Abstract
Purpose
To compare the results of Extracorporeal shock wave (ESWT) with a modified endoscopic plantar fasciotomy technique for the treatment of recalcitrant heel pain.
Method
Sixty-five patients suffering from chronic heel pain that failed to respond to standard nonoperative methods were randomized to undergo either high-energy extracorporeal shock wave therapy (group 1), or modified endoscopic plantar fasciotomy (group 2). The primary outcome measure was the reduction of pain in the two groups from base line to month three post intervention at the first few steps in the morning. In addition, patients' functions were assessed using American Orthopedic Foot and Ankle-Hindfoot Scale (AOFAS) at week three, month three, and month 12 post-intervention, and finally, Roles and Maudsley scores were assessed. The primary analysis was intention-to-treat and involved all patients who were randomly assigned.
Results
Both groups achieved improvement from the base line at 3 weeks, 3 months and 12 months post-intervention. The success rate (Roles and Maudsley score excellent and good) in the ESWT group at month 12 was 70.6 %, while in the fasciotomy group, the success rate was 77.4 % (p = 0.19).
Conclusion
In patients who had experienced failure of conventional treatment of plantar fasciopathy, both endoscopic plantar fasciotomy and shock wave therapy can be potentially helpful lines of management.
Introduction
Plantar fasciopathy is considered one of the most common causes of heel pain, often with severe limitation of activity [25]. Pain on first weight bearing in the morning is a prominent diagnostic feature. Simple foot x-rays are of little utility, because no clinical-radiological correlation exists [10].
Histological examination of chronically painful plantar fascia shows a failed healing response, without histopathological evidence of inflammation. The term plantar fasciopathy seems more accurate than the more common term plantar fasciitis, which implies an inflammatory process [28].
Although the clinical diagnosis is relatively straightforward, the treatment can be difficult and frustrating [7, 33]. The choice of treatment for each individual case remains controversial and is based on the personal experience of the treating physician. There is little argument that conservative treatment is the initial treatment of choice. The scope of conservative treatments suggested includes multiple conservative pharmacological and therapeutic interventions (viz; nonsteroidal anti-inflammatory drugs, heel pads or orthotics, physical therapy, night splints and corticosteroid injections), but none have proven to be effective, nor shown consistent results due to lack of well-designed and well-conducted comparative studies [19]. Most of the patients subsequently improve to the point of symptomatic satisfaction (not necessarily complete relief of symptoms) with one or more of the noninvasive interventions [25]; however, surgical treatment (open or endoscopic release of a portion of plantar fascial insertion onto the calcaneus) is necessary in 10–20 % of patients when symptoms persist [16].
Extracorporeal shock wave therapy (ESWT) emerged in the early 1990s as an effective treatment of insertion tendinopathies. It has been recommended as treatment for chronic plantar fasciopathy in patients unresponsive to conservative treatment [7, 12, 18, 22–25, 31, 32, 34].The mechanism of action of shock waves is not fully understood and has been explained by many theories, including direct stimulation of healing, neovascularization, direct suppressive effects on nociceptors, and a hyperstimulation mechanism that blocks the gate-control mechanism [32].
A prospective randomized study was designed to assess the effectiveness of ESWT for the treatment of recalcitrant plantar fasciopathy, and to compare its outcome with the outcome of modified endoscopic partial plantar fascia release (EPFR). Based on a Medline search and on review of key journals, no previous powered randomized trial has been conducted to compare these two modalities of treatment. There are few randomized trials in orthopedics of a surgical modality compared to a conservative modality. One of these trials was that of the Oslo group, where surgery (open patellar tenotomy) was compared to eccentric exercises in patellar tendinopathy [2].
Materials and methods
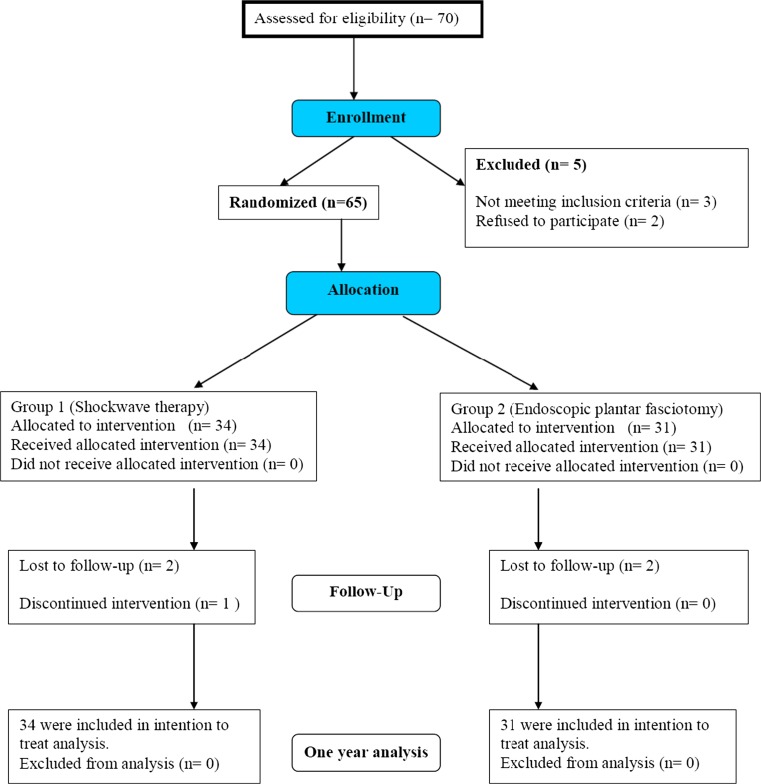
Seventy consecutive patients with unilateral recalcitrant Plantar fasciopathy were enrolled in a prospective study from July 2005 to December 2007. We followed up with 65 patients, who comprised the two study groups, for 12 months post-intervention. Five patients did not complete the one year follow-up (three in group 1 and two in group 2). Figure 1 shows the flow of patients through each stage of the randomization trial according to the CONSORT statement (www.consort-statment.org).
