Abstract
Purpose
Muscle atrophy is a commonly encountered problem in osteoarthritis (OA). The aim of this study was to estimate the amount of muscle atrophy and fatty degeneration of the lower leg muscles related to ankle OA by magnetic resonance imaging (MRI).
Methods
Twenty-one patients with unilateral ankle OA were included in this cohort study. Calf circumference of the affected and healthy lower leg was documented. The degree of OA was classified in conventional radiographs. The cross-sectional areas and fatty degeneration of the muscles of the lower leg were measured in bilateral MRI.
Results
We found a significantly reduced calf circumference of the affected vs. healthy leg (p = 0.016). MRI showed a significantly lower cross-sectional area of the entire lower leg musculature in OA (p = 0.013). Sub-analysis of muscle groups revealed that only the M. soleus had a significant cross-sectional area decrease (p < 0.01). All muscles showed a significant fatty degeneration (p < 0.01).
Conclusions
We conclude that unilateral ankle joint osteoarthritis leads to an overall lower leg muscle atrophy, but significant atrophy of the M. soleus. All muscles of the affected leg undergo a fatty degeneration.
Introduction
Osteoarthritis (OA) is a common degenerative joint disease, affecting nearly 50 million people in the USA [1]. Although the incidence of primary OA in the ankle joint is thought to be lower than in the knee and hip joint, recent studies report a high and increasing incidence of post traumatic, secondary OA in the ankle joint [2–5]. OA is characterised by structural changes of the entire joint organ, such as loss of articular cartilage, subchondral bone sclerosis and cysts, osteophyte formation, and synovitis [6]. The patients report joint stiffness and reduced range of motion (ROM) [7]. However, use-related joint pain, which is relieved by rest, is one of the cardinal features of OA and the most common reason why affected patients seek medical help [8]. It has been suggested that abnormal afferent nociceptive nerves from the degenerated joints trigger a neurotransmitter release at the level of the spinal cord. This inhibits the activity of the alpha-motor neurons, leading to secondary muscle atrophy from a reduction in muscle activity [9], a process also known as arthrogenic muscle inhibition, a reflex atrophy [10].
This is confirmed by previous clinical studies examining calf circumferences in patients with unilateral ankle joint OA showing a reduction on the affected side in comparison to the healthy side [11]. However, it is not known whether this phenomenon is due to a general atrophy of the whole lower leg muscle, or if individual muscle groups are specifically affected.
The aim of this clinical-radiological study was to assess muscle atrophy and degeneration of individual muscle groups of the lower leg in patients suffering from unilateral OA of the ankle joint. MRI was performed to measure the cross-sectional area of the lower leg muscles and to assess muscle tissue degeneration. A comparison of the affected and healthy lower leg was performed. We hypothesised that the clinically found calf atrophy would be caused by selective muscular atrophy of individual muscle groups in the affected lower leg, when compared to the healthy side.
Methods
Patients
We included 21 consecutive patients (11 female, ten male; mean age 57 years, range 35–76 years) with end-stage ankle OA, admitted to the orthopaedic department for total ankle replacement (TAR) due to unilateral post traumatic OA of the ankle joint between May and September 2008. The patient demographics are summarized in Table 1. All patients suffered from a previous fracture around the ankle joint. The mean latency time from the time of fracture to onset of symptoms was 23.5 years (range, 2–56 years). Exclusion criteria consisted of primary OA, any joint or muscle pathologies (e.g. previous muscular trauma, rheumatoid arthritis, diabetes mellitus or neuromuscular diseases) not related to secondary OA, and any injuries of the contralateral non-affected lower extremity.
Table 1.
