Abstract
Culturing the bioluminescent bacterium Photorhabdus luminescens in nutrient broth (NB) is used to recover phase I cells. These phase I cells were highly luminescent for up to 7 h in this media and the luminosity could also be seen with the naked eye after a 15 min eye adjustment period in a dark room. Red pigmentation is a known trait of phase I cells and was visually distinct within the culture media. The color shade of the red pigment varied on nutrient agar and in NB suggesting that the concentration of the pigment produced is dependent upon density of phase I cells within a specified area. The specific growth rate (μ) and doubling time (g) was determined during the logarithmic growth phase to be 0.36 h−1 and 2.1 h, respectively in NB medium. The nematode-bacterium suspension was injected into larvae of Galleria mellonella to test for entomopathogencity. Within 24 h post-injection insect mortality was seen along with dark red pigmentation and extremely high luminosity indicating infection with P. luminescens.
Keywords: Photorhabdus luminescens, Biomass, Specific growth rate, Bioprocessing, Bioluminescence, Entomopathogenic nematodes
Introduction
Photorhabdus luminescens is a Gram-negative, phase-varying enterobacterium that exists in a symbiotic relationship with the entomopathogenic nematode (EPN) Heterorhabditis bacteriophora [1]. Important properties of the phase I variant include pigmentation, bioluminescence and the production of insect toxins and antimicrobials [2, 3]. The growth of P. luminescens in nutrient broth (NB) was studied to determine its physiological properties. In a previous study, cultures of P. luminescens in the late stationary phase were suitable to obtain high recoveries of the nematode H. bacteriophora in liquid culturing [4]. Similarly, transfer of H. bacteriophora dauer juveniles (DJ) into cell-free filtrates of P. luminescens in late logarithmic phase gave recoveries as high as 95% after several days [5]. A low DJ recovery can result in asynchronous nematode populations [6]. In contrast, use of the phase II variant reduced the recoveries of H. bacteriophora cultures [7, 8]. For this reason the phase II form should be avoided. Specificity of culture strains and variants of P. luminescens is critical to mass produce EPNs. Mass production in bioreactors requires culturing under known conditions for both bacteria and EPNs. This study was initiated to produce viable phase I cells for the mass production of H. bacteriophora in a bioreactor [9].
This study underlines the growth characteristics of P. luminescens such as specific growth rates, doubling times and nutrient requirements. Knowing these growth characteristics may allow the elucidation of the symbiotic and pathogenic relationships of P. luminescens [10]. Bacterial luminescence may also be an indicator of insect virulence; however the role of light production in this tritrophic interaction is unclear. In vivo experiments with Manduca sexta showed that P. luminescens rapidly entered exponential growth after infection, and light was produced at the equivalent of late exponential or early stationary phase [11]. The results obtained showed that phase I cells of P. luminescens produced high bioluminescence, up to 7 h in NB medium. The distinct red pigmentation is associated with the phase I variant of this bacterium. The shade of pigment is dependent upon the media and pH.
According to the specific growth rate and doubling time, bioluminescence continued to persist after the logarithmic phase (data not shown). After 7 h of growth, the bacterial culture was then fed to infective juveniles of H. bacteriophora suspended in enriched nutrient broth (eNB) medium. In a trial undertaken to check the entomopathogenicity, the nematode-bacterial suspension was injected into larvae of Galleria mellonella. The death of larvae occurred within 24 h, confirming insect pathogenicity of the mixed suspension. H. bacteriophora has been shown to be an environmentally safe bio-insecticide effective against various crop pests such as the Colorado potato beetle and the sweet potato whitefly [12]. Thus, the study of growth of P. luminescens is an important consideration when mass producing H. bacteriophora for agricultural applications.
Materials and Methods
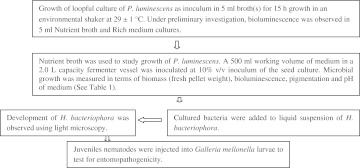
For the mass production of P. luminescens in a bioreactor, the organism was isolated and initially screened for bioluminescence of high luminosity and the appropriate medium was selected for culturing it in larger volume(s). The microorganism’s characteristic of entomopathogenicity for G. mellonella in a symbiotic state with H. bacteriophora is demonstrated. An experimental plan was followed (Scheme 1), for growth and production of phase I cells of P. luminescens for feeding and rearing of H. bacteriophora. Bioluminosity and pigmentation were also used to confirm the presence of phase I cells by utilizing standard methods as described below. The other related growth characteristics of P. luminescens, that may be useful for culturing P. luminescens for rearing of H. bacterioophora, such as pH have been studied.
Scheme 1.
General flow of research protocols
Culture Isolation, Confirmation and Growth Conditions
The gram-negative bacteria P. luminescens was isolated from a light-emitting G. mellonella cadaver infected by H. bacteriophora nematodes that were purchased from ARBICO Organics (Tucson, AZ USA). G. mellonella was obtained from Carolina Biologicals (Burlington, NC USA).