Fig. 1.
Flow of patients through each stage of the randomization trial according to the CONSORT statement (www.consort-statment.org)
Inclusion criteria
Patients included in the study presented with a single site heel pain with local pressure at the origin of proximal plantar fascia on the medial calcanean tuberosity, with:
Failure of at least three lines of conservative treatment measures during the last six months. Conservative treatment included: nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, physical therapy, exercise program (Achilles tendon and plantar fascia stretching exercises) and orthotic devices (heel cup, molded shoe insert, night splint, or cast).
A self-assessment of pain after the first five minutes of walking in the morning that was more than 40mm on the 100mm visual analog scale.
Exclusion criteria
Patients were excluded if they were:
Younger than 18 years, had a local infection, metabolic disorders (especially diabetes) or malignancy, ankle arthritis, generalized polyarthritis, sero-negative arthropathy, ipsilateral or contralateral vascular or neurological abnormalities, history of previous surgery to the affected leg, tarsal tunnel syndrome, recent trauma or foot and ankle deformity or fractures, active anticoagulation therapy or a bleeding disorder, cardiac arrhythmia, a pacemaker or stent, had received a corticosteroid injection within the previous six weeks, had contralateral heel pain of more than 40mm on the visual analogue scale, or were pregnant.
Pretreatment heel radiographs were obtained to exclude the presence of intraosseous lesions, such as calcaneal cyst or subtalar arthritis.
The random allocation sequence was computer generated, using simple randomization. This sequence was then placed into sealed, consecutively numbered, opaque envelopes. After initial assessment, which confirmed the inclusion and exclusion criteria, patients gave their informed consent and were randomly allocated into two groups. The envelopes were kept in a locked cabinet in the office of the surgeons, who later opened the envelopes in order of participant recruitment.
NSAIDs were not allowed concomitantly. If patients did not complete the 12-month follow-up protocol, efforts were made to encourage compliance. Any patient who failed to respond to these efforts was classified as a failure in the outcome assessment. The primary analysis was intention-to-treat and involved all patients who were randomly assigned.
The study population consisted of:
ESWT comprised 34 patients; 18 were male, the mean age was 37.7 ± 9.42 years (range: 23–61 years), and the duration of symptoms was 18.0 ± 10.9 months (range: 6–60 months).
EPFR comprised 31 patients; 22 were male, the mean age was 39.7 ± 8. 79years (range: 26–59 years), and the duration of symptoms was 17.45 ± 8.5 months (range: 7–60 months).
Group 1 (ESWT) (n = 34)
The point of maximum tenderness to pressure was demarcated. Ear protection devices were used. Conscious sedation anesthesia was given to all patients prior to therapy (i.e. no local anesthesia given). The shock wave treatments were applied by means of an OssaTron device (High Medical Technology, Kreuzlingen, Switzerland), a device generating repetitive high-energy shock waves by the electrohydraulic method. The device was adjusted to maximize the focused treatment wave (f2) into the plantar fascia. Each patient received 100 graded shocks (14–18 kV; 0.12– 0.22 mJ/mm2) to assess the effectiveness of the anesthesia, followed by 1,400 shocks at 18 kV (0.22 mJ/mm2), for a total of 1,500 shocks, applied at 4 shocks/second. The total energy delivered was 324.25 J. This power setting was defined as a high-energy treatment protocol [25]. The heel was manipulated against the treatment head throughout the shock wave applications. Shock waves were thus applied to the maximum pain site and a 2 cm radius area surrounding it.
Group 2 (EPPF) (n = 31)
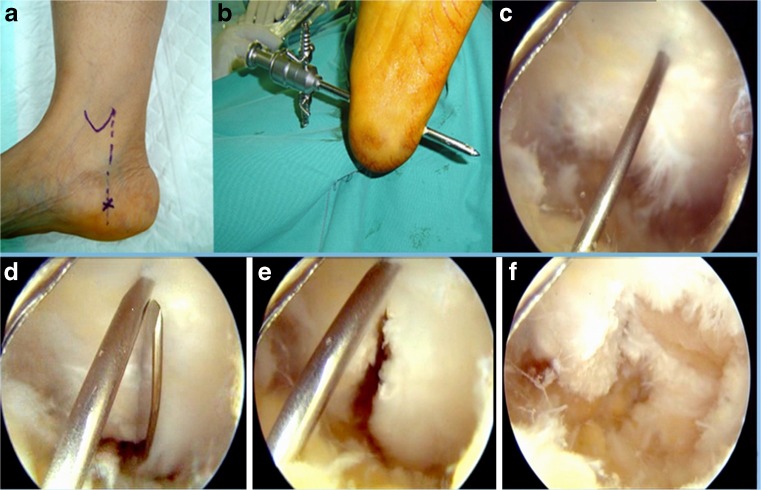
Surgery was performed under general or spinal anesthesia, in the supine position with the foot hanging outside the edge of the table. A pneumatic tourniquet was maintained on the thigh throughout the procedure. A medial portal was developed 1 cm away from the plantar skin along a vertical line passing through the posterior border of the medial malleolus with the foot in neutral position (Fig. 2a). A blunt trocar was then introduced transversely in the subcutaneous tissue, just inferior to the plantar fascia. A lateral portal was made in the lateral side where the trocar emerges. A 5 mm cannula was then introduced through the lateral portal over the trocar. Irrigation fluid was then connected, with the fluid inflow pressure between 50 and 60 mmHg. A 30-degree 4.0 mm endoscope was inserted inside the cannula. A 4.5 motorized incisor blade was then used to debride the subcutaneous tissue until full visualization of the shiny fibers of the plantar fascia was possible. A needle was inserted vertically through the heel skin to act as a landmark for the middle of the plantar fascia (Fig. 2b). A standard scalpel blade No. 11 was then introduced through the medial portal to divide the full thickness of the medial half of the plantar fascia into two leaflets under direct visualization (Fig. 2c and d). The posterior leaflet was then totally debrided using a motorized incisor blade (Fig. 2e). The tunnel was then irrigated and each portal was sutured by one 3-0 prolene stitch (Fig. 2f). Dressing and crepe bandage were then applied. Early ankle and foot mobilization with toe touch weight-bearing in the first week after operation was prescribed to all subjects, progressing to full weight bearing after 2–4 weeks, according to tolerance.
