Abstract
This study aimed at characterizing the insecticidal genes of eight Bacillus thuringiensis isolates that were recovered from the local environment of western Saudi Arabia. The screening for the presence of lepidopteran-specific cry1A family and vip3A genes, dipteran-specific cry4 family and coleopteran-specific cry3A, vip1A and vip2A genes, was carried out by PCR. All eight isolates produced PCR products that confirmed the presence of cry1Aa, cry1Ab, cry1Ac, cry4A, cry4B genes, but not cry3A, vip1A and vip2A genes. However, three isolates only were found to carry vip3A genes as revealed by PCR. The observation of cry1 and cry4 genes suggests that these eight isolates may have dual activity against Lepidoptera and Diptera species, while three isolates possessed vip3 genes in addition to cry1 and cry4 which suggests that these three isolates have toxic crystals and vegetative proteins. The results of this study are interesting in the sense that they may help developing new strategies for controlling insects of economic and medical importance in Saudi Arabia, using B. thuringiensis strains that naturally exist in the local environment instead of the current control strategies that are based solely on chemical insecticides.
Keywords: Bacillus thuringiensis, cry Genes, PCR, vip Genes
The insecticidal activities of Bacillus thuringiensis are mainly related to the production of parasporal inclusions, which are formed by polypeptides known as Cry proteins. These proteins showed entomopathogenic activities against wide spectrum of insect orders [1]. Other virulence factors that may play a role in the toxic activities of B. thuringiensis include vegetative insecticidal protein (vip), phospholipases, proteases, and chitinases [2]. Because of their specificity and safety to the environment, the crystal and vegetative proteins have been widely used for many years as biopesticides for the control of insect pests in agriculture, forestry and in the home [3, 4].
Despite the wide spectrum of B. thuringiensis toxicity against invertebrate, there were a number of crystal-bearing B. thuringiensis strains that exhibited no toxic activities [5], or their activities may threaten by the possible evolution of resistance from susceptible insect host [6]. Thus, there is a great interest for screening B. thuringiensis collection strains [7–9], and/or the isolation of novel strains/toxins [10–12], to discover insecticidal genes with broader insect host range [13].
We managed to isolate eight strains of B. thuringiensis for the first time from the environment of western Saudi Arabia. These isolates were found to be very toxic to Lepidoptera as revealed by toxicity assay [14]. We were unable to investigate the toxicity of these isolates against insects belonging to orders other than Lepidoptera by bioassay. Thus the aim of this study was to predict the insecticidal activity of our eight B. thuringiensis isolates by PCR.
The B. thuringiensis strains analyzed in this work were previously isolated from soil samples and dead larvae of Spodoptera littoralis collected from the environment of Makkah Province, Saudi Arabia. These eight isolates were confirmed by morphological and molecular methods, and finally by toxicity assay against S. littoralis. These strains were identified as B. thuringiesis IBL 200 (GenBank accession number: NK01000211) [14].
Screening of toxicity genes was carried out by PCR. Primers used for the amplification of crystal toxic genes were Lep1A/Lep1B Lepidopteran-specific, Dip1A/Dip1B Dipteran-specific and Col1A/Col1B Coleopteran-specific crystal genes [15]. For amplification of vegetative toxic genes, primers 5-vip1A/3-vip1A, 5-vip2A/3-vip2A [16] and vip3A [17] were used. The sequence of each primer, the target genes and the expected product size are listed in Table 1.
Table 1.
