expressed on the surface of LECs, is an endogenous ligand for CLEC-2. Mice deficient in Pdpn (podoplanin) showed the abnormal connection between blood and lymphatic vessels (Uhrin et al. 2010). In addition, Podoplanin is a highly o-glycosylated protein. Mice null for C1galt1 gene, which lacked T-synthase required for the biosynthesis of O-glycans, displayed defects in the lymphatic-blood vessel separation, a phenotype similar to that of Pdpn knockout mice (Fu et al. 2008). The capacity of podoplanin to induce platelet activation was further demonstrated in a transgenic model where overexpression of a soluble podoplanin-Fc fusion protein under the K14 promoter led to the formation of microthrombi in various organs (Cueni et al. 2010). The hematopoietic signaling proteins SYK (spleen tyrosine kinase) and SLP76 (Src-homology 2 domain-containing leukocyte protein of 76 kDa) have been discovered as downstream mediators of Podoplanin-CLEC2 pathway, and the phenotype of mice deficiency of Syk or Slp76 resemble that of mice with Clec2 or Pdpn deletion (Abtahian et al. 2003). Furthermore, phospholipase Cγ2 (PLCγ2) acts downstream of SYK and SLP76. Mice with a loss of function point mutation in exon 2 of Plcg2 were found to develop the lymphatic-blood vessel shunts (Ichise, Ichise et al. 2009). Interestingly, mice null for Fiaf (fasting-induced adipose factor) or Spred-1/Spred-2 (encoding Spred/Sprouty family proteins) also displayed abnormal partition of lymphatic and blood vessels. The mechanism underlying the biological functions of FIAF and Spreads in the lymphatic-blood vessel separation is still to be elucidated (Backhed et al. 2007; Taniguchi et al. 2007).
Expansion of Lymphatic Vessels
Among the factors responsible for the regulation of lymphatic vessel development, vascular endothelial growth factor-C (VEGF-C) / VEGFR-3-mediated signaling serves as a key pathway. In addition to the secretion of VEGF-C from mesenchymal cells, blood vascular cells including vascular SMCs and BECs also contribute to VEGF-C expression (Hong et al. 2002; Karkkainen et al. 2004). Although both VEGF-C and VEGF-D can bind to and activate VEGFR-3, only VEGF-C is critically required for lymphangiogenesis during embryonic development. Mice null for Vegfc died before birth due to the failure of lymphatic network formation (Karkkainen et al. 2004). Heterozygous Vegfc knockout mice also developed chylous fluid in the abdomen after birth, indicating haploinsufficiency of VEGF-C for lymphangiogenesis (Karkkainen et al. 2004). However, deletion of Vegfd did not affect lymphatic vessel development (Baldwin et al. 2005).
VEGFR-3, also known as Fms-related tyrosine kinase (FLT4) (Pajusola et al. 1992), mediates a critical pathway for lymphatic vessel growth and maintenance (Karkkainen et al. 2000; Karkkainen et al. 2001; Makinen et al. 2001; Zhang, Zhou et al. 2010). It is also crucial for pathological lymphangiogenesis, such as in tumor (He et al. 2004a; He et al. 2005). While VEGFR-3 is initially expressed by all the endothelial cells of primary vascular network in mice during the embryonic development, its expression is primarily in LECs at the later stage of embryogenesis and persists in adult lymphatic vessels (Kaipainen et al. 1995; Zhang, Zhou et al. 2010). It has been found that TBX-1 activates VEGFR-3 transcription in endothelial cells (ECs) by binding to an enhancer element in the Vegfr3 gene (Chen et al. 2010). Mice null for Vegfr3 died at midgestation resulting from the blood vascular defects (Dumont et al. 1998; Hamada et al. 2000). To further investigate biological functions of VEGFR-3, we generated a conditional knockout mouse model targeting its ligand binding domain (LBD) (Zhang, Zhou et al. 2010). Mice homozygous for the LBD deletion (Vegfr3∆LBD/∆LBD) showed only disruption of lymphatic vessel growth, but blood vascular development in both embryo and yolk sac was not affected. Consistently, similar phenotypes were also observed in an independent mutant mouse model in which the kinase activity of VEGFR-3 was ablated by a missense mutation in the tyrosine kinase catalytic domain. Blood vascular development proceeded normally while lymphangiogenesis was disrupted in Vegfr3TKmut/TKmut mice (Zhang, Zhou et al. 2010). Interestingly, in Vegfr3∆LBD/∆LBD mice, lymph sac formation occurred but without lymphatic vessel sprouting due to the loss of ligand sensing ability by LEC expressing the truncated VEGFR-3 (VEGFR-3∆LBD). It is possible that VEGFR-3∆LBD could still be activated and transduces signals for LEC proliferation and survival via VEGFR-3 interacting factors, which may include neuropilin-2 (NRP-2) (Karpanen et al. 2006; Xu et al. 2010), integrin β1 (Zhang et al. 2005), or VEGFR-2 (Nilsson et al. 2010). However, lymph sac was not observed in Vegfr3TKmut/TKmut mice, indicateing that the VEGFR-3 tyrosine kinase activity is indeed required for the lymph sac formation. Although extracellular matrix protein such as collagen-1, via the association with β1 integrin, has been shown to induce c-Src-mediated VEGFR-3 phosphorylation independent of its kinase activity in cultured endothelial cells, further investigation is warranted to demonstrate whether this occurs in vivo and what would be the biological significance of the event. Furthermore, inhibition of NRP-2, a co-receptor of VEGFR-3, using a function-blocking anti-NRP-2 antibody (Xu et al. 2010) or via genetic deletion of Nrp2, suppressed lymphangiogenesis, resulting in hypoplasia of lymphatic vessels (Yuan et al. 2002). In addition, several factors have also been found to modulate VEGFR-3-mediated signals and therefore participate in the regulation of lymphatic vessel development. Soluble VEGFR-2 (sVEGFR-2) has been identified as an endogenous lymphangiogenic inhibitor to suppress VEGFR-3-mediated signals by blocking VEGF-C function (Albuquerque et al. 2009). In contrast, CCBE1 (collagen and calcium-binding EGF domain-containing protein 1) is required for lymphangiogenesis by enhancing VEGF-C function (Hogan et al. 2009; Bos et al. 2011). Furthermore, SPREAD-1 and SPREAD-2 have been shown to suppress VEGFR-3-mediated ERK activation (Taniguchi et al. 2007). While TGF-β negatively regulates VEGFR-3 signaling (Clavin et al. 2008; Oka et al. 2008), ALK-1 mediated signals were found to be required for the lymphatic vessel remodeling at the early postnatal stage in mice (Niessen et al. 2010). RASA1 (RasGTPase-activating protein) was also shown to negatively regulate LEC proliferation via suppressing Ras signal transduction initiated by VEGFR-3 (Lapinski et al. 2012). DLL4 (delta-like 4)- and NOTCH-1-mediated signaling, a crucial pathway for the control of artery specification and angiogenic sprouting of blood vessels, was found to be essential for lymphatic vessel growth by regulating EphrinB2 expression and VFGFR-3-mediated signals (Geudens et al. 2010; Niessen et al. 2011). EphrinB2 was also reported to control VEGFR-3 internalization and its downstream signals (Wang et al. 2010).