Fig. 2.
(a) Intra-operative photograph showing the landmarks of the medial portal. (b) Endoscopic view showing the shiny fibers of the plantar fascia and a needle acting as a landmark for the middle of the plantar fascia. (c) Endoscopic view showing a standard scalpel blade No. 11 introduced through the medial portal. (d) Endoscopic view showing the full thickness of the medial half of the plantar fascia divided into two leaflets. (e) Endoscopic view after debridement of the posterior leaflet. (f) Intra-operative photograph showing the final appearance of the medial portal at the end of the operation
Outcomes
Patients were assessed based on:
Morning Pain: a visual analogue scale ranging from 0 (no pain) to 100 (maximal pain) at baseline, 3 weeks, 12 weeks and 12 months postoperative.
American Orthopedic Foot and Ankle-Hindfoot Scale (AOFAS) [17]. It includes: pain (40 points), function (50 points) and alignment assessment (10 points). Data were recorded at baseline, 3 weeks, 12 weeks and 12 months postoperative.
- Patient subjective assessment: at 3 weeks, 12 weeks and 12 months, patients assessed their overall condition compared to before treatment, according to the criteria of Roles and Maudsley [27] as follows:
- excellent: no pain, full movement, full activity;
- good: occasional discomfort, full movement, full activity;
- acceptable: some discomfort after prolonged activities; and
-
poor: pain-limiting activity.Success is defined as an excellent or good score based on Roles and Maudsley
A telephone follow-up was conducted at 2 years and 3 years after completion of the procedure, the purpose being to ask the patient to rate their results as success or failure, comparing their current status to 12-month follow-up status.
Statistical analysis
PASW version 18 (Chicago, IL, USA) and PASS were used for statistical analysis. The primary efficacy end point was prospectively defined as reduction of pain from baseline to month three post-treatment in a visual pain numeric scale (range from 0—100), during first steps in the morning. Assuming a standard deviation of 2, a sample size of 58 was required to achieve 80 % power to detect a morning pain difference of 15 % as statistically significant; therefore, each group was required at least 29 participants. The primary analysis was intention-to-treat and involved all patients who were randomly assigned.
Descriptive analysis was conducted to explore the characteristics of the participants at baseline. The median, the 25th and the 75th inter-quartile percentiles of the different pain scores, the mean and the standard deviation of age, height, weight, BMI, and the percentages of the gender distribution by intervention type, were calculated.
To compare the different AOFAS sub-scores across the different time periods, Friedman’s analyses were carried out. Post hoc tests were used to compare the scores between a given time period and the one that preceded it. Since post hoc tests were used several times, the significance level was divided by the number of planned comparisons and each two sample test was accordingly performed at the reduced level. A Kruskal–Wallis test was used to compare the AOFAS total score and sub-scores between the two intervention groups at the different time periods, with Cochran’s Q-test for success (categorical data).
Fisher’s exact test was used to compare the number of patients who had an improvement of at least 50 % in AOFAS score at 12 weeks and those who achieved an improvement of at least 80 % at 12 months.
Finally, univariate and multivariate analysis models were used to test for the preferential effect of the age, sex, obesity, duration of symptoms, previous cortisone injection and technique on successful outcome at one year. P values less than 0.05 were considered statistically significant
Results
Baseline demographics and the different scores of the study participants are presented in Table 1. Each group achieved progressive improvement at each follow-up time point, in different parameters measured; records are shown in Table 2.
Table 1.
Comparison of baseline characteristics and various scores across compared groups. Values are medians and interquartile ranges, numbers of participants and their percentages, and means ± SD of age, duration of symptoms and BMI
| ESWT | Fasciotomy | P value | |
|---|---|---|---|
| N = 34 | N = 31 | ||
| Male | 18 (52.9 %) | 22 (70.96 %) | 0.20 |
| Age | 37.7(9.42) | 39.7 (8.79) | 0.40 |
| Mean (SD) Body Weight (kg) | 83.59 (14.09) | 85.65 (14.25) | 0.56 |
| Mean (SD) Height (m) | 1.66 (.09) | 1.68 (.08) | 0.64 |
| Body Mass Index | 29.1 (25-33) | 29.5(27-31) | 0.83 |
| Obese participant (percentage) | 16 (47.05 %) | 14 (45.16 %) | 0.99 |
| Duration of symptoms (months) | 18.0 (10.9) | 17.45 (8.5) | 0.81 |
| Patient number with duration of symptoms >24 months | 8 (23.52 %) | 10 (32.25 %) | 0.58 |
| Patient number that received previous Cortisone injection | 29 (85.3 %) | 29 (90.3 %) | 0.71 |
| Morning pain Score | 71.0 (59.75 -78.0) | 68.0 (54 -78.0) | 0.47 |
| AOFAS Score | 43.00 (40 – 49) | 44.0 ( 42 – 65) | 0.29 |
Values are median and interquartile ranges, means ± SD, or numbers of participants and their percentages.
Table 2.
