Abstract
Bacterial vaginosis (BV) is the most prevalent vaginal infection worldwide and is characterized by reduction of native lactobacilli. Antimicrobial therapy used to cure the disease is often found to be ineffective. We postulate that Bacillus coagulans Unique IS-2 (Unique Biotech Limited, India) might provide an appendage to antimicrobial treatment and improve curing rate. In the present study 40 Indian women diagnosed with BV by the presence of symptoms including white discharge, pH greater than 4.7, burning micturation, itching, soreness and redness at vulva. The subjects were divided in 2 groups probiotic (n = 20) and control (n = 20) based on age (control group, 33 ± 3 years and probiotic group, 32.5 ± 3 years), history of previous vaginosis (control group, 75% or 15/20 and probiotic group, 75% or 15/20) and severity of current vaginosis infection (burning micturation and itching, 35% in each group). Probiotic group subjects were assigned to receive a dose of antibiotic therapy [Ofloxacin–Ornidazole with strength of 200–500 mg per capsule/day for 5 days along with vaginal peccaries (co-kimaxazol) for 3 days] simultaneously with two probiotic capsules (109 CFUs of Bacillus coagulans Unique IS-2 per capsule). The control group received only antibiotic therapy. At the end of the treatment the 80% of probiotic group subjects showed significant positive response as revealed by reduction of vaginosis symptoms compared to the control group which exhibited reduction in 45% subjects only. Thus, the results of present study indicate that strain Bacillus coagulans Unique IS-2 can provide benefits to women being treated with antibiotics to cure an infectious condition.
Keywords: Bacillus coagulans Unique IS-2, Bacterial vaginosis
Introduction
Bacterial vaginosis (BV) is a term used to describe disorders that cause infection or inflammation of the vagina. BV is quite complex, however, very common condition characterized by the substitution of native Lactobacilli by either anaerobic bacteria such as Atopobium, Gardnerella and Mobiluncus [1, 2], or Mycoplasma [3], or aerobic bacteria such as pathogenic Escherichia coli and enterococci [4]. The net result is an elevated pH, production of sialidase by colonizing foreign microbes [5] or amine production by them resulting in white discharge and fishy odor [6]. Vaginal infections can have symptoms, like itching, burning, and vaginal discharge [7]. If vaginosis is left untreated, it leads to some other complications like, pelvic inflammatory disease [8], infertility, pre-term birth, low birth weight etc. [7]. Hence vaginitis is considered to be warning symptoms to prevent occurrence of such complications and should be treated in time. Treatment of BV comprises either oral or vaginal antibiotic supplements, such as metronidazole or clindamycin. However, the curing rate is poor and recurrences are often found to be very common [9]. This has led to the use of long term, low dose suppressive therapy for BV. Other studies also suggest that the incidence of BV increases the risk of sexually transmitted infections [10], and therefore, it is essential to develop new approaches to the preservation of a healthy vagina, particularly therapies which can be readily available, economical and with a higher efficacy in terms of reducing recurrences.
Probiotic bacteria are considered as an alternative choice for BV prevention and treatment due to their capability to produce antimicrobial compounds such as, acetic acid, bacteriocins and hydrogen peroxide, thereby hindering pathogenic bacterial growth [11]. Vaginal probiotic capsules are particularly alluring because of their ease of use and high satisfaction rates against the use of creams and gels. The purpose of this study was to determine the efficacy of oral supplementation of probiotic capsules containing Bacillus coagulans-Unique IS2 in the treatment of BV.
Materials and Methods
Selection of Patients for Clinical Study
Forty women reported at out-patient department in Government Medical College and Hospital Aurangabad, India, with BV were examined. The study was approved by the Institutional Ethics Committee (ref no: ICE/GMCA/114/2008) and all subjects gave written informed consent before any research activity was initiated. The inclusion criteria for the subjects was based on presence of the following symptoms such as, white discharge, positive for whiff test, vaginal pH greater than 4.7 (normal pH between 3.8 and 4.5), along with other symptoms like, burning micturation, itching, soreness and redness at vulva. Subjects were excluded if they were diagnosed as pregnant woman, presence of diseases like IDDM, hypothyroidism, severe obesity or liver disease, diseases which may affect pH of body. Patients who have taken systemic or vaginal antibiotics, probiotics, systemic or vaginal anti-fungal agents or systemic corticosteroids in previous 30 days were also excluded from the study.
Study Groups and Treatments
Participants fulfilling the entry criteria were divided equally (control 20 and probiotic 20). The following observation are noted for age (control group, 33 ± 3 years and probiotic group, 32.5 ± 3), history of previous vaginosis (control group, 75% or 15/20 and probiotic group, 75% or 15/20) and severity of current vaginosis infection (burning micturation and itching, 35% in each group). Study subjects were not blinded to the treatment they received. The control group patients were given standard vaginosis treatment alone (Ofloxacin–Ornidazole with a strength of 200–500 mg per capsule/day for 5 days along with co-kimaxazol vaginal peccaries for 3 days). The probiotic group patients took standard vaginosis treatment plus two capsules of B. coagulans Unique IS-2 (1 × 109 CFU/capsule) twice a day before meals for 90 days.