Remodeling and Maturation of Lymphatic Network
Smooth Muscle Cell Recruitment with Collecting Lymphatics
In addition to the lymphatic capillary network and lymph nodes, a mature lympahtic system includes collecting lymphatics invested with SMCs for contraction to propel lymph and also valves to prevent lymph backflow. The mechanism underlying the remodeling process has attracted intensive studies during the past few years. ANGPT / TIE-2 signaling, a crucial pathway for blood vascular maturation, is also implicated in lymphatic vessel remodeling (Augustin et al. 2009). Defects with lymphatic vessel maturation have been observed in mice null for Angpt2 or Tie1 (our unpublished observation) (Gale et al. 2002; Shimoda et al. 2007; Dellinger et al. 2008). However, the underlying mechanism of ANGPT2 and TIE-1 in lymphatic vessel development is not fully understood. Although it was suggested that ANGPT2 might function via TIE-2, deletion of Tie2 in zebrafish did not affect vascular development due to the compensatory role of TIE-1 (Gjini et al. 2011). In mammals, the role of TIE-2, particularly the interaction of TIE-1 and TIE-2 in the process of lymphatic network formation and remodeling, warrants further investigation. Besides this pathway, a number of other factors have been shown to participate in the regulation of SMC recruitment with collecting lymphatic vessels, including FOXC2, EphrinB2, CLP24 (claudin-like protein of 24 kDa) and AKT1. There was an increase of SMC coverage with lymphatic capillaries in Foxc2 deficient mice (Petrova et al. 2004). Interestingly, it was found that FOXC2 and NFATc1 (nuclear factor of activated T cell) controlled SMC recruitment to the maturing lymphatic vessels via repressing ANGPT2 and PDGF-B expression (Petrova et al. 2004; Norrmen et al. 2009). EphrinB2 is expressed by collecting lymphatic endothelium, and mutant mice lacking its C-terminal PDZ interaction site were shown to have abnormal coverage of SMCs with capillary lymphatics (Makinen et al. 2005). Furthermore, mice null for Clp24 were shown to develop dilated collecting lymphatic vessels with increased SMC clusters (Saharinen et al. 2010). Inhibition of semaphorin-3A (SEMA3A)/ neuropilin-1 (NRP-1) was also shown to result in enhanced perivascular cell coverage with lymphatic vessels and abnormal valve morphology (Jurisic et al. 2012). It is unknown whether there is any direct connection among these distinct signaling pathways. The serine/threonine protein kinase AKT is a major signal transducer (Shiojima and Walsh 2006; Dummler and Hemmings 2007), and is downstream of various receptor protein kinases involved in the regulation of vascular remodeling and maturation, including TIE-2, VEGFR-3, PDGFR-β and EphB4 (Suri et al. 1996; Wang et al. 1998; Adams et al. 1999; Hellstrom et al. 1999; Kim et al. 2000; Papapetropoulos et al. 2000; Kumar et al. 2006; Zhou et al. 2010). We showed that there was insufficient coverage of collecting lymphatic vessels by SMCs in Akt1 deficient mice, but not in Akt2 or Akt3 knockouts (Zhou et al. 2010). It is therefore plausible that AKT1 may coordinate signals from the different pathways during lymphatic vessel remodeling and maturation.
Lymphatic Valve Development
Lymphatic valves are semilunar structures and the valve leaflet is composed of an extracellular matrix core covered by LECs on both sides (Bazigou et al. 2009). Recent studies using genetic mouse models have revealed that several genes play important roles in lymphatic valve development. Defects with lymphatic valve formation have been shown in mice upon suppression of calcineurin/NFATc1 signaling (Norrmen et al. 2009), or with genetic deletion / mutation of the following genes, including Foxc2 (Petrova et al. 2004), Ephrinb2 (Makinen et al. 2005), Itga9 and Fn-EIIIA (Bazigou et al. 2009), Pik3r1 (Mouta-Bellum et al. 2009) and Akt1 (Zhou et al. 2010). Mice deficient in Cx37 (connexin-37), a downstream target of FOXC2, were recently shown to display abnormal valve formation (Kanady et al. 2011; Sabine et al. 2012). GATA2, a zinc finger transcription factor, has also been implicated in lymphatic valve development via regulating the expression of PROX1, FOXC2 and NFATc1 (Kazenwadel et al. 2012). In spite of the new findings, a complete picture of the cellular and molecular cascades in this process is still missing. One needs to employ combined approaches, including genetic mouse models, in vitro 3D cell culture model and imaging technology, for elucidating the mechanism underlying lymphatic valve formation.
Lymphangiogenesis and Tumor Microenvironment
LEC-Leukocyte Interaction in Tumor Microenvironment
Tumor is a complex disease, which involves not only tumor cell per se but also various tumor-associated stromal cells. Tumor-associated LECs recruit leukocytes via secreting various chemokines. Tumor-infiltrating leukocytes can contribute to tumor progression via secreting lymphangoigenic and angiogenic factors to modulate tumor-associated vascular growth (Mantovani et al. 2008; Alitalo 2011; Hanahan and Weinberg 2011). Therefore, there is an active interaction between LECs and immune cells, which forms an important basis for establishing a tumor microenvironment.