Comparison between ESWT and Fasciotomy groups across time
| Test and time in weeks | ESWT | Fasciotomy |
|---|---|---|
| (N = 34) | (N = 31) | |
| Morning pain | ||
| 0 | 71 (59-78) | 68 (54-78) |
| 3 | 40 (34.75-55.25)* | 41 (30-49)* |
| 12 | 30 (20-40.75)* | 30(25-40)* |
| 52 | 15 (5-25)* | 16 (11-25)* |
| <0.017 | <0.017 | |
| AOFAS pain score | ||
| 0 | 0 (0- 0) | 0 (0-20) |
| 3 | 20 (0-30)* | 20 (0-30)* |
| 12 | 30 (20-30)* | 30 (20-30) |
| 52 | 30 (27.5-40)* | 30 (20-30)* |
| <0.017 | <0.017 | |
| AOFAS activity limitation | ||
| 0 | 4 (4 -7) | 4 (4 -7) |
| 3 | 4 (4-7) | 4 (4-7) |
| 12 | 5.5 (4-7)* | 7 (4-7)* |
| 52 | 7 (4-7)* | 7 (7-10)* |
| <0.017 | <0.017 | |
| AOFAS max. walking distance | ||
| 0 | 4 (2- 4) | 4 (2- 4) |
| 3 | 4 (4-5) * | 4 (2-4) |
| 12 | 4(4-5) | 4(4-5) * |
| 52 | 5(4-5) | 5(4-5) * |
| <0.017 | <0.017 | |
| AOFAS walking surface | ||
| 0 | 3 (3-5) | 3 (3-5) |
| 3 | 5 (3-5) * | 5 (3-5) |
| 12 | 5(3-5) | 5(3-5) |
| 52 | 5(5-5) * | 5(5-5) * |
| <0.017 | <0.017 | |
| AOFAS gait abnormality | ||
| 0 | 4 (0- 4) | 4 (0- 4) |
| 3 | 4 (4-8) * | 4 (4-4) |
| 12 | 8(4-8) * | 8(4-8) * |
| 52 | 8(7-8) | 8(8-8) |
| <0.017 | <0.017 | |
| AOFAS sagital motion | ||
| 0 | 8 (8-8) | 8 (8-8) |
| 3 | 8 (8-8) | 8 (8-8) |
| 12 | 8 (8-8) | 8 (8-8) |
| 52 | 8 (8-8) | 8 (8-8) |
| <0.017 | <0.017 | |
| AOFAS hindfoot motion | ||
| 0 | 6 (6-6) | 6 (6-6) |
| 3 | 6 (6-6) | 6 (6-6) |
| 12 | 6 (6-6) | 6 (6-6) |
| 52 | 6 (6-6) | 6 (6-6) |
| <0.017 | <0.017 | |
| AOFAS ankle/hindfoot instability | ||
| 0 | 8 (8-8) | 8 (8-8) |
| 3 | 8 (8-8) | 8 (8-8) |
| 12 | 8 (8-8) | 8 (8-8) |
| 52 | 8 (8-8) | 8 (8-8) |
| <0.017 | <0.017 | |
| AOFAS alignment | ||
| 0 | 10 (10-10) | 10 (10-10) |
| 3 | 10 (10-10) | 10 (10-10) |
| 12 | 10 (10-10) | 10 (10-10) |
| 52 | 10 (10-10) | 10 (10-10) |
| <0.017 | <0.017 | |
| AOFAS Total SCORE | ||
| 0 | 43 (40 – 49) | 44 (42 – 65) |
| 3 | 70 (53.5-79)* | 68 (65-75)* |
| 12 | 80.5 (73-85)* | 77 (72-84)* |
| 52 | 87 (76.75-97)* | 86 (76 -89)* |
| <0.017 | <0.017 | |
| Roles & Maudsley score | ||
| 0 | 4 (4 – 4) | 4 (4-4) |
| 3 | 3 (2 – 3)* | 3 (2-3)* |
| 12 | 2 (2-3) * | 2 (2-3) |
| 52 | 2 (1-3) * | 2 (1-2) * |
| <0.017 | <0.017 | |
| Success (Roles and Maudsely, excellent and good) | ||
| 0 | 1(1-1) | 1(1-1) |
| 3 | 1 (1 – 2)* | 1 (1-1)* |
| 12 | 2 (1 – 2)* | 2 (1-2) |
| 52 | 2 (1-2) | 2 (1-2)* |
| 2 years | 1.5(1-2) | 2 (2-2) |
| 3 years | 1(1-2) | 1(1-2) |
| <0.01 | <0.01 | |
Values are median (25th and 75th percentiles) and proportion (percentages)
*Significantly different from the precedent time period
In the ESWT group, the majority of improvements were achieved and maintained between week three and week 12 post-intervention, and continued to a lesser extent for up to one year. In the EPFR group, the majority of improvements were achieved between week three and month 12 post-intervention.
A minimum of 50 % improvement of AOFAS score at 12 weeks was achieved in 25/34 patients in the ESWT group and 21/31 patients in the EPFR group [Fisher’s exact test, p = 0.785, RR = 0.82 1 (95 % CI = 0.384–1.751), Risk difference 5.7 (95 % CI = 16.3–27.9)]
At 12-month follow-up, a minimum of 80 % improvement of AOFAS score was achieved in 22/34 patients in the ESWT group and 18/31 patients in the EPFR [Fisher’s exact test, p = 0.618, RR = 0.842 (95 % CI = 0.455–1.558), Risk difference 6.6 (95 % CI = 17.1–30.3)]
No significant differences between the ESWT and EPFR groups were detected through the different time periods for any measured parameter, except for the AOFAS maximum walking distance and gait sub-scores, which showed a statistically significant difference between the ESWT group and the EPFR group at 3 weeks (p = 005 and 002, respectively.) (Table 3).
Table 3.