Clinical and radiological variables of subjects
| Case | Sex, age (years) | Side | Mean latency from trauma (months) | AOFAS (points) | VAS (points) | Total ROM healthy ankle (°) | Total ROM OA ankle (°) | Calf circumference healthy leg (cm) | Calf circumference OA leg (cm) | Radiological OA grade |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49, F | R | 30 | 30 | 6 | 60 | 20 | 36 | 34 | 3 |
| 2 | 57, F | R | 2 | 48 | 5 | 55 | 15 | 36 | 35 | 2 |
| 3 | 35, M | L | 2 | 30 | 7 | 60 | 25 | 31 | 28 | 2 |
| 4 | 50, F | L | 29 | 26 | 6 | 60 | 15 | 37 | 35 | 3 |
| 5 | 45, M | R | 21 | 53 | 7 | 50 | 25 | 34 | 31 | 2 |
| 6 | 47, F | R | 5 | 22 | 6 | 50 | 5 | 40 | 36 | 2 |
| 7 | 43, M | R | 8 | 22 | 8 | 55 | 20 | 36 | 34 | 3 |
| 8 | 59, M | R | 23 | 22 | 8 | 50 | 15 | 37 | 36 | 3 |
| 9 | 65, F | L | 5 | 54 | 5 | 50 | 15 | 32 | 30 | 3 |
| 10 | 50, M | L | 2 | 18 | 8 | 50 | 10 | 40 | 38 | 2 |
| 11 | 61, F | R | 12 | 25 | 6 | 65 | 5 | 33 | 32 | 3 |
| 12 | 76, F | L | 40 | 22 | 8 | 60 | 10 | 32 | 31 | 3 |
| 13 | 66, F | R | 30 | 29 | 7 | 60 | 20 | 38 | 35 | 3 |
| 14 | 58, F | L | 30 | 48 | 8 | 60 | 15 | 30 | 28 | 3 |
| 15 | 67, F | R | 4 | 32 | 7 | 60 | 30 | 37 | 35 | 2 |
| 16 | 58, M | L | 54 | 23 | 8 | 50 | 40 | 37 | 34 | 3 |
| 17 | 70, F | R | 45 | 23 | 8 | 50 | 10 | 34 | 32 | 3 |
| 18 | 52, M | L | 20 | 27 | 8 | 70 | 30 | 35 | 32 | 3 |
| 19 | 71, M | R | 56 | 20 | 7 | 55 | 15 | 38 | 36 | 3 |
| 20 | 73, M | L | 46 | 25 | 6 | 45 | 30 | 34 | 33 | 3 |
| 21 | 49, M | R | 29 | 23 | 7 | 60 | 10 | 34 | 32 | 3 |
avg average, M male, F female, AOFAS American Orthopaedic Foot and Ankle Society hindfoot scale, VAS visual analogue scale, OA osteoarthritis
The study was approved by the institutional review board and written informed patient consent was obtained. The study was carried out in accordance with the World Medical Association Declaration of Helsinki.
Clinical assessment
Clinical and radiological assessment was performed before TAR and included the American Orthopaedic Foot & Ankle Society (AOFAS) ankle score [12], and pain status measured by the visual analogue scale (VAS; with use of a 10-cm graded line, with 0 indicating no pain and 10, the worst pain imaginable) [13]. The total ROM for the affected and healthy ankle joint was noted (plantar flexion + dorsiflexion in degrees). The calf circumference was determined in the standing patient with a tape measure (in cm) at the level of the maximal diameter of the calf, as measured by an experienced orthopaedic surgeon (VV).
Radiological examination
All subjects in this case series underwent pre-operative radiological imaging of the foot and ankle consisting of standard anteroposterior and lateral weight-bearing radiographs and MRI of both lower legs.
The extent of OA was graded according to the classification by Morrey and Wiedeman [14]. The MRI investigation of the lower leg musculature was performed bilaterally on a 1.5 Tesla MRI device (Symphony/Avanto, Siemens, Erlangen). Axial T1-weighted turbospin echo sequences (tse2d) with a repetition time of between 2800 ms and 5610 ms, and with an echo time between 70 and 111 ms were obtained. The layer thickness was 8 mm, the interslice gap 0.8 mm, the field of view 19.7 cm, and the image matrix 216 × 512. All measurements were performed by two independent experienced musculoskeletal radiologists using a radiological software dedicated for diagnostic viewing of DICOM data (e-film, Merge, Chicago, USA).
For quantitative analysis, the cross-sectional muscle area was calculated according to the method previously described by Fuchs et al. [15]. First, the level for the measurements within the lower leg had to be determined. For this, the lower leg was divided into three levels: proximal, middle and distal one-third of the lower leg length, as defined from the proximal to the distal tibial joint surface. Next, in the middle 1/3 of the lower leg, the slice which allowed best differentiation of the muscle groups was chosen by visual judgment. For comparability, exactly the same level of measurement was chosen for the affected and healthy lower leg. Next, the following muscle groups of the diseased and healthy lower leg were identified by the presence of intermuscular septa (containing fat tissue) [16]: the anterior tibial muscle group (M. tibialis anterior, M. extensor digitorum et hallucis longus), the peroneal muscle group (M. peroneus longus and brevis) and the deep dorsal muscle group (M. tibialis posterior, M. flexor digitorum longus, M. flexor hallucis longus). In the superficial dorsal muscle group (triceps surae), each of the three muscles (M. gastrocnemius medialis et lateralis and M. soleus) have been measured individually. To calculate the area, the anatomic boundary of the muscle groups (respectively the individual muscles) which was recognized by the intermuscular septa was manually marked (Fig. 1). Based on those markings, the software automatically calculated the area (in cm²).
