Abstract
Purpose
Haemophilic pseudotumour was defined by Fernandez de Valderrama and Matthews as a progressive cystic swelling involving muscle, produced by recurrent haemorrhage into muscles adjacent to the bone. The pseudotumour mainly occurs in the long bones and the pelvis. The treatment of the haemophilic pseudotumour poses a challenge, and extensive clinical experience is essential to appropriately address this serious complication in patients with haemophilia. Consequently, the aim of this study is to present our own clinical experience and treatment results of the haemophilic pseudotumour.
Methods
We retrospectively reviewed the records of 87 patients with bleeding disorders treated between 1967 and 2011 for musculoskeletal complications of congenital bleeding disorders. We identified six patients with a haemophilic pseudotumour who were treated at our department.
Results
The mean age at surgery was 45.9 (range, 40–61) years. The iliac bone was affected in three patients (one right, two left), the right tibia (distal diaphysis) in one, the right thigh in two and the right ulna (proximal part) in one patient. One patient had two pseudotumours. The perioperative course was easily controllable with adequate factor VIII substitution. At the latest follow-up after 8.4 (range, 4–24) years, normal healing with no recurrence was observed.
Conclusions
The haemophilic pseudotumour is a rare but severe complication of hereditary bleeding disorders. In the international literature the resection and postoperative course are described as challenging and difficult, requiring detailed preoperative planning. It is advisable to perform such operations in specialised centres with close co-operation between surgeons and haematologists.
Introduction
The haemophilic pseudotumour was defined by Fernandez de Valderrama and Matthews as a progressive cystic swelling involving muscle, produced by recurrent haemorrhage and accompanied by radiographic evidence of bone involvement [1–3]. Anatomically, it is an encapsulated haematoma with calcification and ossification [2].
Haemophilic pseudotumours occur in 1–2 % of patients with severe haemophilia [2, 4–6]. Clinically, haemophilic pseudotumours usually present as a painless expanding mass growing over years [7–9]. Their most serious sequel is a pathological bone fracture and uncontrollable bleeding. Two types of pseudotumours have been described by Gilbert [7]: a proximal and a distal type. The proximal pseudotumour mainly occurs in the long bones (especially the femur) and the pelvis of the mature skeleton of adult haemophilic patients. The proximal pseudotumour is preceded by a history of trauma and recurrent bleeding, in particular repeated and unresolved bleeding into muscles adjacent to the bone [7], and it develops over many years [3]. Other reports state that there is a “evidence of subperiostal bleeding” and even intraosseous haemorrhage has been reported as the origin of bleeding [3, 7]. In general, the proximal type of haemophilic pseudotumour does not respond to conservative treatment [2].
Distal pseudotumours mostly occur in the young patient with open epiphyseal growth plates [7]. This type of pseudotumour mostly affects the small bones of hands and feet of the immature skeleton and is of intraosseous origin [3, 7, 8]. Distal refers to the peripheral location in the skeleton. The distal pseudotumour develops rapidly and does not result from direct trauma [3, 7]. Conservative treatment with replacement therapy and immobilisation may resolve the distal type [2, 10].
A distinctive type of pesudotumour was described by Fernandez de Valderrama and Matthews, which affects the muscle and has no effect on the adjacent bone [1].
Different therapy options have been described in the treatment of the haemophilic pseudotumour including surgical resection, arterial embolisation, radiotherapy, percutaneous curettage, and filling with fibrin and/or bone graft or hydroxyapatite [2, 3, 10–17]. However, treatment of the haemophilic pseudotumour poses a challenge, and extensive clinical experience is essential to appropriately address this serious complication in patients with haemophilia. Consequently, the aim of this study was to present our own clinical experience and treatment results of the haemophilic pseudotumour.
Patients and methods
We retrospectively reviewed the records of 87 patients with bleeding disorders, who were treated at the Department of Orthopaedic Surgery, Medical University of Vienna, between 1967 and 2011 for musculoskeletal complications of congenital bleeding disorders. We identified six patients with a haemophilic pseudotumour who were treated in our department.
Most of the patients were followed-up regularly by the first author (J.P.) and the senior author (A.W) at the outpatient clinic. The clinical histories and imaging of patients eligible for inclusion in the study were first surveyed by the co-authors (V.S. and J.H.) and additionally evaluated and reviewed by the first author (J.P.). All data including localisation, histological findings, clinical history, symptoms (pain, swelling, neurological findings, coagulopathy), underlying disease, imaging, trauma, treatment regimen and date of the peri- and postoperative course were collected.