Comparison of different scores across ESWT and Fasciotomy groups in the different time periods
| Baseline | 3 weeks | 12 weeks | One year | ||
|---|---|---|---|---|---|
| Morning pain | |||||
| ESWT (n = 34) | 71 (59-78) | 40 (34.75-55.25) | 30 (20-40.75)* | 15 (5-25) | |
| Fasciotomy(n = 31) | 68 (54-78) | 41 (30-49) | 30 (25-40)* | 16 (11-25) | |
| P value | .47 | .45 | .71 | .20 | |
| American Orthopedic Foot and Ankle-Hindfoot Scale (AOFAS) | Pain score | ||||
| ESWT | 0 (0- 0) | 20 (0-30)* | 30 (20-30)* | 30 (27.5-40)* | |
| Fasciotomy | 0 (0-20) | 20 (0- 30) | 30 (20-30) | 30 (20- 30) | |
| .62 | .35 | .40 | .06 | ||
| Activity limitation | |||||
| ESWT | 4 (4 -7) | 4 (4 -7) | 5.5 (4 -7) | 7 (4 -7) | |
| Fasciotomy | 4 (4 -7) | 4 (4 -7) | 7 (4 -7) | 7 (7 -10) | |
| .57 | .90 | .91 | .34 | ||
| Maximum walking distance | |||||
| ESWT | 4 (2- 4) | 4 (4- 5) | 4 (4- 5) | 5 (4- 5) | |
| Fasciotomy | 4 (2- 4) | 4 (2- 4) | 4 (4- 5) | 5 (4- 5) | |
| .79 | .005* | .49 | .87 | ||
| Walking surface | |||||
| ESWT | 3 (3-5) | 5 (3-5) | 5 (3-5) | 5 (5-5) | |
| Fasciotomy | 3 (3-5) | 5 (3-5) | 5 (3-5) | 5 (5-5) | |
| .69 | .75 | .43 | .79 | ||
| Gait abnormality | |||||
| ESWT | 4 (0- 4) | 4 (4- 8) | 8 (4- 8) | 8 (7- 8) | |
| Fasciotomy | 4 (0- 4) | 4 (4- 4) | 8 (4- 8) | 8 (8- 8) | |
| .16 | .002* | .82 | .26 | ||
| Sagital motion | |||||
| ESWT | 8 (8-8) | 8 (8-8) | 8 (8-8) | 8 (8-8) | |
| Fasciotomy | 8 (8-8) | 8 (8-8) | 8 (8-8) | 8 (8-8) | |
| .61 | .57 | .57 | .57 | ||
| Hindfoot motion | |||||
| ESWT | 6 (6-6) | 6 (6-6) | 6 (6-6) | 6 (6-6) | |
| Fasciotomy | 6 (6-6) | 6 (6-6) | 6 (6-6) | 6 (6-6) | |
| .61 | .61 | .61 | .61 | ||
| Ankle/hind foot instability | |||||
| ESWT | 8 (8-8) | 8 (8-8) | 8 (8-8) | 8 (8-8) | |
| Fasciotomy | 8 (8-8) | 8 (8-8) | 8 (8-8) | 8 (8-8) | |
| .99 | .99 | .99 | .99 | ||
| Alignment | |||||
| ESWT | 10 (10-10) | 10 (10-10) | 10 (10-10) | 10 (10-10) | |
| Fasciotomy | 10 (10-10) | 10 (10-10) | 10 (10-10) | 10 (10-10) | |
| .50 | .50 | .50 | .50 | ||
| Total Score | |||||
| ESWT | 43 (40 – 49) | 70 (53.5 – 79) | 80.5 (73 – 85) | 87 (76.75 – 79) | |
| Fasciotomy | 44 (42 – 65) | 68 (65 –75) | 77 (72 – 84) | 86 (76 – 89) | |
| .29 | .59 | .29 | .27 | ||
| Roles and Maudsely score | |||||
| ESWT | 4 (4 – 4) | 3 (2 – 3) | 2(2– 3) | 2 (1 – 3) | |
| Fasciotomy | 4 (4 – 4) | 3(2– 3) | 2 (2 –3) | 2 (1 – 2) | |
| .99 | .75 | .23 | .55 | ||
| Success (Roles and Maudsley excellent and good results) | |||||
| ESWT | - | 14 (41.2 %) | 22 (64.7 %) | 24 (70.6 %) | |
| Fasciotomy | - | 10 (32.3 %) | 16 (51.6 %) | 24 (77.4 %) | |
| .16 | .11 | .19 | |||
*Significant value
Three participants from the ESWT group (one who received cortisone injections and two who were lost to follow-up), and two participants from the EPFR group (both who were lost to follow-up and did not complete the 12 month assessment) were included in the statistics using the last AOFAS score and sub-score, and were rated as failure in the final outcome measure.
The success rates (number of patients who achieved good and excellent scores in the Roles and Maudsley criteria) for the ESWT and EPFR groups at 3 weeks were 14 (41.2 %) and 10 (32.25 %), p = 0.16, respectively. This number increased to 22 (64.7 %) and 16 (51.61 %), p = 0.11, at 12 weeks. At the one year follow-up, the numbers were 24/34 (70.6 %) and 24/31 (77.4 %), p = 0.19 for the ESWT and EPFR groups, respectively (Table 3).
Multivariate statistical analysis indicated that age, obesity, sex, previous cortisone injection, and intervention type has no statistically significantly effect on the success. Patients who had symptoms less than 24 months had better outcomes than those who had symptoms more than 24 months (OR = 0.379,; p = 0.006; 95 % CI = 0.1–0.755).
At 2 years post-intervention, telephone follow-up was conducted to ask the patient to rate their results as success or failure, compared to the previous follow-up time point. We found that 13/26 (50 %) of the ESWT group and 20/25 (80 %) of the EPFR group reported successful outcome (p = 0.026). At 3 years post-intervention, 11/23 (47.8 %) patients in the ESWT group reported success, compared to 20/25 (80 %) in EPFR group (p = 0.021). Five pair-wise comparisons were conducted for the secondary endpoint (success rate). Bonferroni’s correction was done, and p-value was adjusted to a value of 0.01.
Discussion
shock wave therapy in the treatment of proximal plantar fasciopathy has a reported success rate ranging from 34 % to 88 % [7, 38]. Many, but not all, randomized, blinded and controlled multicenter trials have demonstrated that ESWT is more effective than placebo treatment [18, 24, 34]. However, some studies showed disappointing results [6, 13, 33].