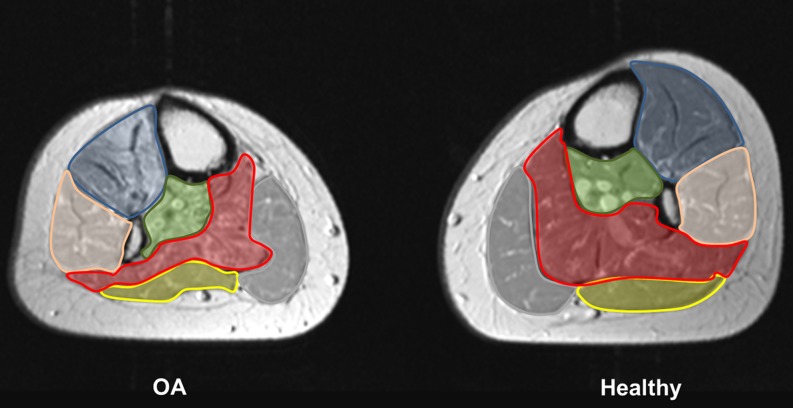
Fig. 1.
Quantitative analysis. This figure represents the affected and healthy lower leg of patient no. 1 from Table 1. To calculate the area, the anatomic boundary of following muscle groups (respectively the individual muscles) were manually marked: the anterior tibial muscle group (blue), the peroneal muscle group (pink), the deep dorsal muscle group (green), the M. gastrocnemius medialis (grey) and lateralis (yellow), and the M. soleus (red)
The qualitative evaluation of the muscle tissue was performed assessing the fatty degeneration which was classified into four grades (Table 2). The measurements were performed at the level of the quantitative measurements (Fig. 2).
Table 2.
Qualitative evaluation of the muscle tissue
| Grade of fatty degeneration | Description of fatty degeneration |
|---|---|
| 0 | None |
| 1 | Up to 30 %, corresponding to one third of muscle tissue |
| 2 | Up to 60 %, corresponding to two thirds of muscle tissue |
| 3 | Greater than 60 %, corresponding to more than two thirds of muscle tissue |
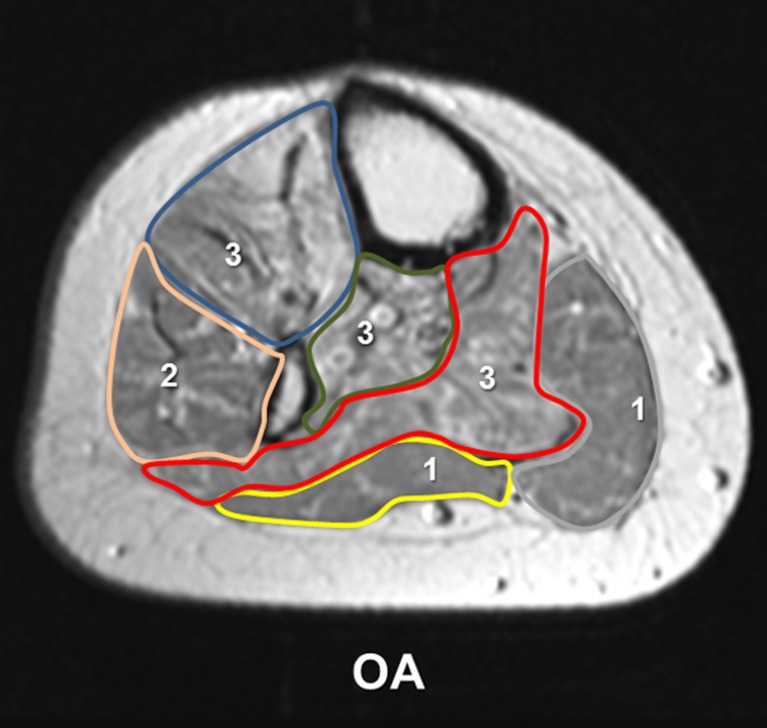
Fig. 2.
Qualitative analysis. This figure represents the affected lower leg of patient no. 1 from Table 1. Fatty muscle degeneration was assessed at the level of the quantitative measurement. The grade of fatty degeneration is displayed within each muscle group: anterior tibial muscle group (blue, 3), the peroneal muscle group (pink, 2), the deep dorsal muscle group (green, 3), the M. gastrocnemius medialis (grey, 1) and lateralis (yellow, 1), and the M. soleus (red, 3)
Statistical analysis
Statistical analysis was performed with use of a standard paired t test by an independent statistician. Power analysis indicated that a total sample size of 21 patients (42 legs) will provide 80 % power (2-tailed α = 0.01, β = 0.20) for determining a significant difference for the cross-sectional area.
Results
Clinical assessment
The mean preoperative AOFAS ankle score was 29.6 points (range, 18.0–54.0) and the mean VAS was 7.0 points (range, 5.0–8.0) (Table 1). The mean total range of motion of the affected ankle joint was 18.1° (range, 5–40) and 56.0° (range, 45–70) on the healthy side. This was statistically significant (p < 0.01). The mean calf circumference on clinical examination was significantly lower (p = 0.016) with 33.2 cm (range, 28–38) on the affected side compared to 35.3 cm (range, 30–40) on the healthy side (Table 1).