Lymphatic Function in Leukocyte Recruitment
LECs secrete a number of chemokines that regulate leukocyte trafficking, including CCL21 (C-C motif ligand 21, also known as secondary lymphoid-tissue chemokine, SLC) and CXCL12. CCL21 is a ligand of CCR7 (also named CD197) expressed by dendritic cells, T and B cells in lymphoid tissues (Hedrick and Zlotnik 1997; Hromas et al. 1997; Nagira et al. 1997). CCL21 expression in LECs was shown to be upregulated by VEGF-C or enhanced fluid flow (Issa et al. 2009; Miteva et al. 2010). Genetic studies showed that the migration of lymphocytes and dendritic cells (DCs) to lymph nodes was defective in mice lack of CCR7 or its ligands (CCL21 and CCL19) (Forster et al. 1999; Luther et al. 2000). CXCL12 is another chemokine expressed by LECs, and its expression can be upregulated by hypoxia (Irigoyen et al. 2007). CXCL12 acts via its receptor CXCR4 for the lymphatic trans-migration of dendritic cells, as evidenced by the partial inhibition of cutaneous DCs to lymph nodes by the CXCR4 antagonist (4-F-benzoyl-TN14003) (Kabashima et al. 2007). LECs also secrete sphingosine-1-phosphate (S1P), a bioactive lipid synthesized by sphingosine kinases, to guide the migration of lymphocytes expressing S1P receptor S1P1 (also named EDG-1) (Schwab and Cyster 2007; Ledgerwood et al. 2008). Interestingly, it has been found that LECs constitutively express NF-κB (Saban et al. 2004), which can be activated by lipopolysaccharides binding to Toll-like receptor 4 expressed in LECs (Kang et al. 2009). Activation of NF-κB pathway up-regulated LEC expression of PROX1 and VEGFR-3, as well as the secretion of several chemoattractants such as CCL2, CCL5 and CX3CL1 from LECs (Flister et al. 2010). This led to an increase in lymphatic vessel growth in inflammatory sites, which could promote leukocyte mobilization, such as dendritic cells and macrophages (Kataru et al. 2009).
In addition, leukocyte trans-lymphatic migration is a dynamically regulated event, involving various junctional adhesion molecules (Johnson and Jackson 2008). LECs express several adhesion molecules, which are the same as those used by leukocyte diapedesis across blood vascular endothelium. Although undetectable or lowly expressed in quiescent LECs, both ICAM-1 and VCAM-1 were markedly upregulated in response to TNFα treatment (Johnson et al. 2006). Adhesion of dendritic cells to activated LECs and trans-lymphatic migration were suppressed by neutralizing antibodies against ICAM-1 or VCAM-1 (Johnson et al. 2006). E-selectin (also named CD62E or ELAM-1, endothelial leukocyte adhesion molecule-1), could also be rapidly upregulated by pro-inflammatory cytokines in LECs (Wyble et al. 1997). E-selectin mediates a reversible tethering of leukocytes to vascular endothelial cells via interaction with PSGL-1 (P-selectin glycoprotein ligand-1) for the initial capture. This interaction can be converted to a tighter adhesion via VCAM-1 and ICAM-1 to facilitate leukocyte transmigration (Hidalgo et al. 2007). Furthermore, LECs can express some specific receptors mediating leukocyte migration, including CLEVER-1 (Prevo et al. 2004; Salmi et al. 2004), mannose receptor (Irjala et al. 2001), Podoplanin (Kerjaschki et al. 2004), and LYVE-1 (Baluk et al. 2007).
Leukocyte Contribution to Lymphangiogenic Vessel Growth
It has been well recognized that there is active lymphangiogenesis at sites of tissue inflammation (Baluk et al. 2005). Hematopoietic cells can be recruited to most tumors, and macrophages are a major component of infiltrating leukocytes in tumor mass (Pollard 2004). Macrophages facilitate tumor progression via the remodeling of intratumor extracellular matrix and induction of intratumor angiogenesis and lymphangiogenesis (Kerjaschki 2005; Condeelis and Pollard 2006). It has been found that macrophages and also granulocytes produce high amount of VEGF-C and VEGF-D (Baluk, Tammela et al. 2005; Kataru et al. 2009). VEGF-C expression can be induced by pro-inflammatory cytokines such as TNFα via the activation of NF-κB pathway (Ristimaki et al. 1998; Baluk et al. 2009). Blockage of VEGFR-1+ macrophage recruitment or inhibition of cyclooxygenase-2 (Cox-2) activity was shown to reduce the level of VEGF-C expression in tumor and therefore suppressed lymph node metastasis (Fischer et al. 2007; Iwata et al. 2007). In addition to the crucial role of VEGFR-3-mediated pathway, integrin α5β1-mediated signaling has been shown to actively participate in inflammatory lymphangiogenesis (Okazaki et al. 2009). There is also evidence showing that a proportion of macrophages could trans-differentiate into LECs in inflamed cornea (Maruyama et al. 2005). In addition, T and B lymphocytes have also been shown to secrete lymphangiogenic regulators, and this is to be discussed in the next section.