Our study shows that ESWT is indeed an effective treatment of chronic plantar fasciopathy. The median VAS morning pain score improved from 71 to 30, and AOFAS increased from 43 to 80.5 by the 3rd month post-intervention, and to 87 at one year. The overall results for the ESWT group at one year were 70.6 % excellent to good. A substantial improvement of symptoms was achieved between 3 weeks to 3 months post-intervention (25/34 patients achieved an improvement of at least 50 %). This improvement was maintained at the one year follow-up; yet, there was a drop of the success level at two and three years, to 50 %, and 48 % respectively.
Our ESWT results reinforced the positive outcomes of many randomized controlled trials that reported a reasonable rate of success using high-energy shock wave therapy for treatment of PPF [20, 24, 37]. Electrohydraulic shock wave generation was the first shock wave method approved by the Food and Drug Administration for musculoskeletal use [14]. High-energy shock waves produce an adequate amount of energy that can produce controlled inflammation which has shown to stimulate many mediators, such as transforming growth factor beta 1 (TGF-B1) and insulin-like growth factor 1 (IGF-I) and initiates the healing process [8]. Whereas some studies demonstrated that poor results were associated with low dose shock wave that did not produce enough energy to stimulate the healing process [33], other studies of low energy shock wave therapy demonstrated reasonable outcome if the study was properly designed [31]. Local anesthetic was not used, as it possibly interferes with hyperstimulation analgesia by altering the tissue effect to ESWT and preventing clinical refocusing (a local anesthetic may inhibit the aiming of the treatment head at the point of maximal tenderness).
Because of the multiple variables inherent in the use of shock wave therapy in the management of plantar fasciopathy, strict comparisons of published results are problematic. On the basis of the preponderance of well-designed studies showing favorable results, it seems that the literature supports a therapeutic benefit and wide safety margin for shock wave therapy for managing chronic plantar fasciopathy [29].
The results of EPFR are also encouraging. In a multi-surgeon prospective analysis of 652 patients treated with endoscopic plantar fasciotomy, 97 % reported that they had heel pain relief [3]. However, a complication rate of 10 % for lateral column overload with calcaneocuboid and mid-tarsal joint pain was reported. The incidence of lateral column overload was high in early reports of EPFR because of complete release of the plantar fascia. The later recommendation of partial release of only the medial two-thirds of the plantar fascia led to a decreased incidence of lateral column overload [35]. Endoscopic fascitomy had less common recurrent pain, neuritis, infection and earlier functional recovery compared with traditional surgery [16, 36].
In EPRF group, the median VAS morning pain score improved from 68 to 30, whereas AOFAS increased from 44 to 77 at the 3rd month post-operative and to 86 at one year follow-up. The overall success rate (excellent to good) at 3 months was 51.61 %, increased to 77 % at one year and was maintained up to the third year, showing 80 % success. A reasonable improvement of symptoms was achieved at 3 months, and progressive improvement occurred until the 1 year follow-up. This is comparable with the results reported by many studies [4, 5, 11, 26].
The goal of partial plantar fasciotomy is to reduce the mechanical overload in the affected area. In the current study, we added debridement of the pathological tissue at the fascial origin and the inflamed periosteum, using the motorized incisor blade. This was expected to improve the final result. Also, we used the heel bisector as a landmark for the middle of the plantar fascia, which is fairly accurate as long as the needle is inserted perpendicular to the heel skin; thereby only 50 % release was achieved, and subsequently lateral column symptoms were not recorded.
We believe that the technique described in the current study is simple, economic, not technically demanding, and does not need special instruments. Also, we found that visualization is better if the endoscope is introduced through the lateral portal, unlike previously described techniques [4, 5, 15, 26]. Proper visualization depends on a water pressure of 50-60 mmHg to inflate the subcutaneous tunnel, and because of the tight nature of the heel fat pad, no fluid extravasation occurs.
No major side effects were observed in our study of the ESWT group. Two patients (6 %) developed parasthesia and two patients developed petechiae and ecchymosis at the treatment area. All made spontaneously full recovery within 1 month. None of the patients in EPFR group had lateral column pain at the end of follow-up, and only two of our early cases developed postoperative swelling that resolved with foot elevation.
Most cases of plantar fasciopathy are self-limited [21]. In this study, trying to avoid the effect of time on healing, we selected patients who had symptoms for more than 6 months, with an average 18 month duration of symptoms. Each of the patients also failed to respond to multiple conservative treatments within the last 6 months prior to enrollment. Rompe et al. [30] reported that plantar fascia stretching exercises were superior to repetitive low-energy radial shock wave therapy for the initial management of acutely presenting plantar fasciopathy (65 % versus 29 % total satisfaction, respectively). Recovery of acute plantar fasciopathy is frequently slow and recurrences are not uncommon, and once the condition is chronic, the response to any form of treatment is less predictable [21].
In our study, correlation analyses showed that patients who had symptoms less than 24 months had better outcomes (OR = 0.379), matching previous studies [1, 23]. Whereas, age, sex, previous cortisone injection, and obesity had no statistically significantly effect on successful outcome. In contrast to other study [9] which showed that diabetes mellitus, psychological issues, and age were found to negatively influence ESWT outcome.
Weil et al. [38] reported similar results for 40 patients that had ESWT, compared to eight patients who underwent percutaneous plantar fasciotomy. In our study, both the ESWT and EPFR groups showed comparable results with respect to the reduction of pain, functional progress and overall rating of the disease state. The lack of statistical significance is not attributable to the absence of a suitable sample size or type-II errors. We believe that ESWT is a comparable method of treatment to that of operative intervention.