Statistical analysis
Standard descriptive statistical analyses were applied to describe the characteristics of patients.
Results
Patients' characteristics
The mean age at surgery was 45.9 (range, 40–61) years. All six patients had severe haemophilia A with a factor VIII activity of <1 %. One patient developed an antibody (inhibitor) against factor VIII. In this patient inhibitor reduction from Bethesda Units 30 to 4 was achieved by elimination therapy according to the Malmö protocol before surgery. All patients were positive for hepatitis C, three were positive for hepatitis B, three patients had chronic liver cirrhosis and died as a consequence of liver failure. One patient was positive for HIV. The details of all patients are summarised in Table 1.
Table 1.
Patient details
| Patient | Type of haemophilia | Virology | Year of surgery | Age at surgery | Localisation | Dimension (cm) | Symptoms | Surgery | FVIII | Remarks | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A <1% | Not available | 1967 | 40 | Thigh R | Not available | Not available | Above knee amputation | Not available | Not available | No recurrence |
| 2 | A <1% | Hep C | 1984 | 43 | Iliac L | 20 x 11.5 | Growing mass over 1.5 years, no pain | Resection | Yes | Infection, fistula after 12y and 17y | No recurrence |
| 3 | A <1% | Hep C, B | 2000 | 40 | Iliac L | 17.5 x 10.1 | Growing mass over 1 year, pain | Resection, cement, pin | Yes | No recurrence | |
| 2007 | 48 | Thigh R | 9.5 x 7.2 | Growing mass over 7 months, no pain | Excision | Yes | Died, liver failure after 3 years | No recurrence | |||
| 4 | A <1% | Hep C | 2000 | 41 | Elbow L | 3.2 x 2 | Paraesthesia over 3 years, pain over 6 months | Resection | Yes | Inhibitor | No recurrence |
| Inhibitor | Died, liver failure after 7.5 years | ||||||||||
| 5 | A <1% | Hep C, B | 2004 | 61 | Iliac R | 22.5 x 15 | Growing mass over 1.5 years, no pain | Resection, cement, pin | Yes | Infection after 4 weeks | No recurrence |
| Died, liver failure after 5 years | |||||||||||
| 6 | A <1% | Hep C, B, HIV | 2000 | 48 | Tibia R | 9.6 x 3.3 | Pain over 4 months | Curettage, cement, LCDCP titanium | Yes | Infection, fistula after 5 months | No recurrence |
Hep hepatitis, R right, L left
Localisation
All subjects were adults with a mature skeleton and had proximal type pseudotumours according to the classification of Gilbert [7].
The iliac bone was affected in three patients (one right, two left), the right tibia (distal diaphysis) in one, the right thigh in two and the right ulna (proximal part) in one patient (Table 1). One patient had two pseudotumours. In one patient the haemophilic pseudotumour affected only the muscle with no effect on the adjacent bone (femur) (patient 3).
Preoperative course
There was no history of heavy trauma, puncture, or intramuscular injection. All patients have had on-demand factor replacement for treatment of haemophilia. Prior to surgery, MRI and X-ray were obtained in four patients, and CT and X-ray in one patient in order to exclude a malignant bone or soft tissue tumour, whereas in one patient no imaging data was available. In this case a high-above-the-knee amputation had already been performed in 1967.