LEC-Tumor Interaction in Lymphatic Metastasis
Distinct Features of Tumor-Associated Lymphangiogenesis
Firstly, although the molecular cascade responsible for lymphatic vessel growth is similar in development and tumor, there are distinct characteristics of tumor-associated lymphangiogenesis. During embryogenesis, mesenchymal cells and blood vascular cells secrete lymphangiogenic factors including VEGF-C to guide lymphatic vessel formation in a highly organized manner. In the case of solid tumor, in addition to BECs and SMCs, tumor cells and tumor-infiltrating leukocytes as well as other tumor-associated stromal cells could secrete lymphangiogenic factors (Alitalo 2011). Therefore, there is no proper formation of lymphangiogenic growth factor gradient in tumor, and tumor-associated lymphatic vessels are disorganized without a hierarchical vascular pattern (He et al. 2005). Secondly, tumor-associated lymphatics are patchy and not homogenously distributed within tumors. Lymphatic vessel growth often occurs in necrotic areas or tumor peripheral regions (Karpanen et al. 2001; Beasley et al. 2002; He et al. 2002). Although most malignant solid tumors express lymphangiogenesis factors including VEGF-C and VEGF-D, only several types of human cancer have been shown to contain lymphatic vessels, including squamous cell carcinomas of the head and neck (HNSCC) (Beasley et al. 2002), primary melanomas (Oliver and Detmar 2002; Straume et al. 2003), and breast cancer (Schoppmann et al. 2001), renal cell carcinoma (Horiguchi et al. 2008), gastric cancer (Lee et al. 2010). Also tumor-associated lymphangiogenesis occurs later than angiogenesis, at least one week behind as shown in animal tumor models (He et al. 2005). The later occurrence of lymphangiogenesis in comparison with angiogenesis was also observed in wound healing (Paavonen et al. 2000). These findings indicate that tumor lymphangiogenesis is regulated not only by lymphangiogenic growth signals, but also by tumor specific microenvironmental factors, which may include hydrostatic pressure due to leaky tumor vessels and mechanical stress exerted by the proliferating tumor cells (Fukumura et al. 2010; Alitalo 2011).
Furthermore, the origin of tumor lymphatic vessels has also attracted a lot of attention. There is evidence showing that bone-marrow (BM) derived cells could participate in lymphangiogenic vessel growth after differentiation into LECs (Maruyama et al. 2005; Jiang et al. 2008; Zumsteg et al. 2009). In tumor-associated lymphangiogenesis, the contribution of BM-derived cells in lymphatic vessels was reported to be approximately 3 % (Jiang et al. 2008; Zumsteg et al. 2009). However, we showed previously that most tumor lymphatics were within 1 mm from tumor margin in an animal tumor model. Tumor-associated lymphatics formed mainly by direct vessel co-option, sprouting and/or splitting from preexisting lymphatic vessels in surrounding tissues (He et al. 2004b).
Finally it is still arguable whether intra-tumoral lymphatic vessels are functional. Tumor-associated lymphatic vessels are only present in some regions (He et al. 2002), and therefore the traditional lymphatic function assay by dye uptake may not be appropriate to address the problem (Padera et al. 2002). Fluid drainage via lymphatics is mainly controlled by interstitial pressure in physiological conditions (Schmid-Schonbein 1990; Swartz et al. 1999), and tumor cell could employ mechanism similar to that of leukocytes during metastatic dissemination (Kozaki et al. 2001). As tumor-associated lymphatic vessel growth is a crucial step during lymphatic metastasis (He et al. 2005), a proportion of intratumoral lymphatics, if not all, have to be functional (Alitalo 2011). Otherwise tumor cells would not have a route to enter into lymphatic vessels and further spread to lymph nodes.
Interaction of Tumor Cells and LECs During Tumor Invasion
To spread from primary tumors and establish metastases in lymph nodes, tumor cells have to detach from tumor mass, interact with LECs to enter lymphatic vessels, survive during the transit in lymphatic vessels, and escape from immune surveillance to form metastatic foci. In spite of the late occurrence of tumor-associated lymphangiogenesis in comparison with angiogenesis, tumor cell spread to regional lymph nodes occurs at early stages of tumor progression as shown in various types of solid tumors (Stacker et al. 2002). A number of factors contribute to the early lymphatic metastasis. First of all, tumor cell per se is one of the major resources of lymphangiogenic factors. LECs respond actively to tumor cells upon their release of lymphangiogenic factors (He et al. 2005). This leads to the activation of LECs in the surrounding tissues and induction of lymphatic vessel growth towards tumor, which facilitates tumor invasion into lymphatics (He et al. 2005). Tumor could also induce the enlargement of collecting lymphatic vessels, which facilitates the transit of tumor cells or emboli to lymph nodes (He et al. 2005). Inhibition of tumor lymphangiogenesis blocked tumor cell entry into lymphatic system and lymphatic metastasis to occur, but did not have any observable effect on tumor growth in lymph nodes (He et al. 2002; He et al. 2005). This further validates the active role of LECs in lymphatic tumor invasion (Fig. 2). However, it should be emphasized that although newly formed lymphatic vessels are necessary for lymphatic metastasis, lymphangiogenesis alone is not sufficient to promote this event (He et al. 2002).
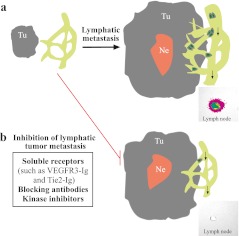
Fig. 2.
Mechanism of lymphatic metastasis and its inhibition. a. Tumor cells invade into the surrounding tissues and co-opt pre-existing lymphatic vessels. Two important events are required for lymphatic metastasis, which are lymphatic vessel sprouting and dilation of collecting vessel diameter. These events facilitates tumor invasion and spread via lymphatic vessels. Shown in panel a is a representative lymph node with metastatic tumor cells overexpressing luciferase, performed using the in vivo bioluminescence imaging system (Caliper Xenogen). b. Inhibition of lymphatic metastasis can be achieved by employing reagents such as soluble receptors against VEGFR-3 and TIE-2 signaling pathways. In the case of VEGFR-3 blockade, tumor lymphangiogenesis, including lymphatic vessel sprouting and vessel dilation, is inhibited. This results in the efficient suppression of tumor cell entry into lymphatic vessels. Interestingly, suppression of TIE-2-mediated pathway could also efficiently block lymphatic metastasis, but do not have appreciable effects on tumor-associated lymphangiogenesis. The mechanism of TIE-2-mediated signaling in tumor metastasis is still to be investigated. Tu, tumor; Ne, necrosis
Secondly, in comparison with the low survival rate of tumor cells in blood circulation, it seems that tumor cells are readily to survive and proliferate in lymph nodes or even in lymphatic vessels when the flow is blocked (Karpanen et al. 2001; He et al. 2005). Lymph node microenvironment may foster tumor metastasis after tumor cells escape immune surveillance, as observed in clinic long time ago (Willis 1973). Although the underlying mechanism is poorly understood, tumor cells become highly invasive, especially with tumor invasion and dissemination via lymphatic vessels, after in vivo selection followed by further propagation of tumor cells from lymph node metastasis (Kozaki et al. 2000). It seems that tumor cells could acquire some capacity of leukocytes for vascular invasion (Kraizer et al. 2001). Expression of CCR7 and CXCR4 has been demonstrated in human cancer cells, and LECs in metastasis-favorite organs express their ligands (CCL21 and CXCL12), which could attract metastatic tumor cells (Gunn et al. 1998; Muller et al. 2001; Kim et al. 2010b). Animal studies further demonstrated that overexpression of CCR7 by B16 murine melanoma cells enhanced the incidence of lymph node metastasis and this increase could be suppressed by treatment with neutralizing anti-CCL21 antibodies (Wiley et al. 2001). CCL21 secreted from lymphatics and also tumor cells has also been suggested to participate in the modulation of tumor microenvironment to be immunotolerant (Shields et al. 2010).