Fifteen patients presented a grade 3 and six patients a grade 2 osteoarthritis on conventional radiographs (mean 2.7, range 2–3).
Radiological examination
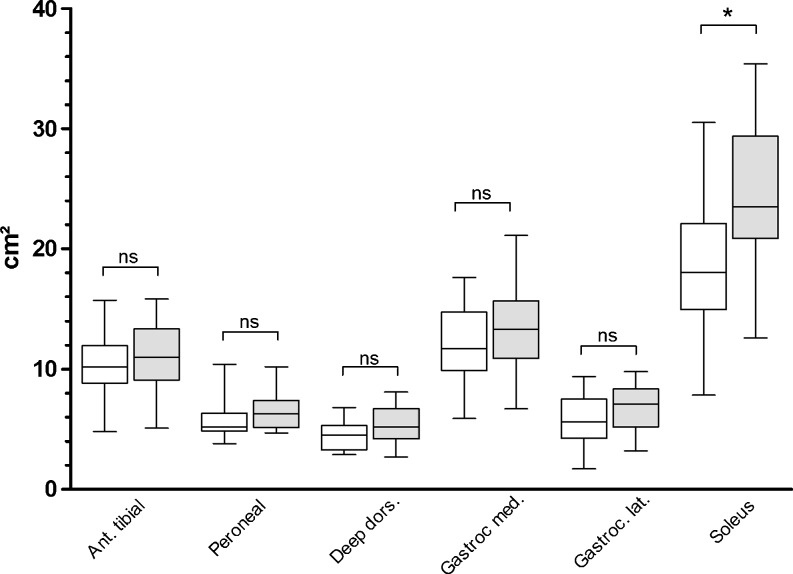
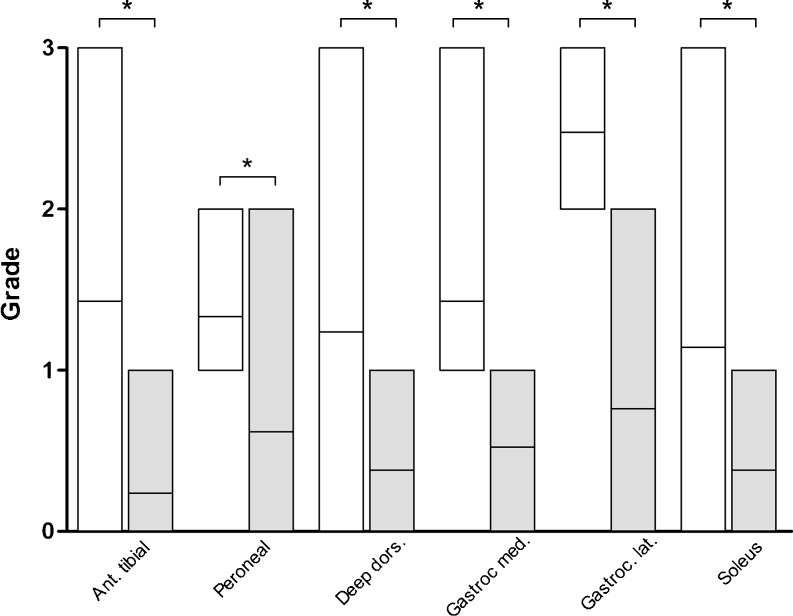
Quantitative MRI analysis of the anterior tibial muscle group, the peroneal muscle group, and the deep dorsal muscle group and individual MRI analyses of the M. gastrocnemius medialis et lateralis showed no significant reduction (p > 0.05) in the muscle cross-sectional area of the affected leg compared to the healthy leg (Table 3, Fig. 3). Measurement of the M. soleus revealed a significant (p < 0.01) reduction in muscle cross-sectional area of the affected leg compared to the healthy leg (Fig. 3). The sum of the values of all individual cross-sectional areas for each individual leg showed a significant difference (p = 0.013) for the affected leg (mean 57 cm², range, 32.5–86) compared to the healthy leg (mean 67.5 cm², range, 44.2–91.9). Qualitative MRI analysis showed a significant fatty degeneration (p < 0.01) in all examined muscle groups of the affected leg compared to the healthy leg (Table 4, Fig. 4).
Table 3.