The assessment of various management approaches is often limited by small sample sizes, heterogeneous study populations, and surrogate outcome measures [32]. There is a growing concern towards checking the impact of treatments on patients´ quality of life, regarding how they feel about their conditions and how they perform their daily life activities. The challenge lies in how to quantify subjective data, and which questions should be addressed by the various instruments assessing health-related quality of life. We chose the widely used American Orthopedic Foot and Ankle-Hindfoot Scale [17] to allow comparison of the data; however, our limitation was that the AOFAS score has not been validated, and translation has not been cross-culturally adapted.
Future research should be focused on carrying out randomized clinical trials that include a sufficient number of patients, comparing different combinations and treatment algorithms, in the medium-to-long term duration.
Conclusion
In patients who had experienced failure of conventional treatment of plantar fasciopathy, shock wave therapy can be a potentially helpful line of management. Our study has revealed comparable results of high-energy ESWT, when compared with EPFR at 3 months and 1 year. However, EPFR has more favorable results afterwards, although not statistically significant. ESWT appears to be a useful noninvasive treatment that may represent a short term prudent and cost-effective alternative for the treatment of resistant plantar fasciopathy that reduces the necessity for surgical procedures.
References
- 1.Bader L, Park K, Gu Y, O'Malley MJ. Functional outcome of endoscopic plantar fasciotomy. Foot Ankle Int. 2012;33(1):37–43. doi: 10.3113/FAI.2012.0037. [DOI] [PubMed] [Google Scholar]
- 2.Bahr R, Fossan B, Løken S, Engebretsen L. Surgical treatment compared with eccentric training for patellar tendinopathy (Jumper's Knee). A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(8):1689–1698. doi: 10.2106/JBJS.E.01181. [DOI] [PubMed] [Google Scholar]
- 3.Barrett SL, Day SV, Pignetti TT, Robinson LB. Endoscopic plantar fasciotomy: A multi-surgeon prospective analysis of 652 cases. J Foot Ankle Surg. 1995;34:400–406. doi: 10.1016/S1067-2516(09)80011-2. [DOI] [PubMed] [Google Scholar]
- 4.Bazaz R, Ferkel RD. Results of endoscopic plantar fascia release. Foot Ankle Int. 2007;28:549–556. doi: 10.3113/FAI.2007.0549. [DOI] [PubMed] [Google Scholar]
- 5.Boyle R, Slater G. Endoscopic plantar fascia release: a case series. Foot Ankle Int. 2003;24:176–179. doi: 10.1177/107110070302400213. [DOI] [PubMed] [Google Scholar]
- 6.Buchbinder R, Ptasznik R, Gordon J, Buchanan J, Prabaharan V, Forbes A. Ultrasound-guided extracorporeal shock wave therapy for plantar fasciitis: a randomized controlled trial. JAMA. 2002;288:1364–1372. doi: 10.1001/jama.288.11.1364. [DOI] [PubMed] [Google Scholar]
- 7.Chen HH, Chen LM, Huang TW. Treatment of painful heel syndrome with shock waves. Clinc Orthop Relat Res. 2001;387:41–46. doi: 10.1097/00003086-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Chen YJ, Wang CJ, Kuender DY, Yur-Ren K, Huang HC, Huang YC, Sun YC, Wang FS. Extracorporeal shock waves promote healing of collagenase-induced Achilles tendonitis and increase TGF-B1 and IGF-I expression. J Orthop Res. 2004;22:854–861. doi: 10.1016/j.orthres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Chuckpaiwong B, Berkson EM, Theodore GH. Extracorporeal shock wave for chronic proximal plantar fasciitis: 225 patients with results and outcome predictors. Foot Ankle Surg. 2009;48(2):148–155. doi: 10.1053/j.jfas.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Cornwall MW, McPoil TG. Plantar fasciitis: etiology and treatment. J Orthop Sports Phys Ther. 1999;29:756–760. doi: 10.2519/jospt.1999.29.12.756. [DOI] [PubMed] [Google Scholar]
- 11.El Shazly O, El Hilaly RA, Abou El Soud MM, El Sayed MN. Endoscopic plantar fascia release by hooked soft-tissue electrode after failed shock wave therapy. Arthroscopy. 2010;26(9):1241–1245. doi: 10.1016/j.arthro.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Furia JP. The safety and efficacy of high energy extracorporeal shock wave therapy in active, moderately active, and sedentary patients with chronic plantar fasciitis. Orthopedics. 2005;28(7):685–692. doi: 10.3928/0147-7447-20050701-17. [DOI] [PubMed] [Google Scholar]
- 13.Haake M, Buch M, Schoellner C, Goebel F, Vogel M, Mueller I, Hausdorf J, Zamzow K, Schade-Brittinger C, Mueller HH. Extracorporeal shock wave therapy for plantar fasciitis: randomised controlled multicentre trial. BMJ. 2003;327:75–79. doi: 10.1136/bmj.327.7406.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henney JE. From the food and drug administration: shock wave for heel pain. JAMA. 2000;284:2711. doi: 10.1001/jama.284.21.2711. [DOI] [PubMed] [Google Scholar]
- 15.Jerosch J, Schunck J, Liebsch D, Filler T. Indication, surgical technique and results of endoscopic fascial release in plantar fasciitis. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):471–477. doi: 10.1007/s00167-004-0496-6. [DOI] [PubMed] [Google Scholar]
- 16.Kinley S, Frascone S, Calderone D, Wertheimer SJ, Squire MA, Wiseman FA. Endoscopic plantar fasciotomy versus traditional heel spur surgery: a prospective study. J Foot Ankle Surg. 1993;32:595–603. [PubMed] [Google Scholar]