Data Collection and Diagnosis
Patients received a multidisciplinary evaluation consisting of physical examination, neuropsychological, psychiatry, and family interview. Study has been initiated only after obtaining informed consent for the current treatment protocol. The diagnosis of BV was assessed based on whiff test, assessment of presence and severity of the symptoms such as white discharge, pH > 4.7, burning micturation, itching, soreness and redness at vulva. All the patients returned for follow-up visit at 90th day. Follow up evaluation included, diagnosis for BV symptoms (described above) and a report of adverse events. The result of the study was analyzed by χ2 test.
Results and Discussion
For this study, 40 subjects were enrolled to participate with the symptoms and signs of BV from Government Medical College and Hospital Aurangabad, India. Table 1 shows the condition of the probiotic (n = 20) and control (n = 20) group subjects at the beginning of the study. All the subjects had exhibited white discharge (probiotic (n = 20) and control (n = 20) subjects), however, they differed in other symptoms such as burning, micturation and itching, followed by soreness. Regardless of the significant progress reported in understanding the epidemiology, pathophysiology, and treatment of BV, scant information is available regarding recurrent BV. Most therapeutic trials evaluate treatment outcome at the completion of treatment after 7th day or at best 28–35 days following the termination of therapy, but longer follow-up is rare [12]. Considering duration of the study, a combinational therapy consisting of conventional antibiotics along with probiotic B. coagulans Unique IS-2 was carried out for 3 months. The strain Unique IS-2 was well identified for its probiotic properties. Selection of this strain was (B. coagulans Unique IS-2) based on its in vitro evaluation for antimicrobial activity against several pathogens (including gram positive and gram negative) and its adherence ability (Adhesion percentage of 58%) [13].
Table 1.
Comparative analysis of vaginosis symptoms in both groups
| Variable | No. of patients | Day 1, n | Day 90, n | ||||
|---|---|---|---|---|---|---|---|
| Probiotic group (n = 20) | Control group (n = 20) | Probiotic group (n = 20) | Control group (n = 20) | χ2 test, P | |||
| Whiff (amine) test | 40 | Positive | 20 (100%) | 20 (100%) | 4 (20%) | 11 (55%) | S, 0.0002 |
| Negative | 0 | 0 | 16 (80%) | 9 (45%) | |||
| White discharge | 40 | Positive | 20 (100%) | 20 (100%) | 4 (20%) | 11 (55%) | S, 0.0002 |
| Negative | 0 | 0 | 16 (80%) | 9 (45%) | |||
| Burning micturation | 14 | Absent | 0 | 0 | 2 (10%) | 0 | NA |
| Mild | 0 | 0 | 5 (25%) | 1 (5%) | |||
| Moderate | 0 | 0 | 0 | 2 (10%) | |||
| Severe | 7 (35%) | 7 (35%) | 0 | 4 (20%) | |||
| Itching | 14 | Presence | 7 (35%) | 7 (35%) | 2 (10%) | 5 (25%) | NA |
| Absence | 0 | 0 | 5 (25%) | 2 (10%) | |||
| pH (> 4.7) | 40 | pH, n (%) | 6.23 ± 4.8, 20 (100%) | 6.3 ± 4.2, 20 (100%) | 6.0 ± 0.0, 20 (100%) | 6.13 ± 3.2, 20 (100%) | NA |
S Significant, NS non significant, NA not applicable
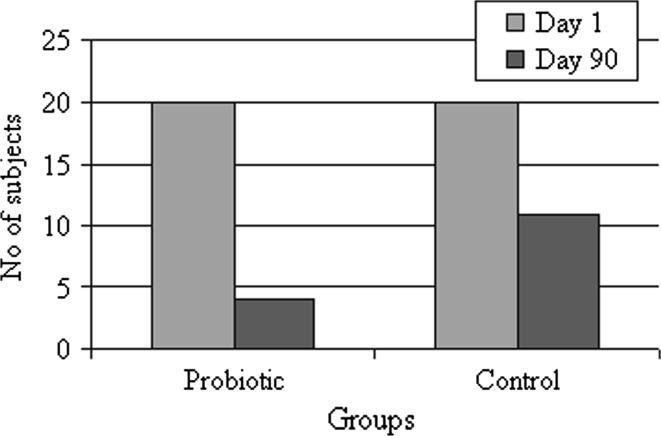
Out of 40 patients who enrolled for the study 20/20 (100%) from probiotic group, 20/20 (100%) from control group returned for follow up at day 90. Among the probiotic group most of the subjects (16/20) were negative for amine (Whiff) test and did not show any white discharge (n = 16), whereas in control group only 9 out of 20 subjects were negative for amine (whiff) test (P = 0.0002, Fig. 1). Other symptoms like, burning micturation was reduced to mild (5/7), absence of itching in 5 subjects and soreness, redness were completely found disappeared in all the tested probiotic subjects. In contrast, control group subjects were suffering with severe burning micturation (4/7) and itching (5/7) (Table 1).