Results of quantitative analysis
| Case | Ant. tibial | Peroneal | Deep dorsal | Gastroc med. | Gastroc lat. | Soleus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | H | P | H | P | H | P | H | P | H | P | H | |
| 1 | 7.1 | 8.4 | 5.1 | 5.8 | 3.4 | 4.6 | 5.9 | 6.7 | 3.1 | 4.0 | 7.84 | 14.8 |
| 2 | 10.7 | 11.0 | 5.1 | 6.2 | 4.5 | 5.4 | 11.7 | 12.0 | 4.7 | 5.8 | 23.3 | 31.3 |
| 3 | 9.4 | 12.5 | 5.1 | 5.8 | 3.2 | 4.0 | 11.2 | 16.3 | 7.3 | 7.7 | 18.2 | 26.7 |
| 4 | 8.3 | 9.4 | 5.9 | 5.2 | 4.8 | 4.4 | 7.3 | 9.0 | 4.2 | 5.4 | 13.9 | 21.0 |
| 5 | 9.9 | 12.0 | 5.2 | 6.6 | 5.3 | 7.1 | 14.3 | 14.2 | 6.7 | 8.2 | 20.9 | 30.6 |
| 6 | 8.9 | 9.2 | 7.4 | 7.6 | 6.6 | 6.9 | 14.2 | 15.8 | 6.9 | 7.2 | 23.6 | 34.2 |
| 7 | 13.4 | 15.8 | 6.0 | 8.0 | 6.5 | 7.2 | 16.2 | 21.1 | 8.1 | 9.8 | 19.4 | 22.1 |
| 8 | 12.6 | 14.2 | 8.1 | 8.1 | 6.8 | 8.1 | 13.5 | 14.3 | 6.8 | 8.4 | 23.7 | 23.5 |
| 9 | 10.3 | 9.2 | 4.8 | 4.8 | 4.7 | 4.9 | 9.9 | 11.3 | 4.9 | 4.3 | 16.9 | 25.1 |
| 10 | 14.5 | 15.5 | 10.4 | 10.2 | 5.3 | 7.8 | 17.6 | 15.0 | 7.7 | 8.0 | 30.5 | 35.4 |
| 11 | 10.2 | 10.2 | 5.2 | 5.6 | 3.8 | 4.5 | 10.4 | 10.1 | 6.0 | 7.1 | 15.7 | 20.0 |
| 12 | 9.4 | 9.0 | 5.0 | 4.8 | 4.0 | 5.4 | 9.9 | 10.5 | 4.6 | 5.1 | 17.6 | 22.9 |
| 13 | 10.3 | 9.8 | 4.9 | 6.8 | 6.5 | 5.2 | 15.2 | 11.9 | 5.6 | 8.3 | 20.4 | 28.6 |
| 14 | 4.8 | 5.1 | 4.8 | 6.3 | 2.9 | 4.1 | 10.5 | 11.4 | 5.3 | 5.3 | 12.8 | 20.7 |
| 15 | 9.5 | 11.5 | 5.3 | 7.2 | 3.2 | 4.2 | 12.9 | 13.3 | 3.7 | 5.9 | 27.9 | 28.4 |
| 16 | 10.2 | 15.1 | 4.7 | 6.3 | 5.0 | 6.2 | 11.1 | 15.5 | 4.3 | 5.3 | 17.8 | 30.1 |
| 17 | 7.1 | 7.7 | 5.4 | 4.7 | 5.1 | 6.5 | 9.2 | 9.3 | 3.4 | 4.2 | 10.7 | 12.6 |
| 18 | 11.3 | 11.8 | 3.8 | 5.1 | 3.8 | 5.7 | 7.1 | 11.3 | 1.7 | 3.2 | 18.0 | 22.3 |
| 19 | 15.7 | 11.9 | 6.7 | 6.8 | 3.6 | 3.8 | 16.5 | 18.1 | 9.4 | 9.7 | 16.4 | 21.5 |
| 20 | 8.8 | 7.1 | 4.6 | 5.1 | 3.1 | 2.7 | 11.9 | 14.3 | 8.5 | 9.1 | 14.2 | 18.5 |
| 21 | 13.2 | 14.4 | 9.7 | 9.8 | 3.0 | 4.2 | 15.5 | 17.6 | 7.8 | 8.8 | 20.6 | 27.4 |
| Mean | 10.3 | 11.0 | 5.8 | 6.5 | 4.5 | 5.4 | 12.0 | 13.3 | 5.7 | 6.7 | 18.6 | 24.7 |
| SD | 2.6 | 2.9 | 1.7 | 1.5 | 1.3 | 1.5 | 3.3 | 3.5 | 2.0 | 2.0 | 5.4 | 6.0 |
| Max | 4.8 | 5.1 | 3.8 | 4.7 | 2.9 | 2.7 | 5.9 | 6.7 | 1.7 | 3.2 | 7.8 | 12.6 |
| Min | 15.7 | 15.8 | 10.4 | 10.2 | 6.8 | 8.1 | 17.6 | 21.1 | 9.4 | 9.8 | 30.5 | 35.4 |
P ankle OA side, H healthy side, SD standard deviation
Cross-sectional muscle area in cm²
Fig. 3.
Results of the quantitative analysis. A significant (*p < 0.01) decrease of the muscle cross-sectional area was only found in the M. soleus of the affected leg (white box), when compared to the healthy leg (grey box). In all other muscle groups the difference was not significant (ns)
Table 4.