Sequence of primers used in PCR screening
| Primer | Sequence | Product size (bp) | Gene(s) recognized | Reference |
|---|---|---|---|---|
| Lep1A | 5′-CCGGTGCTGGATTTGTGTTA-3′ | 490 | cry1Aa, cry1Ab, cry1Ac | [15] |
| Lep1B | 5′-AATCCCGTATTGTACCAGCG-3′ | |||
| Dip1A | 5′-CAAGCCGCAAATCTTGTGGA3-′ | 797 | cry4A, cry4B | [15] |
| Dip1B | 5′-ATGGCTTGTTTCGCTACATC-3′ | |||
| Col1A | 5′-GTCCGCTGTATATTCAGGTG-3′ | 649 | cry3A | [15] |
| Col1B | 5′-CACTTAATCCTGTGACGCCT-3′ | |||
| 5-vip1A | 5′-GGATCCGATGAAAAATATGAAGAA-3′ | 2300 | vip1A | [16] |
| 3-vip1A | 5′-GTCGACTTATCTAGATTTGTTAGGT-3′ | |||
| 5-vip2A | 5′-GGATCCGATGAAAAGAATGGAGGG-3′ | 1300 | vip2A | [16] |
| 3-vip2A | 5′-GTCGACTTAATTTGTTAATAATGTTG-3′ | |||
| vip3A | 5′-ATGAACAAGAATAATACTAAA-3′ | 2300 | vip3A | [17] |
| vip3A | 5′-GCGGCCGCTTACTTAATAGAGAC-3′ |
PCR mixtures were prepared as described by Carozzi et al. [15], briefly, a loopful of cells from an overnight culture, was suspended in 100 μl sterile water and boiled for 10 min. One microliter of this suspension was used as template DNA and was added to 50 μl PCR mix containing 0.25 mmol l−1 dNTPs, 1 mmol l−1 MgCl2, 0.6 mmol l−1 of each primer (Bioneer, Alameda, USA), and 1 unit Taq DNA polymerase (ABgene, Surry, UK). Amplification was done under the following conditions: a 45 s denaturation step at 95°C, anneal for 45 s at 45°C, and extended at 72°C for 1 min, for total of 35 cycles. A total of 20 μl of PCR reaction mix was analyzed by gel electrophoresis on a 0.8% agarose gel (Bioline, London, UK) in Tris–borate buffer, and made visible by ethidium bromide (0.5 μg ml−1) staining and UV transillumination.
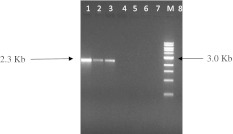
The results showed that all eight isolates were found to produce PCR products with 490 bp when primers Lep1A and Lep1B were used. Similarly, when primers Dip1A and Dip1B were used, the amplicon size of PCR products from all eight isolates was 797 bp. However when primers Col1A and Col1B were used, no PCR products were observed. With regards to vegetative toxic genes, PCR products of 2,300 bp were observed when primers vip3 from three out of eight isolates (Fig. 1), while no PCR products from all eight isolates were observed when primers vip1 and vip2 primers were used.
Fig. 1.
Agarose gel electrophoresis of PCR products amplified from native B. thuringiensis isolates with vip3A forward and reverse primers. Lane M 1 kb DNA ladder, Lanes 1–8B. thuringiensis isolates Bt1 to Bt8
The PCR method we have used was suggested to be a useful rapid screening test to predict the insecticidal activities of new B. thuringiensis isolates [15]. The use of these primers had provided valuable data about the content and the activity of toxic genes in a large number of B. thuringiensis strains worldwide [8–12, 15, 18–21].
The content of cry genes in our isolates appears to be in concordance with the toxicity of these strains against S. littoralis previously reported by Assaeedi et al. [14]. The observation of a 490 bp band with primers Lep1A/Lep1B, confirms the presence of cry1Aa, cry1Ab and cry1Ac genes (Table 2). The cry1 genes family is widely known for their activity against Lepidoptera [12]. The cry1Aa, cry1Ab and cry1Ac are probably the most common profiles of cry1 gene family that are carried by lepidopteran-toxic B. thuringienisis strains [7, 10, 22, 23].
Table 2.
Crystal and vegetative toxic genes of eight Bacillus thurngiensis isolates as determined by PCR
| Bt isolate no. | Source | Crystal genes present | Vegetative genes present |
|---|---|---|---|
| Bt1 | Urban soil | cry1Aa, cry1Ab, cry1Ac, cry4A, cry4B | vip3A |
| Bt2 | Urban soil | cry1Aa, cry1Ab, cry1Ac, cry4A, cry4B | vip3A |
| Bt3 | Agricultural soil | cry1Aa, cry1Ab, cry1Ac, cry4A, cry4B | vip3A |
| Bt4 | Agricultural soil | cry1Aa, cry1Ab, cry1Ac, cry4A, cry4B | – |
| Bt5 | Agricultural soil | cry1Aa, cry1Ab, cry1Ac, cry4A, cry4B | – |
| Bt6 | Dead larvae | cry1Aa, cry1Ab, cry1Ac, cry4A, cry4B | – |
| Bt7 | Dead larvae | cry1Aa, cry1Ab, cry1Ac, cry4A, cry4B | – |
| Bt8 | Dead larvae | cry1Aa, cry1Ab, cry1Ac, cry4A, cry4B | – |
When primers Dip1A/Dip1B were used, PCR profiles suggest that these isolates carry cry4A and cry4B genes, and may have activity against Dipteran species (Table 2). Similar results were reported elsewhere using various sets of primers, and it was observed that insecticidal activity predicted by PCR corresponded with the insecticidal activity of insect bioassay [15, 19]. In this respect we suggest that our eight B. thuringiensis isolates may have dual insecticidal activities against Lepidoptera and Diptera.