- 17.Kitaoka HB, Alexander IJ, Adelaar RS, et al. Clinical rating system for the ankle hindfoot, midfoot, hallux and lesser toes. Foot Ankle Int. 1994;15:349–353. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 18.Kudo P, Dainty K, Clarfield M, Coughlin L, Lavoie P, Lebrun C. Randomized, placebo-controlled, double blind clinical trial evaluating the treatment of plantar fasciitis with an extracorporeal shock wave therapy (ESWT) device: a North American confirmatory study. J Orthop Res. 2006;24:115–123. doi: 10.1002/jor.20008. [DOI] [PubMed] [Google Scholar]
- 19.Lafuente AG, O’Mullony IM, Escriba M, Cura-Ituarte P. Plantar fasciitis: evidence-based review of treatment. Reumatol Clin. 2007;3(4):159–165. doi: 10.1016/S1699-258X(07)73614-8. [DOI] [PubMed] [Google Scholar]
- 20.Malay DS, Pressman MM, Assili A, Kline JT, York S, Buren B, Heyman ER, Borowsky P, LeMay C. Extracorporeal shock wave therapy versus placebo for treatment of chronic proximal plantar fasciitis: results of a randomized, placebo-controlled, double-blinded, multicenter intervention trial. J Foot Ankle Surg. 2006;45(4):196–210. doi: 10.1053/j.jfas.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int. 1998;19:803–811. doi: 10.1177/107110079801901203. [DOI] [PubMed] [Google Scholar]
- 22.Metzner G, Dohnalek C, Aigner E. High-energy extracorporeal shock wavetherapy (ESWT) for the treatment of chronic plantar fasciitis. Foot Ankle Int. 2010;31(9):790–796. doi: 10.3113/FAI.2010.0790. [DOI] [PubMed] [Google Scholar]
- 23.Ogden JA, Alvarez R, Levitt R, Cross GL, Marlow M. Shock wave therapy for chronic proximal plantar fasciitis. Clin Orthop. 2001;387:47–59. doi: 10.1097/00003086-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Ogden JA, Alvarez RG, Levitt RL, Johnson JE, Marlow ME. Electrohydraulic high-energy shock wave treatment for chronic plantar fasciitis. J Bone Joint Surg Am. 2004;86(10):2216–2228. doi: 10.2106/00004623-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Ogden JA, Alveraz RG, Mariow M. shock wave therapy for chronic proximal plantar fasciitis. Foot Ankl Int. 2002;23:301–308. doi: 10.1177/107110070202300402. [DOI] [PubMed] [Google Scholar]
- 26.Ogilvie-Harris D, Lobo J. Endoscopic plantar fascia release. Arthroscopy. 2000;16(3):290–298. doi: 10.1016/S0749-8063(00)90053-7. [DOI] [PubMed] [Google Scholar]
- 27.Roles NC, Maudsley RH. Radial tunnel syndrome. J Bone Joint Surg Br. 1972;54:499–508. [PubMed] [Google Scholar]
- 28.Rompe JD. Plantar fasciopathy. Sports Med Arthrosc. 2009;17:100–104. doi: 10.1097/JSA.0b013e3181a3d60e. [DOI] [PubMed] [Google Scholar]
- 29.Rompe JD, Furia J, Weil L, Maffulli N. Shock wave therapy for chronic plantar fasciopathy. Br Med Bull. 2007;81–82:183–208. doi: 10.1093/bmb/ldm005. [DOI] [PubMed] [Google Scholar]
- 30.Rompe JD, Cacchio A, Weil L, Furia J, Haist J, Reiners V, Schmitz C, Maffulli N. Plantar fascia-specific stretching versus radial shock wave therapy as initial treatment of plantar fasciopathy. J Bone Joint Surg Am. 2010;92:2514–2522. doi: 10.2106/JBJS.I.01651. [DOI] [PubMed] [Google Scholar]
- 31.Rompe JD, Schoellner C, Nafe B. Evaluation of low-energy extracorporeal shock wave application for the chronic plantar fasciitis. J Bone Joint Surg Am. 2002;84:335–341. doi: 10.1302/0301-620X.84B3.12460. [DOI] [PubMed] [Google Scholar]
- 32.Speed CA. Extracorporeal shock wave therapy in the management of chronic soft tissue conditions. J Bone Joint Surg Br. 2004;86:165–171. doi: 10.1302/0301-620X.86B2.14253. [DOI] [PubMed] [Google Scholar]
- 33.Speed CA, Nichols D, Wies J, Humphreys H, Richards C, Burnet S, Hazleman BL. Extracorporeal shock wave therapy for plantar fasciitis. A double blind randomised controlled trial. J Orthop Res. 2003;21:937–940. doi: 10.1016/S0736-0266(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 34.Theodore GH, Buch M, Amendola A, Bachmann C, Fleming LL, Zingas C. Extracorporeal shock wave therapy for the treatment of plantar fasciitis. Foot Ankle Int. 2004;25(5):290–297. doi: 10.1177/107110070402500503. [DOI] [PubMed] [Google Scholar]
- 35.Thorderson DB, Kumar PJ, Hedman TP, Ebramzadeh E. Effect of partial versus complete plantar fasciotomy on the windlass mechanism. Foot Ankle Int. 1997;18:16–20. doi: 10.1177/107110079701800104. [DOI] [PubMed] [Google Scholar]
- 36.Tomczak R, Haverstock B. A retrospective comparison of endoscopic plantar fasciotomy to open fasciotomy with heel spur resection for chronic plantar faciitis/heel spur syndrome. J Foot Ankle Surg. 1995;34:305–311. doi: 10.1016/S1067-2516(09)80065-3. [DOI] [PubMed] [Google Scholar]
- 37.Wang CJ, Wang FS, Yang KD, Weng LH, Ko JY. Long-term results of extracorporeal shock wave treatment for plantar fasciitis. Am J Sports Med. 2006;34(4):592–596. doi: 10.1177/0363546505281811. [DOI] [PubMed] [Google Scholar]
- 38.Weil LS, Jr, Roukis TS, Weil LS, Borrelli AH. Extracorporeal shock wave therapy for the treatment of chronic plantar fasciitis: indications, protocol, intermediate results, and a comparison of results to fasciotomy. J Foot Ankle Surg. 2002;41(3):166–172. doi: 10.1016/S1067-2516(02)80066-7. [DOI] [PubMed] [Google Scholar]