Results of qualitative analysis: grade of fatty degeneration according to Table 1
| Case | Ant. tibial | Peroneal | Deep dorsal | Gastroc med. | Gastroc lat. | Soleus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | H | P | H | P | H | P | H | P | H | P | H | |
| 1 | 3 | 0 | 2 | 1 | 3 | 0 | 1 | 0 | 1 | 0 | 3 | 1 |
| 2 | 1 | 1 | 2 | 1 | 1 | 0 | 2 | 1 | 3 | 0 | 2 | 0 |
| 3 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 |
| 4 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 2 | 1 |
| 5 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 3 | 1 |
| 6 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 |
| 7 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 1 |
| 8 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 3 | 2 |
| 9 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 |
| 10 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 3 | 1 |
| 11 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 3 | 1 |
| 12 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 3 | 1 |
| 13 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 |
| 14 | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 3 | 2 |
| 15 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 0 |
| 16 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 1 |
| 17 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 3 | 1 |
| 18 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 2 | 1 |
| 19 | 3 | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 2 | 0 | 3 | 0 |
| 20 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 2 | 1 |
| 21 | 1 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 0 |
| Mean | 1.3 | 0.4 | 1.4 | 0.5 | 1.4 | 0.2 | 1.3 | 0.6 | 1.1 | 0.4 | 2.5 | 0.8 |
| SD | 0.8 | 0.5 | 0.6 | 0.5 | 0.8 | 0.4 | 0.5 | 0.6 | 0.7 | 0.5 | 0.5 | 0.6 |
| Max | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Min | 3 | 1 | 3 | 1 | 3 | 1 | 2 | 2 | 3 | 1 | 3 | 2 |
Fig. 4.
Results of the qualitative analysis. A significant (*p < 0.01) fatty degeneration of the muscle tissue was found in all muscle groups of the affected leg (white box), when compared to the healthy leg (grey box)
Discussion
Muscle atrophy is one of the major problems in osteoarthritis (OA) causing muscle volume loss and weakness. This study reports for the first time the objective and accurate quantification of lower leg muscle atrophy in ankle joint OA by MRI examination. All patients involved in this study group suffered from high grade unilateral OA of the ankle joint, as indicated by the low mean AOFAS hindfoot score, the high mean VAS, the low ROM of the OA ankle joint, and the high grade degenerative changes as shown on conventional radiographs. A significant atrophy of the soleus muscle of the affected leg was demonstrated. All examined muscle groups of the affected leg showed a significantly greater fatty degeneration than the healthy leg.
The gradual decline in muscle strength and size associated with OA has been attributed to an impairment of the central nervous system, the so-called arthrogenous muscle inhibition or reflex atrophy [9, 10]. It is believed that abnormal joint afferent nerves from the osteoarthritic joint lead to neurotransmitter release at the level of the spinal cord, causing a reduction in the activity of alpha-motor neurons [9]. However, the exact mechanism of OA-related muscle atrophy remains unclear. Previous studies on muscle pathology in conjunction with OA focus on the hip and knee and on muscle force measurements. It has been shown that in knee OA the quadriceps strength is usually reduced by 14–45 % compared to control subjects [17–19]. Sirca et al. reported that OA of the hip was strongly associated with a type II fiber atrophy of the gluteal muscles [18]. Nakamura et al. also reported selective type II fiber atrophy in hip and knee OA [20]. For ankle OA, Valderrabano et al. showed that affected lower legs compared to legs with no OA had a significant atrophy on clinical examination [11]. Torque measurements showed a dorsiflexion/plantar flexion weakness, while surface electromyography showed a significant shift towards lower EMG frequencies, and significantly reduced EMG intensity for the anterior tibial, medial gastrocnemius, and long peroneus muscles during maximal voluntary muscle contraction. Interestingly, the mean electromyography intensity was not significantly changed in the soleus muscle.
Using MRI imaging, we differentiated whether individual muscle groups are involved in the process of atrophy. Only the M. soleus of the affected lower leg showed a significant atrophy, compared to the healthy side. The M. soleus is an uniarticular muscle and forms the gastrocsoleus complex with the biarticular muscles M. gastrocnemius medialis and lateralis. It is attached to the middle third of the Achilles tendon where it acts as a primary plantar flexor of the foot. Furthermore, it is an auxiliary muscle in supination [21] and inversion of the heel [22]. Campbell et al. observed that the medial portion of the M. soleus primarily acts as a plantar flexor, whereas the lateral portion mainly stabilises the lower leg on unstable surfaces [23]. Due to OA pain ankle joint movement becomes restricted and movements facilitated by the M. soleus are avoided. As a uniarticular muscle this disuse results in muscle atrophy. The M. gastrocnemius medialis and lateralis were not significantly affected. A possible explanation is that in contrast to the M. soleus those muscles are not only involved in ankle joint movement, but also contribute to knee joint flexion and stabilization. While ankle joint movement is restricted in OA, these muscles are still activated by knee joint motion. Similar data was obtained in a study investigating the development of atrophy of the M. quadriceps femoris after a period of immobilisation due to injury to the anterior cruciate knee ligament. Atrophy was mainly determined for the M. vastus medialis and lateralis which are also uniarticular muscles. As a biarticular muscle, the M. rectus femoris was, similarly to this study, affected to a lesser degree [24].
