Abstract
OBJECTIVES
ARID1A is a recently identified tumor suppressor participating in chromatin remodeling. Somatic inactivating mutations of ARID1A and loss of its expression occur most frequently in ovarian clear cell and endometrioid carcinomas and uterine endometrioid carcinomas. Since endometriosis is thought to be a precursor of most ovarian clear cell and endometrioid carcinomas, we undertook an analysis of ARID1A expression of these tumors arising within an endometriotic cyst (endometrioma).
MATERIALS/METHODS
Our immunohistochemical study set consisted of 47 endometriotic cysts containing clear cell carcinoma in 24 cases, well-differentiated ovarian endometrioid carcinoma in 20 and mixed clear cell and endometrioid carcinoma in 3.
RESULTS
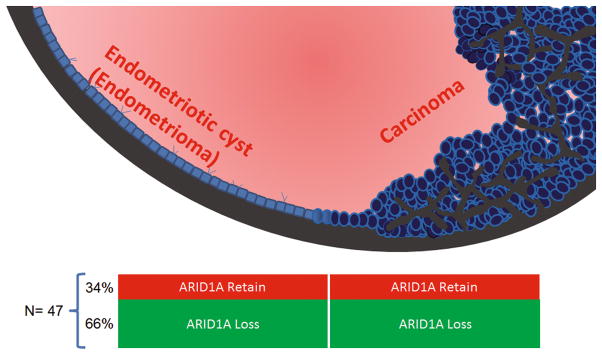
ARID1A loss was observed in 31 (66%) of 47 carcinomas and therefore these cases were informative for determining the temporal sequence of loss of ARID1A expression in tumor progression. In 16 of the 47 cases, ARID1A immunoreactivity was retained in both the endometriotic cyst and the carcinoma and thus these cases were not informative. All of the 31 informative cases showed loss of ARID1A immunoreactivity in the carcinoma and in the endometriotic cyst epithelium in direct continuity with the carcinoma but not in the cyst epithelium that was not adjacent to the tumor.
CONCLUSIONS
The findings in this study provide cogent evidence that loss of ARID1A function as shown by loss of expression, presumably due to mutations, is an early molecular event, occurring before malignant transformation, in the development of the majority of ovarian clear cell and endometrioid carcinomas arising in endometriomas.
Introduction
The dualistic model of ovarian carcinogenesis, broadly divides all epithelial tumors into two groups, designated type I and type II. Clear cell and endometrioid carcinomas along with low-grade serous and mucinous carcinomas are included in the type I group (1, 2) and appear to develop from well-established precursor lesions such as cystadenomas, atypical proliferative (borderline) tumors and endometriosis. In contrast, high-grade serous carcinoma, malignant mixed mesodermal tumors (carcinosarcomas) and undifferentiated carcinomas are included in the type II group and appear to develop from precursor lesions in the fallopian tube that pass through a stage of intraepithelial carcinoma, so-called “serous tubal intraepithelial carcinoma (STIC)” (3–6). The association of endometriosis with ovarian clear cell and endometrioid carcinoma has been known for over three decades and documentation of malignant transformation was first reported as far back as 1925 (7). Accordingly, investigators have invoked endometriosis as the precursor of these tumors based on clinicopathological, epidemiologic and more recently molecular studies (8). Molecular genetic alterations such as PTEN deletion and microsatellite instability, which occur in clear cell and endometrioid carcinomas, can be detected in the epithelial cells of endometriotic cysts (8, 9) and a clonal relationship between endometriosis and endometriosis-related ovarian carcinomas has been demonstrated in several studies (10–13). Moreover, gene expression profiling studies have shown that ovarian clear cell and endometrioid carcinomas more closely resemble endometrial as opposed to colonic, ovarian surface and fallopian tube epithelium (14). A large pooled analysis of case control studies surveying more than 20,000 women demonstrated that even self-reported endometriosis is a risk factor in the development of ovarian clear cell and endometrioid carcinomas, providing cogent clinical evidence that endometriosis is associated with these tumors (15). Despite the mounting evidence linking endometriosis with ovarian endometrioid and clear cell carcinomas, the molecular pathogenesis involved in tumor initiation remains largely unknown.