Another contributing factor for lymphatic metastasis is the increased lymphangiogenesis in sentinel lymph nodes draining a tumor (Hirakawa et al. 2005; Van den Eynden et al. 2007; Rinderknecht and Detmar 2008; Ruddell et al. 2008). Besides the lymphangiogenic factors transported with lymph from tumor, lymphocytes in lymph nodes also actively participate in the regulation of lymph node-associated lymphatic vessel growth. The follicular B cells could produce VEGF to stimulate sinusoidal lymphangiogenesis in lymph nodes that drain the inflamed tissues (Angeli et al. 2006; Shrestha et al. 2010). Lymphangiogenesis was decreased in B cell-deficient mice in the trachea of M. pulmonis infected animal model (Baluk, Tammela et al. 2005). Interestingly, T cells have been found to modulate lymphangiogenesis in a negative manner via secreting IFN-γ (Kataru et al. 2011). These findings indicate that multiple cells could contribute to the activation of LECs via secreting lymphangiogenic regulators, and actively participate in lymphatic tumor metastasis.
Targeting LECs to Suppress Tumor Metastasis
In spite of the different cellular sources, the key molecular players in tumor lymphangiogenesis are the same as those revealed from genetic studies. The VEGF-C / VEGFR-3 signaling pathway is essential for tumor-associated lymphangiogenesis, and lymphatic tumor metastasis can be efficiently suppressed when VEGFR-3 signaling pathway is blocked. This has been demonstrated in several studies by employing exogenous reagents, including the use of soluble VEGFR-3-Ig to trap its ligands (Karpanen et al. 2001; He et al. 2002; Krishnan et al. 2003), or the blocking antibodies against VEGFR-3 (Roberts et al. 2006; Tammela et al. 2008) or its co-receptor NRP-2 (Caunt et al. 2008) (Fig. 2). The crucial role of VEGFR-3-mediated signaling in tumor lymphangiogenesis was also demonstrated using a genetic mouse model with an inactivation point mutation in VEGFR-3 kinase domain (Vegfr3TKmut/WT) (He et al. 2004b; Zhang et al. 2010). There was no lymphatic vessel growth detected in tumors when they were implanted subcutaneously in Vegfr3TKmut/WT mice due to the dominant negative effect of the mutant VEGFR-3 (He et al. 2004b).
Furthermore, we have recently revealed an important role of ANGPT1/TIE-2 signaling pathway in lymphatic metastasis (Holopainen et al. 2009). The involvement of ANGPT1 in lymphatic vessel growth was first demonstrated in a genetic model (Gale et al. 2002). Mice deficient of Angpt2 displayed defects in lymphatic organization, and this could be rescued when a cDNA encoding ANGPT1 was placed in the Angpt2 locus (Gale et al. 2002; Dellinger et al. 2008). Consistently, recombinant ANGPT1 protein and adenoviral vector-mediated overexpression of ANGPT1 were shown to induce lymphatic vessel sprouting and hyperplasia (Morisada et al. 2005; Tammela et al. 2005). The lymphangiogenic effect of ANGPT1 was also shown in a transgenic mouse model, where overexpression of ANGPT1 or ANGPT2 in pancreatic β-cells was shown to induce peri-insular lymphangiogenesis (Fagiani et al. 2011). Both ANGPT1 and ANGPT2 bind to TIE-2, and TIE-2-mediated signaling is crucial for blood vascular maturation and the maintenance of its integrity (Augustin et al. 2009). We showed that tumor cells could secrete ANGPT1 and systemic treatment using ANGPT1 delivered via an adenoviral vector increased the rate of lymph node metastasis (Holopainen et al. 2009). Inhibition of TIE-2-mediated signaling via systemic delivery of soluble TIE-2 suppressed lymphatic and hematogenous tumor metastasis (Holopainen et al. 2009). However, tumor associated lymphangiogenesis was not affected by blocking TIE-2 signaling. This is different from that of VEGFR-3 signaling inhibition, where tumor-associated lymphangiogenesis could be suppressed (He et al. 2002; He et al. 2005). The detailed mechanism of ANGPT1/TIE-2 signaling in lymphatic metastasis is yet to be investigated. It is possible that activation of TIE-2 signaling pathway by ANGPT1 promotes the normalization of tumor-associated lymphatic vessels so that tumor cell could spread efficiently via lymphatics and establish metastatic foci in lymph nodes. Furthmore, ANGPT2 was also shown to promote tumor lymphangiogenesis and lymphatic metastasis (Schulz et al. 2011). ANGPT2-blocking antibodies were shown to suppress tumor lymphangiogenesis and lymph node metastasis (Holopainen et al. 2012). Interestingly, ANGPT2 was also shown to promote glioma cell invasion (Hu et al. 2003; Hu et al. 2006) and breast cancer metastasis (Imanishi et al. 2007; Imanishi et al. 2011) by up-regulation and activation of matrix metalloprotease 2 (MMP-2), which was mediated via αvβ1integrin pathway but independent of TIE-2 signaling.