MRI and computertomography have been previously used to assess the cross sectional area of leg muscles in OA. Using MRI imaging, Arokoski et al. reported a significantly reduced muscle cross-sectional area of the thigh and pelvic muscles in patients with unilateral hip OA [25]. In patients with hip OA, Rasch et al. showed that the cross-sectional area of hip extensors, flexors, and adductors, as well as knee extensors and flexors in the OA limb, was significantly reduced on computertomographic imaging [26].
To the best of our knowledge no study exists which describes fatty muscle degeneration in OA of the leg. However, MRI has been previously used to determine the muscle quality of the rotator cuff [15, 27, 28]. In a prospective study on 38 patients, Gladstone et al. assessed the fatty infiltration of the rotator cuff before and after surgical repair [29]. Surgical repair did not lead to improvement of muscle degeneration and a failed repair resulted in significantly more progression. He concluded that repairs should be performed before more significant deterioration in the cuff musculature in order to optimize outcomes.
TAR is commonly performed in patients suffering from OA of the ankle joint. Good functional results and decrease of pain have been demonstrated [30–32]. However, it is still unclear whether the encountered muscle atrophy is reversible after TAR implantation. At one-year follow up after TAR surgery, Valderrabano et al. demonstrated a significant improvement in muscle function (torque, EMG intensity), while the calf circumference decreased insignificantly [33]. Whether the cross-sectional muscle area is a prognostic factor for the outcome after TAR is not yet known and will be assessed by an upcoming follow-up study.
This study has several limitations. As the overall incidence of post traumatic ankle joint OA is low, the study included only a limited number of patients. However, a power analysis performed prior to the study confirmed that the number of involved patients would be sufficient to find a significant difference. All patients had advanced OA on conventional radiographs. Therefore no conclusion can be made as to whether patients with beginning mild OA might show similar muscle atrophy. The MRI analysis of the individual muscles of each muscle group of the lower leg was limited. The individual muscles of the anterior tibial muscle group, the peroneal muscle group, and the deep dorsal muscle group could not be clearly separated on MRI. Thus the muscles were grouped and analysed together.
We conclude that unilateral ankle joint osteoarthritis leads to an overall lower leg muscle atrophy, but significant atrophy of the M. soleus. All muscles of the affected leg undergo a significant fatty degeneration.
Acknowledgements
The authors want to thank Thomas Egloff and Thorsten Wischer for support in evaluation of the MRI images.
References
- 1. Centers for Disease Control and Prevention (CDC) (2010) Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2007–2009. MMWR Morb Mortal Wkly Rep 59(39):1261–1265 [PubMed]
- 2.Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M. Increasing number and incidence of low-trauma ankle fractures in elderly people: Finnish statistics during 1970–2000 and projections for the future. Bone. 2002;31(3):430–433. doi: 10.1016/S8756-3282(02)00832-3. [DOI] [PubMed] [Google Scholar]
- 3.Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467(7):1800–1806. doi: 10.1007/s11999-008-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med. 2006;34(4):612–620. doi: 10.1177/0363546505281813. [DOI] [PubMed] [Google Scholar]
- 5.Horisberger M, Valderrabano V, Hintermann B. Posttraumatic ankle osteoarthritis after ankle-related fractures. J Orthop Trauma. 2009;23(1):60–67. doi: 10.1097/BOT.0b013e31818915d9. [DOI] [PubMed] [Google Scholar]
- 6.Nuki G. Osteoarthritis: a problem of joint failure. Z Rheumatol. 1999;58(3):142–147. doi: 10.1007/s003930050164. [DOI] [PubMed] [Google Scholar]
- 7.Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332(7542):639–642. doi: 10.1136/bmj.332.7542.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidd BL. Osteoarthritis and joint pain. Pain. 2006;123(1–2):6–9. doi: 10.1016/j.pain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Hurley MV, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis. 1997;56(11):641–648. doi: 10.1136/ard.56.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zacharova G, Knotkova-Urbancova H, Hnik P, Soukup T. Nociceptive atrophy of the rat soleus muscle induced by bone fracture: a morphometric study. J Appl Physiol. 1997;82(2):552–557. doi: 10.1063/1.365614. [DOI] [PubMed] [Google Scholar]
