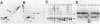Abstract
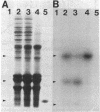
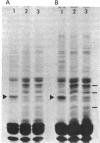
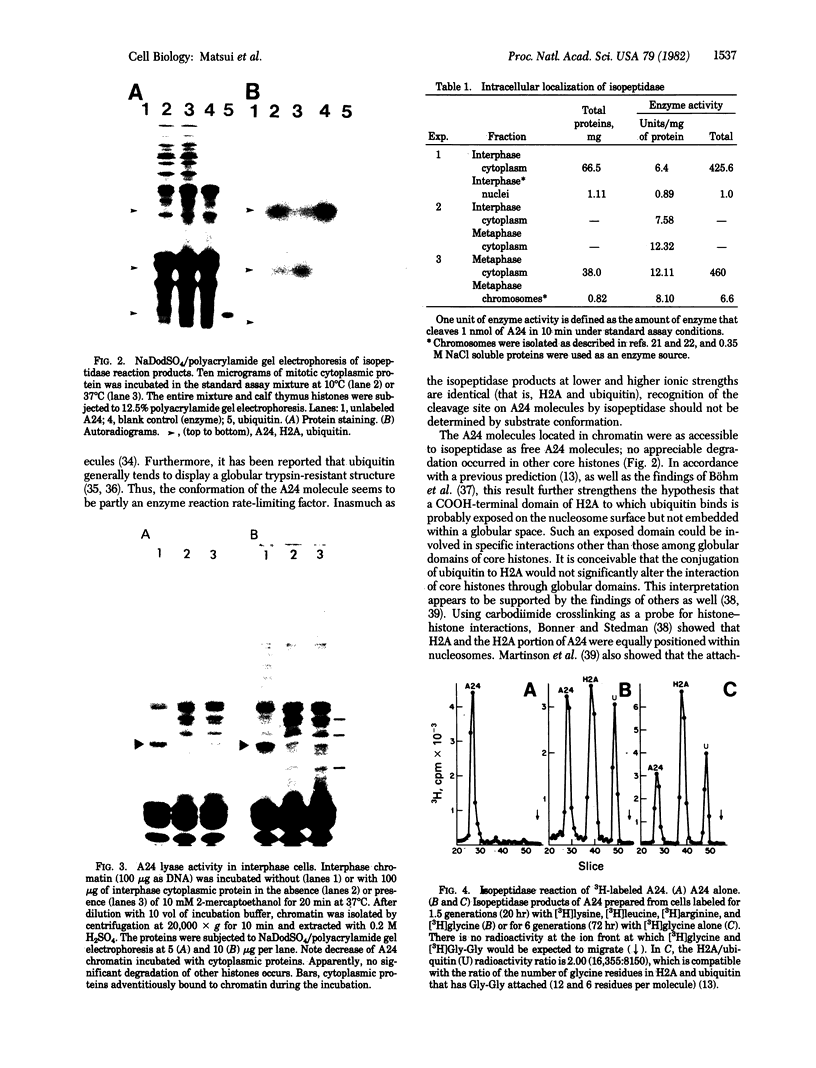
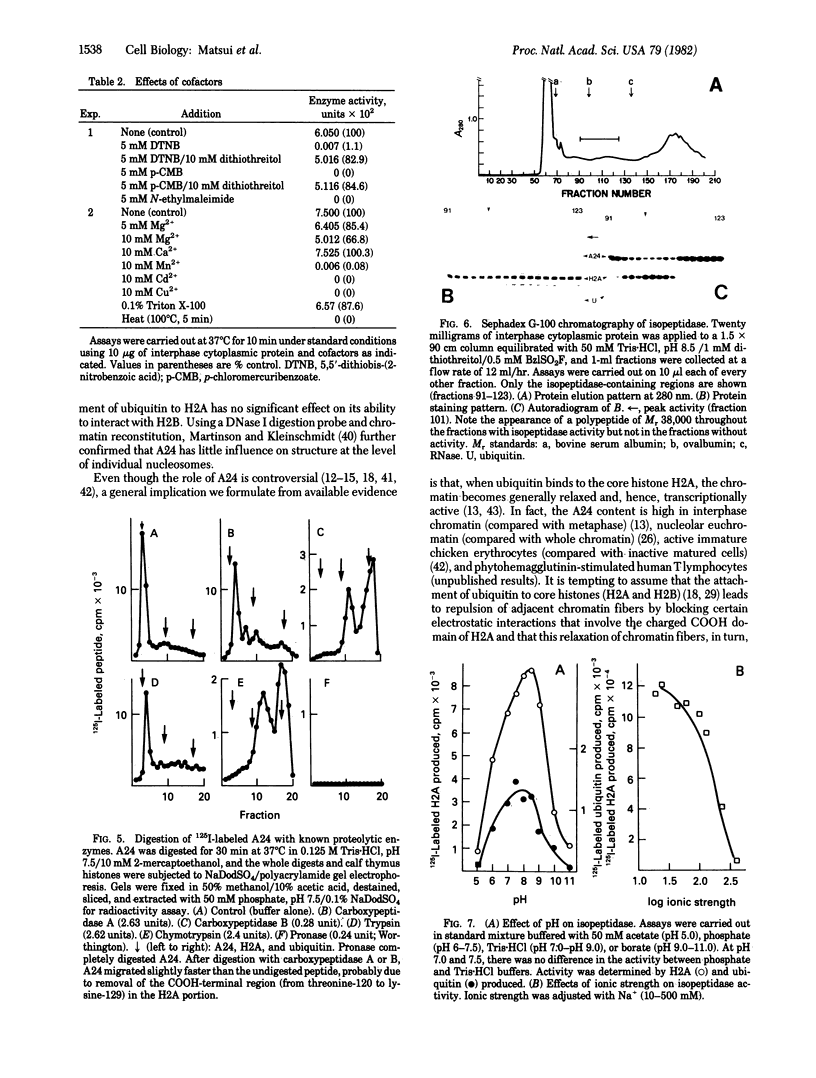
In an attempt to clarify the regulatory mechanism that accounts for the shift of protein A24 in the mitotic cycle, we demonstrated the existence of an enzyme, provisionally termed isopeptidase, that cleaves A24 stoichiometrically into histone H2A and ubiquitin. Properties of this enzyme are (i) most eukaryotes, including mammals, amphibia, chicken, and yeast, contain isopeptidase in the cytoplasm; (ii) a significant increase in enzyme binding to chromatin occurs when cells enter mitosis; (iii) Escherichia coli does not contain isopeptidase; (iv) isopeptidase has a molecular weight of 38,000; (v) at an ionic strength that induces globular conformation of H2A, isopeptidase activity is repressed; (vi) a SH group is an essential cofactor; and (vii) most divalent cations (except Mg2+ and Ca2+) are inhibitory. In view of the stoichiometric conversion of A24 into H2A and ubiquitin by isopeptidase in vitro, A24 probably contains a Gly-Gly dipeptide in isopeptide linkage but no other intervening polypeptides. Since ubiquitin in various eukaryotes binds to protein other than H2A, and is proteolytically released, isopeptidase probably acts on isopeptide bonds in general and not uniquely on those of A24. Inasmuch as isopeptidase is present throughout the cell cycle, the level of A24 in chromatin appears to be controlled by a balance between isopeptidase and an as yet unestablished H2A-ubiquitin ligase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. W., Ballal N. R., Goldknopf I. L., Busch H. Protein A24 lyase activity in nucleoli of thioacetamide-treated rat liver releases histone 2A and ubiquitin from conjugated protein A24. Biochemistry. 1981 Mar 3;20(5):1100–1104. doi: 10.1021/bi00508a009. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Histone 1 is proximal to histone 2A and to A24. Proc Natl Acad Sci U S A. 1979 May;76(5):2190–2194. doi: 10.1073/pnas.76.5.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Böhm L., Crane-Robinson C., Sautière P. Proteolytic digestion studies of chromatin core-histone structure. Identification of a limit peptide of histone H2A. Eur J Biochem. 1980 May;106(2):525–530. doi: 10.1111/j.1432-1033.1980.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Cary P. D., King D. S., Crane-Robinson C., Bradbury E. M., Rabbani A., Goodwin G. H., Johns E. W. Structural studies on two high-mobility-group proteins from calf thymus, HMG-14 and HMG-20 (ubiquitin), and their interaction with DNA. Eur J Biochem. 