To summarize, in addition to BECs, there is accumulating evidence pointing to LECs as another type of tumor-associated stromal cells for targeting. At least in animal tumor models, it is achievable to target the key molecular players for blocking lymphatic tumor metastasis. Translation of these findings into clinical applications requires more in-depth studies. One needs to develop methods for the early detection of tumor initiation before its spread, as inhibition of lymphangiogenesis may not have much benefit when tumor metastasis has already occurred. For preventative purpose, one also needs to have better understanding of the effect of lymphangiogenic inhibition on the physiological functions of lymphatic vessels.
Acknowledgements
The studies in my laboratory are currently supported by grants from the National Natural Science Foundation of China (31071263, 30771069, 30930028), the Ministry of Science and Technology of China (2012CB947600), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Abtahian F, Guerriero A, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299(5604):247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Wilkinson GA, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13(3):295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque RJ, Hayashi T, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15(9):1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17(11):1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- Angeli V, Ginhoux F, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Young Koh G, et al. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Backhed F, Crawford PA, et al. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc Natl Acad Sci USA. 2007;104(2):606–611. doi: 10.1073/pnas.0605957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin ME, Halford MM, et al. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol Cell Biol. 2005;25(6):2441–2449. doi: 10.1128/MCB.25.6.2441-2449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Tammela T, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115(2):247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204(10):2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Yao LC, et al. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119(10):2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Xie S, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17(2):175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley NJ, Prevo R, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62(5):1315–1320. [PubMed] [Google Scholar]
- Bertozzi CC, Schmaier AA, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116(4):661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos FL, Caunt M, et al. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ Res. 2011;109(5):486–491. doi: 10.1161/CIRCRESAHA.111.250738. [DOI] [PubMed] [Google Scholar]
- Bowles J, Schepers G, et al. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227(2):239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Carramolino L, Fuentes J, et al. Platelets play an essential role in separating the blood and lymphatic vasculatures during embryonic angiogenesis. Circ Res. 2010;106(7):1197–1201. doi: 10.1161/CIRCRESAHA.110.218073. [DOI] [PubMed] [Google Scholar]
- Caunt M, Mak J, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13(4):331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Chen L, Mupo A, et al. Tbx1 regulates Vegfr3 and is required for lymphatic vessel development. J Cell Biol. 2010;189(3):417–424. doi: 10.1083/jcb.200912037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Bertozzi C, et al. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest. 2012;122(6):2006–2017. doi: 10.1172/JCI57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavin NW, Avraham T, et al. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol. 2008;295(5):H2113–2127. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Cueni LN, Chen L, et al. Podoplanin-Fc reduces lymphatic vessel formation in vitro and in vivo and causes disseminated intravascular coagulation when transgenically expressed in the skin. Blood. 2010;116(20):4376–4384. doi: 10.1182/blood-2010-04-278564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger M, Hunter R, et al. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev Biol. 2008;319(2):309–320. doi: 10.1016/j.ydbio.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35(Pt 2):231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282(5390):946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- Fagiani E, Lorentz P, et al. Angiopoietin-1 and −2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer Res. 2011;71(17):5717–5727. doi: 10.1158/0008-5472.CAN-10-4635. [DOI] [PubMed] [Google Scholar]
- Fischer C, Jonckx B, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131(3):463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Flister MJ, Wilber A, et al. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood. 2010;115(2):418–429. doi: 10.1182/blood-2008-12-196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Schubel A, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99(1):23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Francois M, Caprini A, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456(7222):643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- Fu J, Gerhardt H, et al. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118(11):3725–3737. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D, Duda DG, et al. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17(3):206–225. doi: 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N, Thurston G, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3(3):411. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Geudens I, Herpers R, et al. Role of delta-like-4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler Thromb Vasc Biol. 2010;30(9):1695–1702. doi: 10.1161/ATVBAHA.110.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjini E, Hekking LH, et al. Zebrafish Tie-2 shares a redundant role with Tie-1 in heart development and regulates vessel integrity. Dis Model Mech. 2011;4(1):57–66. doi: 10.1242/dmm.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95(1):258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K, Oike Y, et al. VEGF-C signaling pathways through VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood. 2000;96(12):3793–3800. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- He Y, Kozaki K, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94(11):819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- He Y, Karpanen T, et al. Role of lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta. 2004;1654(1):3–12. doi: 10.1016/j.bbcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- He Y, Rajantie I, et al. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64(11):3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- He Y, Rajantie I, et al. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65(11):4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- Hedrick JA, Zlotnik A. Identification and characterization of a novel beta chemokine containing six conserved cysteines. J Immunol. 1997;159(4):1589–1593. [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, et al. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126(14):3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Peired AJ, et al. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26(4):477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Kodama S, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201(7):1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BM, Bos FL, et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41(4):396–398. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- Holopainen T, Huang H, et al. Angiopoietin-1 overexpression modulates vascular endothelium to facilitate tumor cell dissemination and metastasis establishment. Cancer Res. 2009;69(11):4656–4664. doi: 10.1158/0008-5472.CAN-08-4654. [DOI] [PubMed] [Google Scholar]
- Holopainen T, Saharinen P, et al. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst. 2012;104(6):461–475. doi: 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Harvey N, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225(3):351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Horiguchi A, Ito K, et al. Intratumoral lymphatics and lymphatic invasion are associated with tumor aggressiveness and poor prognosis in renal cell carcinoma. Urology. 2008;71(5):928–932. doi: 10.1016/j.urology.2007.11.076. [DOI] [PubMed] [Google Scholar]