- 11.Valderrabano V, Tscharner V, Nigg BM, Hintermann B, Goepfert B, Fung TS, Frank CB, Herzog W. Lower leg muscle atrophy in ankle osteoarthritis. J Orthop Res. 2006;24(12):2159–2169. doi: 10.1002/jor.20261. [DOI] [PubMed] [Google Scholar]
- 12.Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349–353. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 14.Morrey BF, Wiedeman GP., Jr Complications and long-term results of ankle arthrodeses following trauma. J Bone Joint Surg Am. 1980;62(5):777–784. [PubMed] [Google Scholar]
- 15.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599–605. doi: 10.1016/S1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 16.Zanetti M, Gerber C, Hodler J. Quantitative assessment of the muscles of the rotator cuff with magnetic resonance imaging. Invest Radiol. 1998;33(3):163–170. doi: 10.1097/00004424-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Pap G, Machner A, Awiszus F. Strength and voluntary activation of the quadriceps femoris muscle at different severities of osteoarthritic knee joint damage. J Orthop Res. 2004;22(1):96–103. doi: 10.1016/S0736-0266(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 18.Sirca A, Susec-Michieli M. Selective type II fibre muscular atrophy in patients with osteoarthritis of the hip. J Neurol Sci. 1980;44(2–3):149–159. doi: 10.1016/0022-510X(80)90123-9. [DOI] [PubMed] [Google Scholar]
- 19.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, Wolinsky FD. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127(2):97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Suzuki K. Muscular changes in osteoarthritis of the hip and knee. Nihon Seikeigeka Gakkai Zasshi. 1992;66(5):467–475. [PubMed] [Google Scholar]
- 21.Janda V. Muscle function testing. London: Butterworths; 1983. [Google Scholar]
- 22.Michael RH, Holder LE. The soleus syndrome. A cause of medial tibial stress (shin splints) Am J Sports Med. 1985;13(2):87–94. doi: 10.1177/036354658501300202. [DOI] [PubMed] [Google Scholar]
- 23.Campbell KM, Biggs NL, Blanton PL, Lehr RP. Electromyographic investigation of the relative activity among four components of the triceps surae. Am J Phys Med. 1973;52(1):30–41. [PubMed] [Google Scholar]
- 24.Einsingbach T. Funktionelle Behandlung be Knieinstabilitäten. In: Krankengymnastik Jg. 38. Heft. 1986;6:411–430. [Google Scholar]
- 25.Arokoski MH, Arokoski JP, Haara M, Kankaanpaa M, Vesterinen M, Niemitukia LH, Helminen HJ. Hip muscle strength and muscle cross sectional area in men with and without hip osteoarthritis. J Rheumatol. 2002;29(10):2185–2195. [PubMed] [Google Scholar]
- 26.Rasch A, Bystrom AH, Dalen N, Berg HE. Reduced muscle radiological density, cross-sectional area, and strength of major hip and knee muscles in 22 patients with hip osteoarthritis. Acta Orthop. 2007;78(4):505–510. doi: 10.1080/17453670710014158. [DOI] [PubMed] [Google Scholar]
- 27.Hedtmann A, Heers G. Imaging in evaluating rotator cuff tears. Orthopade. 2007;36(9):796–809. doi: 10.1007/s00132-007-1138-8. [DOI] [PubMed] [Google Scholar]
- 28.Spencer EE, Jr, Dunn WR, Wright RW, Wolf BR, Spindler KP, McCarty E, Ma CB, Jones G, Safran M, Holloway GB, Kuhn JE. Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging. Am J Sports Med. 2008;36(1):99–103. doi: 10.1177/0363546507307504. [DOI] [PubMed] [Google Scholar]
- 29.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 30.Hintermann B, Valderrabano V, Knupp M, Horisberger M. The HINTEGRA ankle: short- and mid-term results. Orthopade. 2006;35(5):533–545. doi: 10.1007/s00132-006-0941-y. [DOI] [PubMed] [Google Scholar]
- 31.Valderrabano V, Pagenstert G, Horisberger M, Knupp M, Hintermann B. Sports and recreation activity of ankle arthritis patients before and after total ankle replacement. Am J Sports Med. 2006;34(6):993–999. doi: 10.1177/0363546505284189. [DOI] [PubMed] [Google Scholar]
- 32.Valderrabano V, Hintermann B, Dick W (2004) Scandinavian total ankle replacement: a 3.7-year average followup of 65 patients. Clin Orthop Relat Res (424):47–56 [PubMed]
- 33.Valderrabano V, Nigg BM, Tscharner V, Frank CB, Hintermann B. J. Leonard Goldner award 2006. Total ankle replacement in ankle osteoarthritis: an analysis of muscle rehabilitation. Foot Ankle Int. 2007;28(2):281–291. doi: 10.3113/FAI.2007.0281. [DOI] [PubMed] [Google Scholar]