ARID1A is a recently identified tumor suppressor encoding the protein BAF250a, which participates in forming SWI/SNF chromatin remodeling complexes. This gene is frequently mutated in ovarian clear cell and endometrioid carcinomas as well as in uterine endometrioid carcinomas compared to other common types of human cancer (16–19). The great majority of ARID1A mutations are non-sense, frameshift, and in-frame mutations, leading to loss of expression of BAF250a. Accordingly, loss of expression of ARID1A (BAF250a) immunoreactivity can be used as a surrogate marker for ARID1A inactivating mutations in formalin fixed, paraffin embedded tissue (19). Indeed, several reports have analyzed ARID1A staining patterns in a variety of human cancers and demonstrated that loss of ARID1A expression, like its mutation, occurs most frequently in ovarian clear cell, endometrioid carcinomas and uterine endometrioid carcinomas compared to the other types of ovarian and uterine carcinomas (19, 20).
Given that ARID1A functions as a tumor suppressor that appears to be important in the genesis of endometrium-related carcinomas (21), the present study was undertaken to determine the ARID1A immunostaining pattern in both clear cell and endometrioid carcinoma as well as in the endometriotic cyst and in endometriosis at distant sites. Our results provide important insight into the early events involved in the initiation of these tumors.
Materials and Methods
Tissue Materials
Paraffin embedded whole tissue sections from 47 ovarian endometriotic cysts (endometriomas) containing clear cell or endometrioid carcinomas were obtained from the Departments of Pathology at the Johns Hopkins Hospital, Seirei Mikatahara General Hospital and Hirosaki University Hospital, Japan. All tumors were FIGO stage I and arose in endometriotic cysts. They included 24 clear cell carcinomas, 20 endometrioid carcinomas, and 3 mixed clear cell and endometrioid carcinomas. All the endometrioid carcinomas analyzed were low-grade except one (case 27 in Table 1), which contained high-grade areas. The junction between normal-appearing endometriotic cyst epithelium and carcinoma was selected in all cases and in four of these, cystic epithelium distant from the carcinoma was also evaluated. Eight cases were bilateral, for which the uterine corpus tumor and contralateral ovarian tumor were also examined in terms of ARID1A expression; however the tumor was counted as one in the final analysis as there were no discrepancy between the bilateral tumors in terms of ARID1A expression. All cases studies were re-reviewed by at least two surgical pathologists to confirm the diagnosis. Clinicopathological information such as patient age, size and location of the tumor and the presence of concurrent corpus carcinoma were gathered from the clinical and pathology records. We also assessed discrete endometriosis foci in 15 cases that were remote from the endometriotic cyst and carcinoma. In addition, we examined 4 ovarian endometriomas without carcinoma and 6 cases of peritoneal endometriosis as controls. The acquisition of the tissue material was approved by the Institutional Review Boards.
Table 1.
ARID1A immunoreactivity in ovarian endometriotic cysts and associated ovarian clear cell and well-differentiated endometrioid carcinomas.