PCR analysis of all eight isolates yielded no detectable cry3A gene products when primers Col1A/Col1B were used (Table 2). This result may suggest that these eight isolates do not carry cry3A gene. Similarly, Chak et al. [22] reported the absence of cry3 genes in 225 isolates of B. thuringiensis in Taiwan. The observation of no PCR products, may also suggests that the genes are gone undetected [15].
The vip3A genes are toxic to Lepidoptera and other species, with different mode of action from cry genes [24]. We found that (37.5%) of our eight isolates carried vip3A genes (Table 2). Similarly, the low frequency of vip3A genes was reported elsewhere [25]. The coleopteran-specific vip1 and vip2 genes were not detected in any of the eight isolates (Table 2). Altogether the absence of cry3A, vip1A and vip2A genes further suggests that these isolates may have no insecticidal activity against coleopteran species.
In conclusion, the findings of this study are very interesting and need further investigation since dengue fever is widely common in Makkah region [26], thus, if the cry4 genes of these eight isolates are active against Aedes aegypti, it would be best developing control strategies of this pest in particular, or other insects of economic importance in general, using locally derived B. thuringiensis strains instead of the current control strategies that are based solely on chemical insecticides.
References
- 1.Frankenhuyzen K. Insecticidal activity of Bacillus thuringiensis crystal proteins. J Invertebr Pathol. 2009;101:1–16. doi: 10.1016/j.jip.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay A, Bhatnagar NB, Bhatnagar R. Bacterial insecticidal toxins. Crit Rev Microbiol. 2004;30:33–54. doi: 10.1080/10408410490270712. [DOI] [PubMed] [Google Scholar]
- 3.Crickmore N. Beyond the spore—past and future developments of Bacillus thuringiensis as a biopesticide. J Appl Microbiol. 2006;101:616–619. doi: 10.1111/j.1365-2672.2006.02936.x. [DOI] [PubMed] [Google Scholar]
- 4.Roh JY, Jae YC, Ming SL, Byung RJ, Yeon HJ. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J Microbiol Biotechnol. 2007;17:547–559. [PubMed] [Google Scholar]
- 5.Yasutake K, Binh ND, Kagoshima K, Uemori A, Ohgushi A, Maeda M, Mizuki E, Yu YM, Ohba M. Occurrence of parasporin-producing Bacillus thuringiensis in Vietnam. Can J Microbiol. 2006;52:272–365. doi: 10.1139/w05-134. [DOI] [PubMed] [Google Scholar]
- 6.Herrero S, Oppert B, Ferré J. Different mechanisms of resistance to Bacillus thuringiensis toxins in the Indian meal moth. Appl Environ Microbiol. 2001;67:1085–1089. doi: 10.1128/AEM.67.3.1085-1089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Boets A, Rie J, Ren G. Characterization of cry1, cry2, and cry9 genes in Bacillus thuringiensis isolates from China. J Invertebr Pathol. 2003;82:63–71. doi: 10.1016/S0022-2011(02)00202-1. [DOI] [PubMed] [Google Scholar]
- 8.Seifinejad A, Salehi Jouzani GR, Hosseinzadeh A, Abdmishani C. Characterization of Lepidoptera-active cry and vip genes in Iranian Bacillus thuringiensis strain collection. Biol Control. 2008;44:216–226. doi: 10.1016/j.biocontrol.2007.09.010. [DOI] [Google Scholar]
- 9.Hernández-Rodríguez CS, Boets A, Rie J, Ferré J. Screening and identification of vip genes in Bacillus thuringiensis strains. J Appl Microbiol. 2009;107:219–225. doi: 10.1111/j.1365-2672.2009.04199.x. [DOI] [PubMed] [Google Scholar]
- 10.Armengol G, Escobar MC, Maldonado ME, Orduz S. Diversity of Colombian strains of Bacillus thuringiensis with insecticidal activity against dipteran and lepidopteran insects. J Appl Microbiol. 2007;102:77–88. doi: 10.1111/j.1365-2672.2006.03063.x. [DOI] [PubMed] [Google Scholar]
- 11.Gao M, Li R, Dai S, Wu Y, Yi D. Diversity of Bacillus thuringiensis strains from soil in China and their pesticidal activities. Biol Control. 2008;44:380–388. doi: 10.1016/j.biocontrol.2007.11.011. [DOI] [Google Scholar]