- Hromas R, Kim CH, et al. Isolation and characterization of Exodus-2, a novel C-C chemokine with a unique 37-amino acid carboxyl-terminal extension. J Immunol. 1997;159(6):2554–2558. [PubMed] [Google Scholar]
- Hu B, Guo P, et al. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci USA. 2003;100(15):8904–8909. doi: 10.1073/pnas.1533394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Jarzynka MJ, et al. Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the alphavbeta1 integrin and focal adhesion kinase signaling pathway. Cancer Res. 2006;66(2):775–783. doi: 10.1158/0008-5472.CAN-05-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise H, Ichise T, et al. Phospholipase Cgamma2 is necessary for separation of blood and lymphatic vasculature in mice. Development. 2009;136(2):191–195. doi: 10.1242/dev.025353. [DOI] [PubMed] [Google Scholar]
- Imanishi Y, Hu B, et al. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007;67(9):4254–4263. doi: 10.1158/0008-5472.CAN-06-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi Y, Hu B, et al. Angiopoietin-2, an angiogenic regulator, promotes initial growth and survival of breast cancer metastases to the lung through the integrin-linked kinase (ILK)-AKT-B cell lymphoma 2 (Bcl-2) pathway. J Biol Chem. 2011;286(33):29249–29260. doi: 10.1074/jbc.M111.235689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irigoyen M, Anso E, et al. Hypoxia alters the adhesive properties of lymphatic endothelial cells. A transcriptional and functional study. Biochim Biophys Acta. 2007;1773(6):880–890. doi: 10.1016/j.bbamcr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Irjala H, Johansson EL, et al. Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium. J Exp Med. 2001;194(8):1033–1042. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa A, Le TX, et al. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009;69(1):349–357. doi: 10.1158/0008-5472.CAN-08-1875. [DOI] [PubMed] [Google Scholar]
- Iwata C, Kano MR, et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67(21):10181–10189. doi: 10.1158/0008-5472.CAN-07-2366. [DOI] [PubMed] [Google Scholar]
- Jiang S, Bailey AS, et al. Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One. 2008;3(11):e3812. doi: 10.1371/journal.pone.0003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Jackson DG. Cell traffic and the lymphatic endothelium. Ann N Y Acad Sci. 2008;1131:119–133. doi: 10.1196/annals.1413.011. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Clasper S, et al. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203(12):2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurisic G, Maby-El Hajjami H et al (2012) “An Unexpected Role of Semaphorin3A/Neuropilin-1 Signaling in Lymphatic Vessel Maturation and Valve Formation”. Circ Res 2012 Jun 20. doi:10.1161/CIRCRESAHA.112.269399 [DOI] [PMC free article] [PubMed]
- Kabashima K, Shiraishi N, et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol. 2007;171(4):1249–1257. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92(8):3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanady JD, Dellinger MT, et al. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol. 2011;354(2):253–266. doi: 10.1016/j.ydbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Lee SP, et al. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood. 2009;113(11):2605–2613. doi: 10.1182/blood-2008-07-166934. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Ferrell RE, et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet. 2000;25(2):153–159. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98(22):12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Egeblad M, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61(5):1786–1790. [PubMed] [Google Scholar]
- Karpanen T, Heckman CA, et al. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 2006;20(9):1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- Kataru RP, Jung K, et al. Critical role of CD11b + macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113(22):5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- Kataru RP, Kim H, et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34(1):96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Kazenwadel J, Secker GA, et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood. 2012;119(5):1283–1291. doi: 10.1182/blood-2011-08-374363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005;115(9):2316–2319. doi: 10.1172/JCI26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Regele HM, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15(3):603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim HG, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86(1):24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- Kim H, Nguyen VP, et al. Embryonic vascular endothelial cells are malleable to reprogramming via Prox1 to a lymphatic gene signature. BMC Dev Biol. 2010;10:72. doi: 10.1186/1471-213X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Koh YJ, et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010;70(24):10411–10421. doi: 10.1158/0008-5472.CAN-10-2591. [DOI] [PubMed] [Google Scholar]
- Kim H, Kataru RP, et al. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol. 2012;33(7):350–356. doi: 10.1016/j.it.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Kozaki K, Miyaishi O, et al. Establishment and characterization of a human lung cancer cell line NCI- H460-LNM35 with consistent lymphogenous metastasis via both subcutaneous and orthotopic propagation. Cancer Res. 2000;60(9):2535–2540. [PubMed] [Google Scholar]
- Kozaki K, Koshikawa K, et al. Multi-faceted analyses of a highly metastatic human lung cancer cell line NCI-H460-LNM35 suggest mimicry of inflammatory cells in metastasis. Oncogene. 2001;20(31):4228–4234. doi: 10.1038/sj.onc.1204561. [DOI] [PubMed] [Google Scholar]
- Kraizer Y, Mawasi N, et al. Vascular endothelial growth factor and angiopoietin in liver regeneration. Biochem Biophys Res Commun. 2001;287(1):209–215. doi: 10.1006/bbrc.2001.5548. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Kirkin V, et al. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63(3):713–722. [PubMed] [Google Scholar]
- Kumar SR, Singh J, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169(1):279–293. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapinski PE, Kwon S et al (2012) “RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice”. J Clin Invest 122(2):733–747 [DOI] [PMC free article] [PubMed]
- Ledgerwood LG, Lal G, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9(1):42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- Lee K, Park do J, et al. Increased intratumoral lymphatic vessel density correlates with lymph node metastasis in early gastric carcinoma. Ann Surg Oncol. 2010;17(1):73–80. doi: 10.1245/s10434-009-0707-y. [DOI] [PubMed] [Google Scholar]
- Lin FJ, Chen X, et al. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest. 2010;120(5):1694–1707. doi: 10.1172/JCI40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Tang HL, et al. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97(23):12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Jussila L, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7(2):199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Makinen T, Adams RH, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19(3):397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Ii M, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115(9):2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miteva DO, Rutkowski JM, et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106(5):920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisada T, Oike Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105(12):4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- Mouta-Bellum C, Kirov A, et al. Organ-specific lymphangiectasia, arrested lymphatic sprouting, and maturation defects resulting from gene-targeting of the PI3K regulatory isoforms p85alpha, p55alpha, and p50alpha. Dev Dyn. 2009;238(10):2670–2679. doi: 10.1002/dvdy.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Nagira M, Imai T, et al. Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J Biol Chem. 1997;272(31):19518–19524. doi: 10.1074/jbc.272.31.19518. [DOI] [PubMed] [Google Scholar]