| Case | Endometrioma | Ovarian clear cell carcinoma | Ovarian endometrioid carcinoma |
|---|---|---|---|
| 1 | Loss | Loss | Not present |
| 2 | Loss | Loss | Not present |
| 3 | Loss | Loss | Not present |
| 4 | Loss | Loss | Not present |
| 5 | Loss | Loss | Not present |
| 6 | Loss | Loss | Not present |
| 7 | Loss | Loss | Not present |
| 8 | Loss | Loss | Not present |
| 9 | Loss | Loss | Not present |
| 10 | Loss | Loss | Not present |
| 11 | Loss | Loss | Not present |
| 12 | Loss | Loss | Not present |
| 13 | Loss | Loss | Not present |
| 14 | Loss | Loss | Not present |
| 15 | Loss | Loss | Not present |
| 16 | Loss | Loss | Not present |
| 17 | Loss | Loss | Not present |
| 18 | Loss | Loss | Not present |
| 19 | Loss | Not present | Loss |
| 20 | Loss | Not present | Loss |
| 21 | Loss | Not present | Loss |
| 22 | Loss | Not present | Loss |
| 23 | Loss | Not present | Loss |
| 24 | Loss | Not present | Loss |
| 25 | Loss | Not present | Loss |
| 26 | Loss | Not present | Loss |
| 27 | Loss | Not present | Loss |
| 28 | Loss | Not present | Loss |
| 29*± | Loss | Loss | Loss |
| 30* | Loss | Loss | Loss |
| 31 | Loss | Not present | Loss |
| 32 | Retained | Retained | Not present |
| 33* | Retained | Retained | Retained |
| 34 | Retained | Not present | Retained |
| 35 | Retained | Not present | Retained |
| 36 | Retained | Not present | Retained |
| 37 | Retained | Not present | Retained |
| 38 | Retained | Not present | Retained |
| 39 | Retained | Not present | Retained |
| 40 | Retained | Retained | Not present |
| 41 | Retained | Retained | Not present |
| 42 | Retained | Retained | Not present |
| 43 | Retained | Retained | Not present |
| 44 | Retained | Retained | Not present |
| 45 | Retained | Not present | Retained |
| 46 | Retained | Not present | Retained |
| 47 | Retained | Not present | Retained |
Shaded cases represent those non-informative cases (ARID1A staining in all lesions).
cases containing both ovarian endometrioid and clear cell carcinomas.
cases containing endometrioid borderline tumors
Immunohistochemistry
Immunohistochemical analysis was performed on whole tissue sections obtained from one or two representative tumor blocks containing both endometriotic cyst and carcinoma. A polyclonal rabbit anti-ARID1A (BAF250A) antibody (Sigma-Aldrich, HPA005456) was used, the specificity of which was previously confirmed by Western blotting (19). Antigen retrieval was performed by submerging the tissue sections in citrate buffer (pH 6.0) and then in a steamer for 10 minutes. The sections were then incubated with the rabbit antibody at a dilution of 1:200 at 4°C overnight. A positive reaction was detected by the EnVision+System (Dako, Carpinteria, CA). Only nuclear staining was scored and tumor stromal cells served as positive internal controls. Since previous studies demonstrated that loss of nuclear expression generally correlated with mutation of the ARID1A gene (18, 19), absence of nuclear staining was interpreted as a presumable ARID1A mutation. Loss of ARID1A immunoreactivity was defined as undetectable ARID1A staining in more than 95% of epithelial cells, while retained ARID1A expression was defined as staining in greater than 95% of epithelial cells.
Results
Immunohistochemistry was used to analyze ARID1A expression in 47 ovarian endometriotic cysts, which contained clear cell carcinoma in 24 cases, endometrioid carcinoma in 20 cases and mixed clear cell and endometrioid carcinoma in three (case 29, 30, and 33). Patient age ranged from 31 to 71 years with a mean of 52 years and a median of 47 years. None of the patients received neoadjuvant chemotherapy before surgery. Tumors ranged from 4 to 17 cm, with a mean of 10.5 cm. Loss of ARID1A expression was detected in 31 (66%) of 47 carcinomas and the endometriotic cyst epithelium immediately adjacent to the carcinoma (Table 1). Of these, epithelium lining the cyst wall that was distant from the carcinoma was available in four cases; the cystic epithelium in those areas retained ARID1A immunoreactivity. Cases in which there was loss of expression in both tumors and cyst epithelium were informative because loss of ARID1A expression in the carcinoma allows one to determine if ARID1A loss occurred early (in the endometriotic cyst epithelium and the carcinoma) or relatively late (only in the carcinoma). Of the 47 cases, ARID1A immunoreactivity was retained in both endometriotic cysts and the carcinomas in 16 (34%) and these cases were therefore not informative.