- 12.Saadaoui I, Rouis S, Jaoua S. A new Tunisian strain of Bacillus thuringiesis kurstaki having high insecticidal activity and δ-endotoxin yield. Arch Microbiol. 2009;191:341–348. doi: 10.1007/s00203-009-0458-y. [DOI] [PubMed] [Google Scholar]
- 13.Franco-Rivera A, Benintende G, Cozzi J, Baizabal-Aguirre VM, Valdez-Alarcon JJ, Lopez-Meza JE. Molecular characterization of Bacillus thuringiensis strains from Argentina. Antonie van Leeuwenhoek. 2004;86:87–92. doi: 10.1023/B:ANTO.0000024913.94410.05. [DOI] [PubMed] [Google Scholar]
- 14.Assaeedi ASA, Osman GEH, Abulreesh HH. The occurrence of Bacillus thuringiensis in the arid environments. Aust J Crop Sci. 2011;5:1185–1190. [Google Scholar]
- 15.Carozzi NB, Kramer VC, Warren GW, Evola S, Koziel MC. Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl Environ Microbiol. 1991;57:3057–3061. doi: 10.1128/aem.57.11.3057-3061.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Xu W, Yuan M, Tang M, Chen J, Pang Y. Expression of vip1/vip2 genes in Escherichia coli and Bacillus thuringiensis and analysis of their signal peptides. J Appl Microbiol. 2004;97:757–765. doi: 10.1111/j.1365-2672.2004.02365.x. [DOI] [PubMed] [Google Scholar]
- 17.Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, Koziel MG. vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum activities against lepidopteran insects. Proc Natl Acad Sci USA. 1996;93:5389–5394. doi: 10.1073/pnas.93.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Procar M, Caballero P. Molecular and insecticidal characterization of a Bacillus thuringiensis strain isolated during a natural epizootic. J Appl Microbiol. 2000;89:309–316. doi: 10.1046/j.1365-2672.2000.01115.x. [DOI] [PubMed] [Google Scholar]
- 19.Martínez C, Caballero P. Contents of cry genes and insecticidal toxicity of Bacillus thuringiensis strains from terrestrial and aquatic habitats. J Appl Microbiol. 2002;92:745–752. doi: 10.1046/j.1365-2672.2002.01579.x. [DOI] [PubMed] [Google Scholar]
- 20.Beard CE, Court L, Boets A, Mourant R, Rie J, Akhurst RJ. Unusually high frequency of genes encoding vegetative insecticidal proteins in an Australian Bacillus thuringiensis collection. Curr Microbiol. 2008;57:195–199. doi: 10.1007/s00284-008-9173-1. [DOI] [PubMed] [Google Scholar]
- 21.Song L, Gao M, Dai S, Wu Y, Yi D, Li R. Specific activity of Bacillus thuringiensis strain against Locusta migratoria manilensis. J Invertebr Pathol. 2008;98:176–196. doi: 10.1016/j.jip.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Chak KF, Chao DC, Tseng MY, Kao SS, Tuan SJ, Feng TY. Determination and distribution of cry-type genes of Bacillus thuringiensis isolates from Taiwan. Appl Environ Microbiol. 1994;60:2415–2420. doi: 10.1128/aem.60.7.2415-2420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bravo A, Sarabia S, Lopez L, Ontiveros H, Abarca C, Ortiz A, Ortiz M, Lina L, Villalobos F, Pena G, Nunes M, Soberón M, Quintero R. Characterization of cry genes in a Mexican Bacillus thuringiensis strain collection. Appl Environ Microbiol. 1998;64:4965–4972. doi: 10.1128/aem.64.12.4965-4972.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MK, Walters FS, Hart H, Palekar N, Chen J-S. The mode of action of the Bacillus thuringiensis vegetative insecticidal protein vip3A differs from that of Cry1AB δ-endotoxin. Appl Environ Microbiol. 2003;69:4648–4657. doi: 10.1128/AEM.69.8.4648-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrieta G, Hernandez AG, Espinoza AM. Diversity of Bacillus thuringiensis strains isolated from coffee plantations infested with the coffee berry borer Hypothenemus hampei. Rev Biol Trop. 2004;52:757–764. [PubMed] [Google Scholar]
- 26.Health statistical year book. Riyadh: Ministry of Health Press; 2008. [Google Scholar]