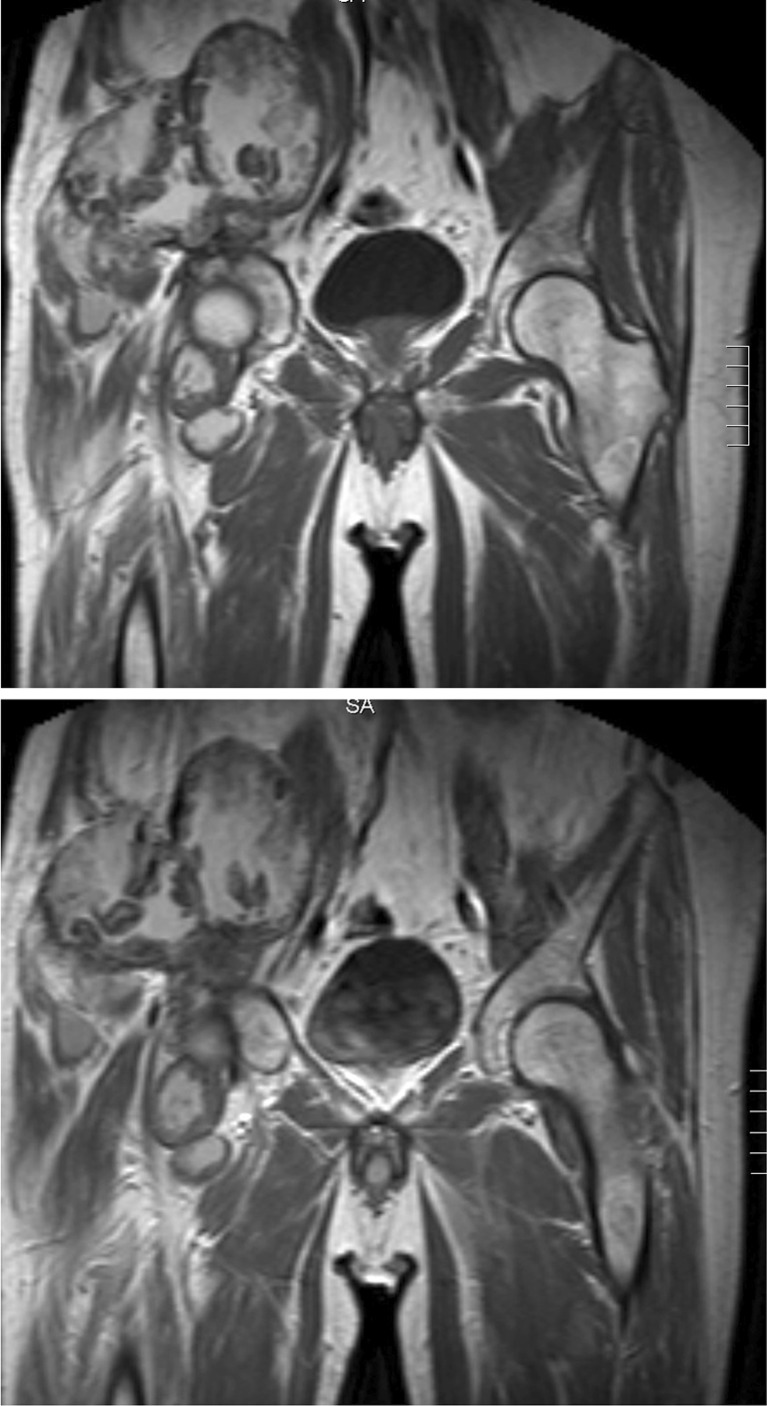
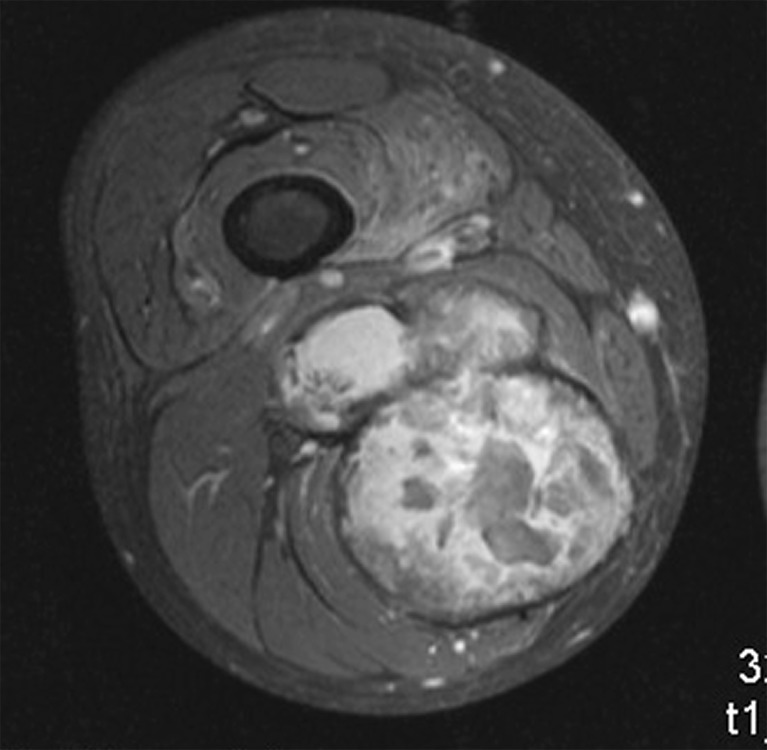
Diagnosis could easily be established due to typical clinical findings and through plain radiographs and/or MRI. The knowledge of the underlying disease and the long clinical history led to diagnosis. Typical findings in MRI were a rim enhancement with a rather minor uptake after contrast agent application. The large haemophilic pseudotumours were mainly poly-lobulated (Figs. 1 and 2). None of the patients required biopsy or embolisation before surgery.