Analysis of the 31 informative cases revealed loss of ARID1A immunoreactivity in both the carcinoma and normal-appearing endometriotic cyst epithelium adjacent to the neoplasms in all cases. On the other hand, loss of ARID1A staining in the carcinomas but not in the adjacent endometriotic cysts was never observed. In the two mixed clear cell/endometrioid carcinoma cases (case 30 and 31) that were informative (Table 1) there was loss of ARID1A in the both the tumor components and in the adjacent endometriotic cyst epithelium (Fig. 1). In the remaining mixed case that was not informative, ARID1A expression was observed in both the endometriotic cyst and the different components of the carcinoma. Endometriotic cyst epithelium showing focal nuclear atypia, so called “atypical endometriosis” was occasionally noted and these foci also exhibited the same ARID1A staining pattern as the normal-appearing endometriotic cyst epithelium. Generally, ARID1A staining was either completely lost or retained, but in one case (case 32) intratumoral heterogeneity was observed as there was focal clonal loss of ARID1A immunoreactivity both in the endometriotic cyst epithelium and in the neoplasm. In this case, we recorded it as “retained” because the majority of epithelial cells retained ARID1A immunoreactivity. In all cases in which the epithelial cells of the endometriotic cysts and tumors lost ARID1A immunoreactivity, the stromal cells adjacent to the lesions were intensely positive, thus serving as internal positive controls. The association of ARID1A expression in the tumors and adjacent epithelium is summarized in Table 2 and illustrated in Figure 2. There was no significant difference between clear cell and endometrioid carcinomas as far as the patterns of ARID1A expression are concerned (p> 0.05, ANOVA test). All of the endometriosis foci in 15 cases that were remote from the endometriotic cyst and carcinoma, all endometriotic cyst cases without carcinoma and all the cases with discrete peritoneal endometriosis without evidence of associated carcinoma exhibited a high level of ARID1A expression (Fig. 3).
Fig. 1.
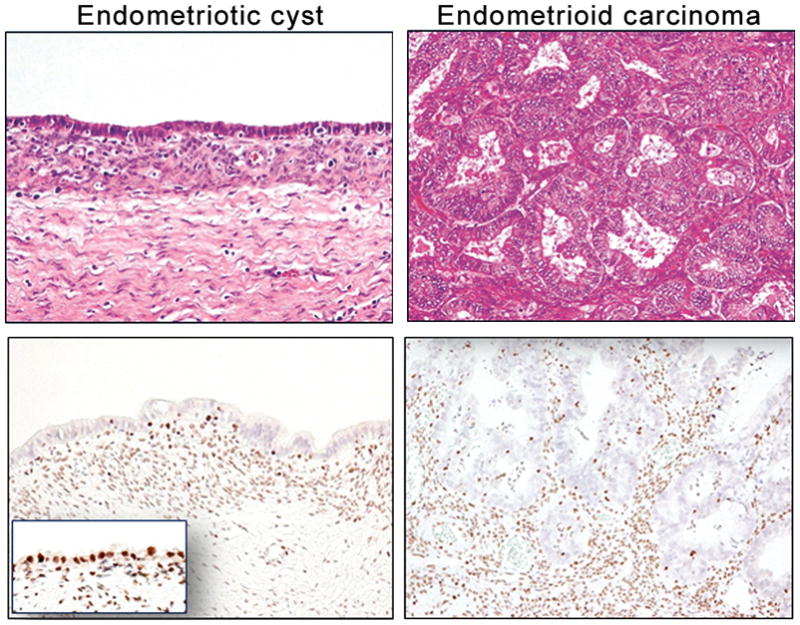
ARID1A immunoreactivity in a representative case (No. 32) containing an endometriotic cyst and associated well-differentiated endometrioid carcinoma. ARID1A immunoreactivity is undetectable in both carcinoma and adjacent normal-appearing endometriotic cyst epithelium while the epithelium remote from the carcinoma is intensely positive for ARID1A (inset). The stromal cells are also strongly positive.
Fig. 2.
A schematic presentation summarizes the percentage of cases showing ARID1A staining status in carcinomas arising from endometriotic cysts. A total of 47 cases were analyzed and two thirds of them show ARID1A loss in both endometriotic cyst and carcinoma.
Fig. 3.