Fig. 1.
Summary of subjects suffering with BV before (day 1) and after (day 90) treatment
Oral supplementation of probiotic is also effective as most of the urogenital bacterial microflora originates from the GI tract [14]. Intestinal passage of these probiotic strains led to a beneficial impact on the vaginal microflora. This may have occurred due to strains themselves ascending to the vagina from the rectal area or altering the ability of the pathogens to transfer to this niche [14]. Thus, the probiotic supplementation along with standard antibiotic treatment was more beneficial as reported by Anukam et al. [7].
In the present study, 80% (16/20) of probiotic group subjects were recorded towards improvement in minimizing BV recurrence as per consent. Similar observation was reported by Shirodkar et al. [15], wherein Lactobacillus sporogenes (B. coagulans) alleviates the symptoms caused by non specific vaginitis.
Conclusions
This study concludes that B. coagulans Unique IS-2 is quite effective in minimizing recurrence of vaginal infections and consequent symptoms such as, white discharge, burning, micturation, itching and soreness. Hence, oral supplementation of probiotic capsule containing strain Unique IS-2 could be a solution for efficient treatment of bacterial vaginosis. The study however, had following limitations of being a preliminary study with a small number of patients and also an open trial. Further studies are needed to establish significant effect in large number of patients before it is accepted as an alternative mode of treatment of patients with symptoms of BV.
References
- 1.Hillier SL. The complexity of microbial diversity in bacterial vaginosis. New Eng J Med. 2005;353:1886–1887. doi: 10.1056/NEJMp058191. [DOI] [PubMed] [Google Scholar]
- 2.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. New Eng J Med. 2005;353:899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 3.French JI, McGregor JA, Parker R. Readily treatable reproductive tract infections and preterm birth among black women. Am J Obstet Gynecol. 2006;194(6):1717–1726. doi: 10.1016/j.ajog.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Donder GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Int J Obstet Gynaecol. 2002;109:34–43. doi: 10.1111/j.1471-0528.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- 5.Cauci S, Guaschino S, Driussi S, Santo D, Lanzafame P, Quadrifoglio F. Correlation of local interleukin-8 with immunoglobulin A against Gardnerellavaginalis hemolysin and with prolidase and sialidase levels in women with bacterial vaginosis. J Infect Dis. 2002;185:1614–1620. doi: 10.1086/340417. [DOI] [PubMed] [Google Scholar]
- 6.Hapsari ED, Hayashi M, Matsuo H. Clinical characteristics of vaginal discharge in bacterial vaginosis diagnosed by Nugent’s criteria. Clin Exp Obstet Gynecol. 2006;33:5–9. [PubMed] [Google Scholar]
- 7.Anukam KC, Osazuwa E, Osemene GI, Ehigiagbe F, Bruce AW, Reid G. Clinical study comparing probiotic lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006;8:2772–2776. doi: 10.1016/j.micinf.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Ness RB, Kip KE, Soper DE, Stamm CA, Rice P, Richter HE. Variability of bacterial vaginosis over 6- to 12-month intervals. Sex Trans Dis. 2006;33(6):381–385. doi: 10.1097/01.olq.0000204748.89222.33. [DOI] [PubMed] [Google Scholar]
- 9.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 10.Myer L, Denny L, Telerant R, Souza M, Wright TC, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192:372–1380. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 11.Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo controlled study. Am J Obstet Gynecol. 2010;203:120.1–120.6. doi: 10.1016/j.ajog.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Roger L, Cook, Redondo-lopez V, Schmitt C, Meriwether C, Sobel JD. Clinical, microbiological, and biochemical factors in recurrent bacterial vaginosis. J Clin Microbiol. 1992;30(4):870–877. doi: 10.1128/jcm.30.4.870-877.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudha R, Chauhan P, Dixit K, Babu SM, Jamil K. Molecular typing and probiotic attributes of a new strain of Bacilluscoagulans–Unique IS-2: a potential biotherapeutic agent. Genetic Eng Biotechnol J. 2010;7:1–20. [Google Scholar]
- 14.Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, Bruce AW. Oral use of Lactobacillusrhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol Med Microbiol. 2003;35(2):131–134. doi: 10.1016/S0928-8244(02)00465-0. [DOI] [PubMed] [Google Scholar]
- 15.Shirodkar NV, Sankholkar PC, Ghosh S, Nulkar SM. Multi-centre clinical assessment of myconip vaginal tablets in non-specific vaginitis. Indian Pract. 1980;33:207–210. [Google Scholar]