Fig. 3.
Factor VIII levels
Fig. 4.
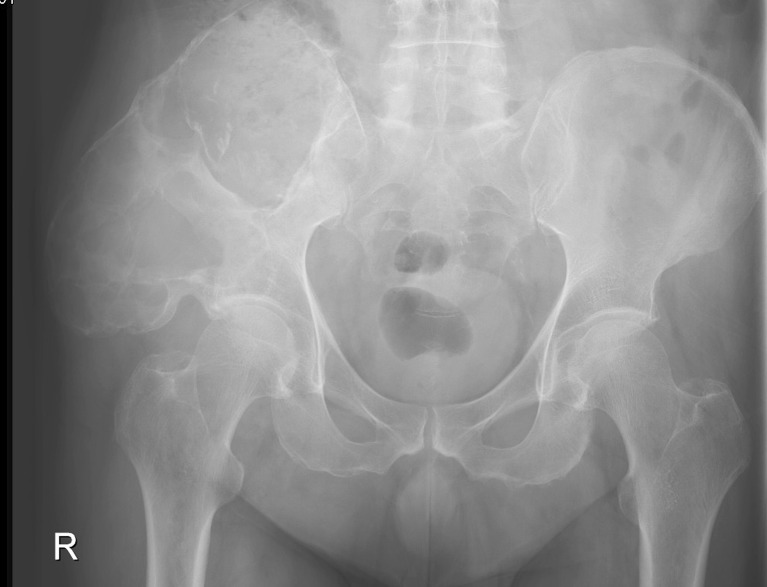
Radiograph with expansive osteolytic tumour mass with minor calcification of the right iliac bone (patient 5)
The clinical symptoms are described in Table 1, i.e. growing mass over 13.7 (7–18) months (patients 2, 3 and 5), and pain over four to six months (patients 4 and 6). Impaired walking was found in all patients, mainly due to impairment of adjacent joints. Neurological findings were present in one case due to compression of the ulnar nerve (patient 4). Conservative treatment with F VIII on demand was turned into F VIII prophylaxis after diagnosis of the pseudotumour.
Surgery
In all cases surgery was performed by the head of the department or by a senior consultant, both with vast experience in haemophilic surgery. We performed a resection of the haemophilic pseudotumour of the iliac bone in three cases, of the right distal tibia in one, of the right thigh in one, and of the left proximal ulna with transposition of the ulnar nerve in one patient.
The reconstruction of the iliac bone was a compound osteosynthesis with 3-mm pins and bone cement. In one case with involvement of the acetabulum, cancellous bone graft was interposed.
The reconstruction of the tibia was also performed as a compound osteosynthesis with an LCDCP titanium plate and bone cement. The resection consisted of an excision or curettage, i.e. an intralesional resection according to tumour resection criteria.
The perioperative factor substitution regimen was prescribed by haemophilia experts from the Department of Medicine I, Clinical Division of Haematology and Haemostaseology. One patient (with low-titre inhibitors after therapy according to the Malmö protocol) had continuous infusion with FVIII 500 IU h-1. The other factor substitutions were administered via bolus therapy (60–80 IU kg-1). The factor VIII levels during surgery and the postoperative course are shown in Fig. 3. During surgery and in the first week after surgery all patients received replacement therapy to reach almost normal FVIII levels.
Fig. 1.
MRI of the right iliac bone with T1-weighted image of a poly-lobulated mass with fibrous capsule (a) and rim enhancement after application of contrast agent (b) (patient 5)
Postoperative course
In three cases the postoperative course was complicated by deep infection (patients 2, 5 and 6). One patient (patient 5) required removal of the compound filling (pins and bone cement) of the right iliac bone due to bacterial spread of a septic intravenous catheter. In this case the microbiological analysis detected Klebsiella pneumoniae. One patient (patient 6) was in a weakened state of health and had a decreased CD4 count due to HIV. Infection occurred five months postoperatively requiring removal of the LCDCP plate, temporary vacuum treatment and plastic reconstruction with a soleus flap. One patient had an infection with a fistula 12 and 17 years after surgery and underwent conservative treatment. This infection was caused by Staphylococcus aureus. Both infections (with the Klebsiella pneumoniae and Staphylococcus aureus) were treated with antibiotics according to the antibiogram.