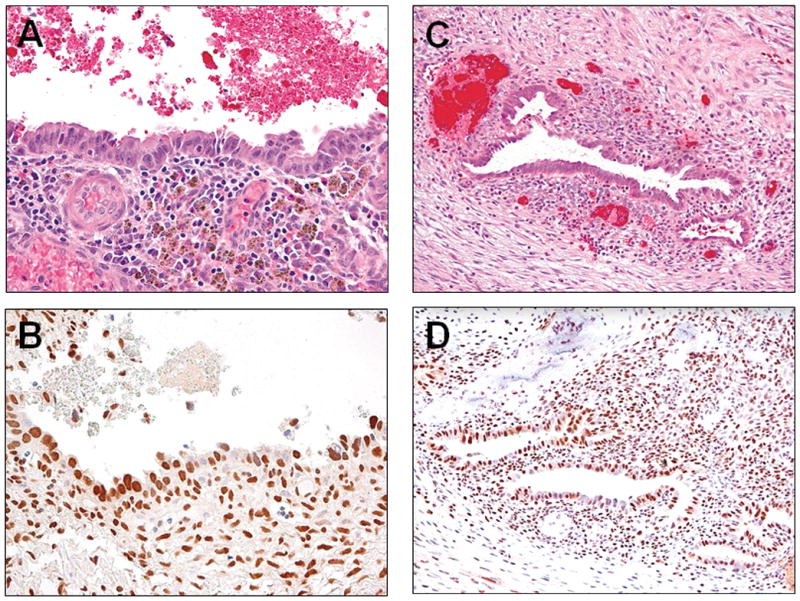
ARID1A expression in an endometriosis remote from the carcinoma (A and B) and an endometriotic cyst without concurrent carcinoma (C and D). ARID1A immunoreactivity is retained in the epithelial cells as well as in the stromal cells.
Discussion
Inactivating ARIDIA mutations are the most common molecular genetic alteration reported thus far in ovarian clear cell and endometrioid carcinomas. These mutations result in loss of expression of the protein encoded by ARID1A (BAF250a) which normally suppresses cellular proliferation through a p53-dependent transcription regulation of several tumor suppressors including CDKN1A (encoding p21) and SMAD3 (21). It is still not clear, however, as to whether ARID1A mutation occurs prior to, or after tumor initiation. Delineating the “timing” of ARID1A mutations is not only of biologic interest but also has important clinical implications. In this study, we provide cogent evidence that loss of ARID1A expression consistently occurs in the normal-appearing epithelium of endometriotic cysts that is immediately adjacent to the clear cell or endometrioid carcinoma arising in the cyst but not in the epithelium of the endometriotic cyst that is distant from the carcinoma. In addition, endometriotic cysts and peritoneal endometriosis that are not associated with carcinomas exhibit retention of ARID1A expression. These findings together with a recent report (22) suggest a temporal relationship of ARID1A inactivation occurring early in tumor progression in clear cell and endometrioid carcinoma. Our findings are consistent with a study demonstrating that ARID1A negative clear cell carcinomas were more frequently associated with the presence of adjacent endometriotic cysts than those that were positive for ARID1A staining (23).
Like ARID1A mutation, inactivation of the PTEN tumor suppressor gene and activation of PIK3CA due to somatic mutations have also been reported as early events in the development of endometriosis-related carcinomas since loss of PTEN is observed in endometriotic cysts associated with and without carcinoma (13) and activating PIK3CA mutation is detected in endometriotic cysts associated with carcinoma (12, 23 ). Although the factors that might be responsible for the development of mutations of ARID1A, PIK3CA and PTEN in endometriotic cyst epithelium remain unclear, it is conceivable that “incessant menstruation” associated with endometriosis leads to repeated episodes of hemorrhage and inflammation which may facilitate the accumulation of oxygen free radical species and other genotoxic molecules within the cysts that contribute to malignant transformation (24). High concentration of free iron in the fluid of endometriotic cysts has been proposed to be responsible for iron-induced persistent oxidative stress and for induction of mutations in endometriotic cyst epithelium (25).
In this study, we found that 66% of early stage ovarian cystic clear cell and endometrioid carcinomas lost ARID1A expression, which is rather higher compared to the 45% in a previous report examining ARID1A expression in 212 ovarian clear cell and endometrioid carcinomas (18). That epidemiological study reported frequent association of loss of ARID1A expression with self-reported endometriosis but clearly did include histologic confirmation. Although this may be solely due to different the antibodies used in different studies, our manuscript refers only to clear cell and endometrioid ovarian carcinomas which occur within endometriomas, excluding the adenofibromatous tumors which might have a lower rate of ARID1A loss, and instead be associated with other molecular aberrations.