- Niessen K, Zhang G, et al. ALK1 signaling regulates early postnatal lymphatic vessel development. Blood. 2010;115(8):1654–1661. doi: 10.1182/blood-2009-07-235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen K, Zhang G, et al. The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood. 2011;118(7):1989–1997. doi: 10.1182/blood-2010-11-319129. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Bahram F, et al. VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. 2010;29(8):1377–1388. doi: 10.1038/emboj.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmen C, Ivanov KI, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol. 2009;185(3):439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmen C, Tammela T, et al. Biological basis of therapeutic lymphangiogenesis. Circulation. 2011;123(12):1335–1351. doi: 10.1161/CIRCULATIONAHA.107.704098. [DOI] [PubMed] [Google Scholar]
- Oka M, Iwata C, et al. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood. 2008;111(9):4571–4579. doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Ni A, et al. alpha5beta1 Integrin blockade inhibits lymphangiogenesis in airway inflammation. Am J Pathol. 2009;174(6):2378–2387. doi: 10.2353/ajpath.2009.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16(7):773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- Paavonen K, Puolakkainen P, et al. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol. 2000;156(5):1499–1504. doi: 10.1016/S0002-9440(10)65021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padera TP, Kadambi A, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296(5574):1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- Pajusola K, Aprelikova O, et al. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines [published erratum appears in Cancer Res 1993 Aug 15;53(16):3845] Cancer Res. 1992;52(20):5738–5743. [PubMed] [Google Scholar]
- Papapetropoulos A, Fulton D, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275(13):9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- Pennisi D, Gardner J, et al. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet. 2000;24(4):434–437. doi: 10.1038/74301. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21(17):4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10(9):974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Prevo R, Banerji S, et al. Rapid plasma membrane-endosomal trafficking of the lymph node sinus and high endothelial venule scavenger receptor/homing receptor stabilin-1 (FEEL-1/CLEVER-1) J Biol Chem. 2004;279(50):52580–52592. doi: 10.1074/jbc.M406897200. [DOI] [PubMed] [Google Scholar]
- Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008;216(2):347–354. doi: 10.1002/jcp.21494. [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Narko K, et al. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem. 1998;273(14):8413–8418. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- Roberts N, Kloos B, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66(5):2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- Ruddell A, Kelly-Spratt KS, et al. p19/Arf and p53 suppress sentinel lymph node lymphangiogenesis and carcinoma metastasis. Oncogene. 2008;27(22):3145–3155. doi: 10.1038/sj.onc.1210973. [DOI] [PubMed] [Google Scholar]
- Saban MR, Memet S, et al. Visualization of lymphatic vessels through NF-kappaB activity. Blood. 2004;104(10):3228–3230. doi: 10.1182/blood-2004-04-1428. [DOI] [PubMed] [Google Scholar]
- Sabine A, Agalarov Y, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22(2):430–444. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Helotera H, et al. Claudin-like protein 24 interacts with the VEGFR-2 and VEGFR-3 pathways and regulates lymphatic vessel development. Genes Dev. 2010;24(9):875–880. doi: 10.1101/gad.565010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M, Koskinen K, et al. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood. 2004;104(13):3849–3857. doi: 10.1182/blood-2004-01-0222. [DOI] [PubMed] [Google Scholar]
- Schepers GE, Teasdale RD, et al. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3(2):167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70(4):987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Schoppmann SF, Birner P, et al. “Lymphatic microvessel density and lymphovascular invasion assessed by anti-podoplanin immunostaining in human breast cancer.”. Anticancer Res. 2001;21(4A):2351–2355. [PubMed] [Google Scholar]
- Schulz P, Fischer C, et al. Angiopoietin-2 drives lymphatic metastasis of pancreatic cancer. FASEB J. 2011;25(10):3325–3335. doi: 10.1096/fj.11-182287. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8(12):1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Shields JD, Kourtis IC, et al. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328(5979):749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- Shimoda H, Bernas MJ, et al. Abnormal recruitment of periendothelial cells to lymphatic capillaries in digestive organs of angiopoietin-2-deficient mice. Cell Tissue Res. 2007;328(2):329–337. doi: 10.1007/s00441-006-0360-8. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20(24):3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Hashiguchi T, et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J Immunol. 2010;184(9):4819–4826. doi: 10.4049/jimmunol.0903063. [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Geng X, et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24(7):696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Baldwin ME, et al. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16(9):922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- Straume O, Jackson DG, et al. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res. 2003;9(1):250–256. [PubMed] [Google Scholar]
- Suri C, Jones PF, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Inoue O, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285(32):24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MA, Kaipainen A, et al. Mechanics of interstitial-lymphatic fluid transport: theoretical foundation and experimental validation. J Biomech. 1999;32(12):1297–1307. doi: 10.1016/s0021-9290(99)00125-6. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105(12):4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454(7204):656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Kohno R, et al. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol Cell Biol. 2007;27(12):4541–4550. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrin P, Zaujec J, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115(19):3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- Eynden GG, Vandenberghe MK, et al. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin Cancer Res. 2007;13(18 Pt 1):5391–5397. doi: 10.1158/1078-0432.CCR-07-1230. [DOI] [PubMed] [Google Scholar]
- Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010;24(19):2115–2126. doi: 10.1101/gad.1955910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, et al. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakayama M, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465(7297):483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27(6):1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98(6):769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, et al. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21(3):318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21(7):1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HE, Gonzalez EB, et al. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93(21):1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- Willis RA. The spread of tumours in the human body. London: Butterworths; 1973. [Google Scholar]
- Wyble CW, Hynes KL, et al. TNF-alpha and IL-1 upregulate membrane-bound and soluble E-selectin through a common pathway. J Surg Res. 1997;73(2):107–112. doi: 10.1006/jsre.1997.5207. [DOI] [PubMed] [Google Scholar]
- Xu Y, Yuan L, et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol. 2010;188(1):115–130. doi: 10.1083/jcb.200903137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Moyon D, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129(20):4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- Zhang X, Groopman JE, et al. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. J Cell Physiol. 2005;202(1):205–214. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou F, et al. VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res. 2010;20(12):1319–1331. doi: 10.1038/cr.2010.116. [DOI] [PubMed] [Google Scholar]
- Zhou F, Chang Z, et al. Akt/Protein kinase B is required for lymphatic network formation, remodeling, and valve development. Am J Pathol. 2010;177(4):2124–2133. doi: 10.2353/ajpath.2010.091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumsteg A, Baeriswyl V, et al. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One. 2009;4(9):e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]