At the latest follow-up after 8.4 (range, 4–24) years normal healing with no recurrence of a haemophilic pseudotumour was observed in all patients.
Histological findings
Histological findings were similar in all cases, i.e. synovial membranes with histiocytes with haemosiderin deposits, giant cells, and foreign body granuloma reaction.
Discussion
Apart from the multicentre study of Magallon et al. [11] only one comparatively large series of analyses [18] on the surgical treatment of haemophilic pseudotumours has been reported. To the best of our knowledge, our current report presents one of the largest groups of patients with severe haemophilia in whom surgery was performed for haemophilic pseudotumours in a single center.
According to Magallon et al. [11] the incidence of haemophilic pseudotumour does not significantly differ among patients with mild, moderate, or severe haemophilia. The author's explanation is that in patients with severe haemophilia the frequent factor administration could act as a protective mechanism, whereas the less frequent replacement therapy in moderate and mild haemophiliacs would not provide this protection. In our series all patients had severe haemophilia A with factor activity of less than 1 %. Previous authors have reported that the haemophilic pseudotumour, in particular the proximal type, results from repeated and unresolved intramuscular bleeding episodes. Consequently, early diagnosis and adequate therapy of intramuscular haematomas ought to prevent the development of haemophilic pseudotumours [2, 3, 10]. However, opinions differ as to whether a manifest haemophilic pseudotumour of the proximal type can resolve with factor treatment alone. In our cohort, factor VIII substitution was available for all the subjects included, except for the patient with amputation in 1967. In this case, amputation was performed to avoid uncontrollable bleeding.
Nevertheless, conservative treatment with factor replacement and prolonged immobilisation can lead to resorption of the mass, in particular the distal type located in small bones of the hand and foot [2, 10]. Rodriguez-Merchan [2] suggested that in children with distal pseudotumours conservative treatment should be attempted before considering surgical excision. In our cohort, no patient was affected by a pseudotumour of the distal type.
Radiotherapy can induce inflammation and fibrosis resulting in vessel constriction and consequently reduce the mass [15]. It can thus be considered as an alternative for unresectable pseudotumours or if surgical intervention is contraindicated [2]. There is much variation in the international literature regarding the irradiation therapy with a broad range of doses (5–30 Gy) with various fraction protocols (0.5–2 Gy applied in 5 to 16 sessions) [15]. Preoperative radiation is not recommended, since the induced fibrosis can complicate the operation [3]. There is no wide agreement on the adequate management of pseudotumours, so treatment can differ from case to case and from centre to centre. In the series of Magallon et al. [11], surgery was associated with the best results. Buchowski et al. [6] also stated that surgical treatment seemed to deliver the best results. According to Rodriguez-Merchan [2], surgical treatment of proximal pseudotumours is indicated, if no regression is achieved despite adequate replacement therapy for eight to 12 weeks, and if there is a risk of skin necrosis, pathological fracture, compression of neurovascular structures, or spontaneous perforation. In our department surgery was carried out to prevent rupture and massive bleeding or pathological bone fracture. In one case surgery was done to prevent neurological deficiency of the ulnar nerve.
Iwata et al. [9] reported on two cases of surgical resection of two haemophilic pseudotumours of the iliac bone. One pseudotumour was removed partially and the patient showed a favourable postoperative course. The other pseudotumour was removed totally and the patient died of postoperative bleeding complications. Rodriguez-Merchan [2] reports on a mortality rate of 20 % after surgical removal. However, in our cases, and even for the patient with inhibitor, the perioperative course was easily controllable from the perspective of bleeding events. In no case pre- and intraoperative embolization was necessary. In our patients we administered adequate factor VIII replacement therapy to reach normal factor VIII levels during surgery and in the postoperative period.