The great majority of endometriosis-related carcinomas are either clear cell or endometrioid carcinomas; however, in this study, we report three cases of mixed clear cell and endometrioid carcinoma suggesting that they share a common precursor arising in the endometriotic cyst or that they arise from independent clones of endometriotic epithelial cells. In other words, the concurrent loss of ARID1A immunoreactivity in both clear cell and endometrioid carcinoma components in two endometriotic cysts implies that differentiation into distinct types of carcinoma takes place after loss of ARID1A expression, presumably due to ARID1A mutations. Even though a recent report showing that loss of ARID1A expression in 15% of endometriomas in women without ovarian clear cell and endometrioid carcinomas but not in their associated peritoneal endometriosis and eutopic endometrium may be perceived a contrasting finding to our small series performed on whole sections where only 4 benign endometriomas were examined and all were positive for ARID1A, it seems that staining was also absent in the stroma of their 3 cases in that microarray-based study, and the difference may well be for technical reasons(26). Accordingly we think that our results along with theirs raise the possibility that loss of ARID1A expression may be an early event in the development of these tumors.
In summary, we compared the ARID1A expression pattern in stage I ovarian clear cell and endometrioid carcinomas arising in endometriotic cysts, and found loss of ARID1A immunoreactivity in both the endometriotic cyst immediately adjacent to the carcinomas and in the carcinomas in all informative cases. In contrast, endometriotic epithelium in the cyst that was distant from the carcinoma expressed ARID1A. Thus, like mutations in PTEN and PIK3CA, loss of ARID1A expression, presumably due to mutations, is an early molecular event in the development of the majority of ovarian clear cell and endometrioid carcinomas. It is of interest that separate foci of endometriosis, either in the absence of a tumor or in cases in which a tumor is present, express ARID1A. On the other hand, clear cell and endometrioid carcinomas almost always arise within endometriotic cysts as opposed to discrete foci of endometriosis and loss of ARID1A expression is limited to the endometriotic tissue immediately adjacent to the carcinoma. Thus, we conjecture that formation of an endometriotic cyst is required for the development of most clear cell and endometrioid carcinomas. Future studies of women with endometriosis, particularly endometriotic cysts, should evaluate if molecular testing including ARID1A expression and mutation as well as molecular changes in PTEN and PIK3CA (by analyzing endometrioma content, for example) is useful in identifying women at a higher risk of developing clear cell or endometrioid carcinoma (27).
Table 2.
Summary of loss of ARID1A staining in endometriomas and associated adjacent carcinomas in 48 cases.
| Total case No. | Informative cases* | Loss in endometriotic cystand carcinoma | |
|---|---|---|---|
| CCC only | 24 | 18(75%) | 18 (100%) |
| EMC only | 20 | 11(55%) | 11 (100%) |
| CCC andEMC | 3 | 2(67%) | 2(100%) |
| Total | 47 | 31(66%) | 31 (100%) |
Informative case means those showing ARID1A loss in endometriotic cyst and/or carcinoma. CCC, ovarian clear cell carcinoma; EMC, ovarian well-differentiated endometrioid carcinoma %, loss of ARID1A immunoreactivity in carcinomas in informative cases.
Acknowledgments
This study is supported by an NIH/NCI grant R21CA165807, an OSB1 grant from HERA Women’s Cancer Foundation, NSC 100-2320-B-002-081 and Endometriosis Foundation of America (EFA).
Footnotes
This study is presented at United States-Canadian Academy of Pathology meeting at Vancouver, Canada, March 2012.
Disclosure of Funding: This study is supported by an NIH/NCI grant R21CA165807, an OSB1 grant from HERA Women’s Cancer Foundation, NSC 100-2320-B-002-081 and Endometriosis Foundation of America (EFA).