1980 Dec;112(3):577–580. doi: 10.1111/j.1432-1033.1980.tb06123.x. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Heller H., Elias S., Haas A. L., Hershko A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickbush T. H., Watson D. K., Moudrianakis E. N. A chromatin-bound proteolytic activity with unique specificity for histone H2A. Cell. 1976 Dec;9(4 Pt 2):785–792. doi: 10.1016/0092-8674(76)90141-0. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldknopf I. L., Taylor C. W., Baum R. M., Yeoman L. C., Olson M. O., Prestayko A. W., Busch H. Isolation and characterization of protein A24, a "histone-like" non-histone chromosomal protein. J Biol Chem. 1975 Sep 25;250(18):7182–7187. [PubMed] [Google Scholar]
- Goldknopf I. L., Wilson G., Ballal N. R., Busch H. Chromatin conjugate protein A24 is cleaved and ubiquitin is lost during chicken erythropoiesis. J Biol Chem. 1980 Nov 25;255(22):10555–10558. [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Keevert J. B. Absence of histone F1 in a mitotically dividing, genetically inactive nucleus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2672–2676. doi: 10.1073/pnas.72.7.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyanes V. J., Matsui S., Sandberg A. A. The basis of chromatin fiber assembly within chromosomes studied by histone-DNA crosslinking followed by trypsin digestion. Chromosoma. 1980;78(1):123–135. doi: 10.1007/BF00291911. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Cell cycle-specific changes in histone phosphorylation associated with cell proliferation and chromosome condensation. J Cell Biol. 1974 Feb;60(2):356–364. doi: 10.1083/jcb.60.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Heller H., Haas A. L., Rose I. A. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. T., Dayhoff M. O. Amino-terminal sequence identity of ubiquitin and the nonhistone component of nuclear protein A24. Biochem Biophys Res Commun. 1977 Jan 24;74(2):650–655. doi: 10.1016/0006-291x(77)90352-7. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A. M., Martinson H. G. Structure of nucleosome core particles containing uH2A (A24). Nucleic Acids Res. 1981 Jun 11;9(11):2423–2431. doi: 10.1093/nar/9.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lake R. S. F1-histone phosphorylation in metaphase chromosomes of cultured Chinese hamster cells. Nat New Biol. 1973 Apr 4;242(118):145–146. doi: 10.1038/newbio242145a0. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Goidl J. A., Salzman N. P. F1-histone modification at metaphase in Chinese hamster cells. Exp Cell Res. 1972 Jul;73(1):113–121. doi: 10.1016/0014-4827(72)90108-5. [DOI] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. High-resolution fractionation of nucleosomes: minor particles, "whiskers," and separation of mononucleosomes containing and lacking A24 semihistone. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3244–3248. doi: 10.1073/pnas.77.6.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D. B., Paik W. K., Borun T. W. The relationship of histone phosphorylation to deoxyribonucleci acid replication and mitosis during the HeLa S-3 cell cycle. J Biol Chem. 1973 Aug 25;248(16):5660–5667. [PubMed] [Google Scholar]
- Martinson H. G., True R., Burch J. B., Kunkel G. Semihistone protein A24 replaces H2A as an integral component of the nucleosome histone core. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1030–1034. doi: 10.1073/pnas.76.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. I., Fuke M., Busch H. Fidelity of ribosomal ribonucleic acid synthesis by nucleoli and nucleolar chromatin. Biochemistry. 1977 Jan 11;16(1):39–45. doi: 10.1021/bi00620a007. [DOI] [PubMed] [Google Scholar]
- Matsui S. I., Seon B. K., Sandberg A. A. Disappearance of a structural chromatin protein A24 in mitosis: implications for molecular basis of chromatin condensation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6386–6390. doi: 10.1073/pnas.76.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. I., Weinfeld H., Sandberg A. A. Quantitative conservation of chromatin-bound RNA polymerases I and II in mitosis. Implications for chromosome structure. J Cell Biol. 1979 Feb;80(2):451–464. doi: 10.1083/jcb.80.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. I., Yoshida H., Weinfeld H., Sandberg A. A. Induction of prophase in interphase nuclei by fusion with metaphase cells. J Cell Biol. 1972 Jul;54(1):120–132. doi: 10.1083/jcb.54.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S., Busch H. Isolation and characterization of rDNA-containing chromatin from nucleoli. Exp Cell Res. 1977 Oct 1;109(1):151–161. doi: 10.1016/0014-4827(77)90054-4. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L. Nucleosomes: the structural quantum in chromosomes. Am Sci. 1978 Nov-Dec;66(6):704–711. [PubMed] [Google Scholar]
- Schlesinger D. H., Goldstein G., Niall H. D. The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells. Biochemistry. 1975 May 20;14(10):2214–2218. doi: 10.1021/bi00681a026. [DOI] [PubMed] [Google Scholar]
- Tanphaichitr N., Moore K. C., Granner D. K., Chalkley R. Relationship between chromosome condensation and metaphase lysine-rich histone phosphorylation. J Cell Biol. 1976 Apr;69(1):43–50. doi: 10.1083/jcb.69.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Kohn K. W., Bonner W. M. Metabolism of ubiquitinated histones. J Biol Chem. 1981 Jun 10;256(11):5916–5920. [PubMed] [Google Scholar]