Other reports on the surgical treatment of haemophilic pseudotumours are scanty (case or radioimaging reports) [19–24]. Plain radiography has a broad spectrum of osteolysis patterns and varies from circumscribed osteolysis to a huge destruction with or without calcification, with or without periostal reaction or rim thickening [23] (Fig. 4). On MRI a heterogeneous pattern of signal intensities is seen. The haemophilic pseudotumour is surrounded by a thick fibrous capsule with a hypointense signal on T1- and T2- weighted images. The internal signal intensities are non homogeneous with hypo-, iso- or hyperintense signal on T1-weighted images reflecting the presence of blood products [4, 23]. It is difficult to distinguish haemophilic pseudotumour from an infection or neoplasm. In our collective, MRI had a rim enhancement with a rather minor uptake of contrast agent. The knowledge of the underlying disease, the long clinical history and MRI findings with contrast agent may help in diagnosis.
Fig. 2.
MRI of the right thigh with T1-weighted image of a poly-lobulated tumour mass with fibrous capsule and minor contrast agent uptake (patient 3)
Preoperative biopsy is regarded as contraindicated [2] and puncture is not considered to be a safe procedure, leading to fistula formation and infection [11].
The surgical resection of the haemophilic pseudotumour is a complex and demanding procedure with unpredictable postoperative course including many possible complications, requiring detailed preoperative planning. There is no concise explanation for the high incidence of infection in haemophilic patients. Infection could be caused by self-injection of factor concentration with skin contamination, HIV and hepatitis C. Pour et al. [25] recently stated in their report that patients with hepatitis C undergoing joint arthroplasty had a higher rate of surgical complications. The authors stated that the reason is not known.
One should be aware of an unpredictable outcome of this complex procedure. To minimise possible complications, we recommend the surgical treatment of the haemophilic pseudotumour in multi-specialist centres with close cooperation between all disciplines. Patients should be educated about aseptic self-administration of factor, be informed of a possible failure and complications and be monitored at regular intervals.
In conclusion, our experience shows that surgical treatment of the haemophilic pseudotumour is feasible and safe during the intra- and postoperative course, when performed in specialised centres.
Acknowledgments
We thank Tanja Altreiter (Medical University of Vienna, Department of Medicine I) and Reinhard Schuh (Medical University of Vienna, Department of Orthopaedics) for proofreading this manuscript.
References
- 1.Fernandez de Valderrama JA, Matthews JM. The haemophilic pseudotumor or haemophilic subperiostal haematoma. J Bone Joint Surg. 1965;47-B:256–265. [PubMed] [Google Scholar]
- 2.Rodriguez-Merchan EC. The haemophilic pseudotumour. Int Orthop. 1995;19(4):255–260. doi: 10.1007/BF00185235. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Merchan EC. Haemophilic cysts (pseudotumours) Haemophilia. 2002;8(3):393–401. doi: 10.1046/j.1365-2516.2002.00609.x. [DOI] [PubMed] [Google Scholar]
- 4.Jaovisidha S, Ryu KN, Hodler J, Schweitzer ME, Sartoris DJ, Resnick D. Hemophilic pseudotumor: spectrum of MR findings. Skeletal Radiol. 1997;26(8):468–474. doi: 10.1007/s002560050268. [DOI] [PubMed] [Google Scholar]
- 5.Sundaram M, Wolverson MK, Joist JH, Riaz MA, Rao BJ. Case report 133. Hemophilic pseudotumor of ilium and soft tissues. Skeletal Radiol. 1981;6(1):54–57. doi: 10.1007/BF00347349. [DOI] [PubMed] [Google Scholar]
- 6.Buchowski JM, Cascio BM, Streiff MB, Frassica FJ. Resection and reconstruction of a massive femoral hemophilic pseudotumor. Clin Orthop Relat Res. 2005;430:237–242. doi: 10.1097/01.blo.0000137545.37824.b0. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert MS. Characterizing the hemophilic pseudotumor. Ann NY Acad Sci. 1975;240:311–315. doi: 10.1111/j.1749-6632.1975.tb53365.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahlberg AK. On the natural history of hemophilic pseudotumor. J Bone Joint Surg Am. 1975;57(8):1133–1136. [PubMed] [Google Scholar]