References
- 1.Shih I-M, Kurman RJ. Ovarian tumorigenesis- a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho KR, Shih IM. Ovarian cancer. Annu Rev Pathol Mech Dis. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 4.Piek JM, Verheijen RH, Kenemans P, Massuger LF, Bulten H, van Diest PJ. BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol Oncol. 2003;90:491. doi: 10.1016/s0090-8258(03)00365-2. [DOI] [PubMed] [Google Scholar]
- 5.Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn E, Kurman RJ, Shih IM. Ovarian cancer is an imported disease: fact or fiction? Curr Obstet Gynecol Rep. 2012;1:1–9. doi: 10.1007/s13669-011-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg. 1925;10:1–72. [Google Scholar]
- 8.Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecologic Oncology. 2012;124:164–9. doi: 10.1016/j.ygyno.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Ali-Fehmi R, Khalifeh I, Bandyopadhyay S, et al. Patterns of loss of heterozygosity at 10q23. 3 and microsatellite instability in endometriosis, atypical endometriosis, and ovarian carcinoma arising in association with endometriosis. Int J Gyn Pathol. 2006;25:223–9. doi: 10.1097/01.pgp.0000192274.44061.36. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Morland SJ, Hitchcock A, Thomas EJ, Campbell IG. Allelotyping of endometriosis with adjacent ovarian carcinoma reveals evidence of a common lineage. Cancer Res. 1998;58:1707–12. [PubMed] [Google Scholar]
- 11.Prowse AH, Manek S, Varma R, et al. Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. Int J Cancer. 2006;119:556–62. doi: 10.1002/ijc.21845. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto S, Tsuda H, Takano M, Iwaya K, Tamai S, Matsubara O. PIK3CA mutation is an early event in the development of endometriosis-associated ovarian clear cell adenocarcinoma. J Pathol. 2011;225:189–94. doi: 10.1002/path.2940. [DOI] [PubMed] [Google Scholar]
- 13.Sato N, Tsunoda H, Nishida M, et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–6. [PubMed] [Google Scholar]
- 14.Marquez RT, Baggerly KA, Patterson AP, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–26. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 15.Pearce CL, Templeman C, Rossing MA, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–94. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones S, Li M, Parsons DW, Zhang X, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33:100–3. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones S, Wang TL, Shih Ie M, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–31. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. New Engl J Med. 2010;363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan B, Mao TL, Panuganti PK, et al. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35:625–32. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowery WJ, Schildkraut JM, Akushevich L, et al. Loss of ARID1A-associated protein expression is a frequent event in clear cell and endometrioid ovarian cancers. Int J Gyn Cancer. 2012;22:9–14. doi: 10.1097/IGC.0b013e318231f140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan B, Wang TL, Shih Ie M. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011;71:6718–27. doi: 10.1158/0008-5472.CAN-11-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod Pathol. 2012;25:615–24. doi: 10.1038/modpathol.2011.189. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. PIK3CA mutations and loss of ARID1A protein expression are early events in the development of cystic ovarian clear cell adenocarcinoma. Virchows Archiv: an international journal of pathology. 2012;460:77–87. doi: 10.1007/s00428-011-1169-8. [DOI] [PubMed] [Google Scholar]
- 24.Vercellini P, Crosignani P, Somigliana E, et al. The ‘incessant menstruation’ hypothesis: a mechanistic ovarian cancer model with implications for prevention. Hum Reprod. 2011;26:2262–73. doi: 10.1093/humrep/der211. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi K, Mandai M, Toyokuni S, et al. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:32–40. doi: 10.1158/1078-0432.CCR-07-1614. [DOI] [PubMed] [Google Scholar]
- 26.Samartzis EP, Samartzis N, Noske A, et al. Loss of ARID1A/BAF250a-expression in endometriosis: a biomarker for risk of carcinogenic transformation? Mod Pathol. 2012;25:885–92. doi: 10.1038/modpathol.2011.217. [DOI] [PubMed] [Google Scholar]
- 27.Mandai M, Suzuki A, Matsumura N, et al. Clinical management of ovarian endometriotic cyst (chocolate cyst): diagnosis, medical treatment, and minimally invasive surgery. Curr Obstet Gynecol Rep. 2012;1:16–24. [Google Scholar]