- 9.Iwata H, Oishi Y, Itoh A, Ishiguro N, Yamaga H, Miyamoto N, Kamiya T. Surgical excision of hemophilic pseudotumor of the ilium. Clin Orthop Relat Res. 1992;284:234–238. [PubMed] [Google Scholar]
- 10.Chakal F, Viso R, Fernández Palazzi F. Percutaneous treatment of haemophilic digital pseudo tumours. Int Orthop. 2005;29(3):197–198. doi: 10.1007/s00264-005-0653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magallón M, Monteagudo J, Altisent C, Ibáñez A, Rodríguez-Pérez A, Riba J, Tusell J, Martín-Villar J. Hemophilic pseudotumor: multicenter experience over a 25-year period. Am J Hematol. 1994;45(2):103–108. doi: 10.1002/ajh.2830450202. [DOI] [PubMed] [Google Scholar]
- 12.Valentino LA, Martinowitz U, Doolas A, Murali P. Surgical excision of a giant pelvic pseudotumour in a patient with haemophilia A. Haemophilia. 2006;12(5):541–544. doi: 10.1111/j.1365-2516.2006.01318.x. [DOI] [PubMed] [Google Scholar]
- 13.Sagarra M, Lucas M, Torre D. E., Almagro D, González R, García T, Menéndez A, González A. Successful surgical treatment of haemophilic pseudotumour, filling the defect with hydroxyapatite. Haemophilia. 2000;6(1):55–56. doi: 10.1046/j.1365-2516.2000.00344.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Merchan EC. The haemophilic pseudotumour. Haemophilia. 2002;8(1):12–16. doi: 10.1046/j.1365-2516.2002.00577.x. [DOI] [PubMed] [Google Scholar]
- 15.Espandar R, Heidari P, Rodriguez-Merchan EC. Management of haemophilic pseudotumours with special emphasis on radiotherapy and arterial embolization. Haemophilia. 2009;15(2):448–457. doi: 10.1111/j.1365-2516.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 16.Caviglia HA, FernAndez-Palazzi F, Gilbert MS. Haemophilic pseudotumours of the limbs and their percutaneous treatment. Haemophilia. 2002;8(3):402–406. doi: 10.1046/j.1365-2516.2002.00641.x. [DOI] [PubMed] [Google Scholar]
- 17.Subasi M, Dirier A, Kapukaya A, Uludağ A, Karadayi B, Cebesoy O. Successful treatment of hemophilic hand pseudotumors by only radiotherapy. Ann Plast Surg. 2007;59(3):338–340. doi: 10.1097/01.sap.0000251487.58054.9d. [DOI] [PubMed] [Google Scholar]
- 18.Xue F, Sun C, Sui T, Zhang L, Jiang L, Yang R. Hemophilic pseudotumor in Chinese patients: a retrospective single-centered analysis of 14 cases. Clin Appl Thromb Hemost. 2011;17(3):279–282. doi: 10.1177/1076029610366433. [DOI] [PubMed] [Google Scholar]
- 19.Hermann G, Yeh HC, Gilbert MS. Computed tomography and ultrasonography of the hemophilic pseudotumor and their use in surgical planning. Skeletal Radiol. 1986;15(2):123–128. doi: 10.1007/BF00350205. [DOI] [PubMed] [Google Scholar]
- 20.Hilgartner MW, Arnold WD. Hemophilic pseudotumor treated with replacement therapy and radiation. Report of a case. J Bone Joint Surg Am. 1975;57(8):1145–1146. [PubMed] [Google Scholar]
- 21.Pettersson H, Ahlberg A. Computed tomography in hemophilic pseudotumor. Acta Radiol Diagn (Stockh) 1982;23(5):453–457. doi: 10.1177/028418518202300503. [DOI] [PubMed] [Google Scholar]
- 22.Takedani H, Mikami S, Kawasaki N, Abe Y, Arai M, Naka H, Yoshioka A. Excision of pseudotumour in a patient with haemophilia A and inhibitor managed with recombinant factor VIIa. Haemophilia. 2004;10(2):179–182. doi: 10.1111/j.1365-2516.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 23.Kerr R. Imaging of musculoskeletal complications of hemophilia. Semin Musculoskelet Radiol. 2003;7(2):127–136. doi: 10.1055/s-2003-41346. [DOI] [PubMed] [Google Scholar]
- 24.Pruzansky JS, Gilbert MS, Garcia RA, Gilbert RS. Intraosseous pseudotumor of the distal radius in a patient with hemophilia: case report. J Hand Surg Am. 2012;37(3):532–537. doi: 10.1016/j.jhsa.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 25.Pour AE, Matar WY, Jafari SM, Purtill JJ, Austin MS, Parvizi J. Total joint arthroplasty in patients with hepatitis C. J Bone Joint Surg Am. 2011;93(15):1448–1454. doi: 10.2106/JBJS.J.00219. [DOI] [PubMed] [Google Scholar]