The larvae of G. mellonella were inoculated with H. bacteriophora and upon death (in 24 to 48 h) red pigmented larvae were subjected to luminosity measurements. The cadavers that exhibited high luminosity were then dissected and the infected hemolymph was used to inoculate NA, NBTA and MAC agar plates. After 48 h incubation at 27°C the isolated red colonies on NA were suspended in sterile distilled water and the luminosity was confirmed by measuring in a luminometer. The phase I culture of P. luminescens was confirmed by observing dark red to purple colonies on NBTA medium and reddish colonies on MAC agar medium. However, P. luminescens is a non lactose fermenter and the red colonies (on MAC agar) are misleading as lactose fermentating bacteria appear red on MAC as well. Phase II cells are transparent on MAC therefore assuming the color (light pink) of the medium.
Media Used
Nutrient broth(NB) contained per liter: 3 g beef extract; 5 g enzymatic digest of gelatin.
Rich medium contained per liter: 5.0 g peptone; 3.0 g yeast extract (YE); 3.0 ml glycerol; 1.0 g sodium chloride; 5 mg magnesium sulfate and 10 g trehalose (if added).
MacConkey medium (MAC) contained per liter: 17.0 g pancreatic digest of gelatin; 1.5 g pancreatic digest of casein; 1.5 g peptic digest of animal tissue; 10.0 g lactose; 1.5 g bile salts; 5 g sodium chloride; 13.5 g agar; 0.03 g crystal red; 0.001 g crystal violet.
NBTA medium contained per liter: 8.0 g nutrient agar; 25 mg bromothymol blue; 40 mg 2,3,5-triphenyltetrazolium chloride.
Enriched nutrient broth (eNB) contained per liter: 3 g beef extract; 5 g enzymatic digest of gelatin. Lipid addition was 1.0% w/v, 50:50 mixture of canola and olive oils.
Cell Biomass
Cell biomass was determined by measuring the absorbance of cultures at 600 nm using a Spectronic 20D+, Milton Roy Co. spectrophotometer. The cell weight was measured after centrifuging 2 vol of culture in 1.5 ml Eppendorf tubes at 10,000 rpm for 8 min. The pellet mass was recorded as cell biomass (g) and this fraction was reported as gram percent volume. Since the balance was an analytical balance and measured up to 100 mg with accuracy, we could obtain repetitive results of biomass (fresh pellet weight) of the culture.
Phase Variation and Identification of Phase Variants
NBTA and MAC agar media was used to confirm phase variation [13]. Phase I cells appear as brick/dark red colonies on NBTA and red colonies on MAC agar, whereas phase II cells appear yellow on NBTA and transparent on MAC [13].
Bioluminescence of P. luminescens
Culture samples were prepared by suspending two loop full of identified phase I cells into 1.5 ml distilled water. Bioluminescence was measured using a Turner Biosystems Modulus™ luminometer single-tube reader. Broth cultures were measured for bioluminescence. Luminosity of the retained supernatants was measured after centrifugation at 8,000 rpm for 10 min.
Growth Parameters
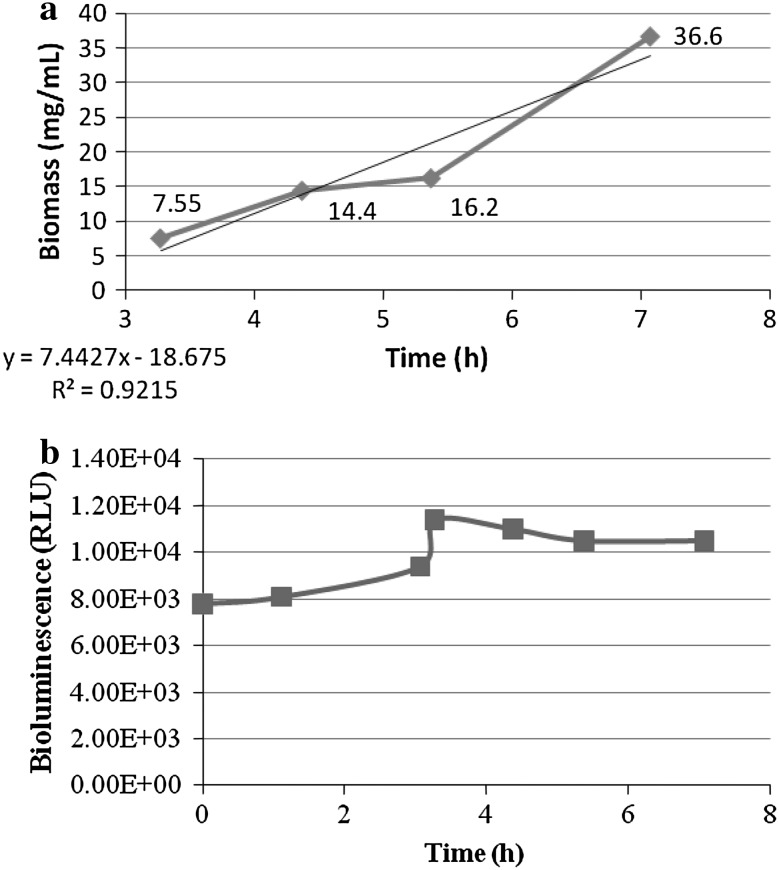
The biomass from the actively growing culture in NB medium was sampled (5 ml) periodically and measured up to 7.07 h. Bioluminescence was also measured in these samples. The specific growth rate (μ) and generation time (g) of the organism were computed from the slope of exponential growth, assuming there are no limiting factors, by the formulae [14] as under:
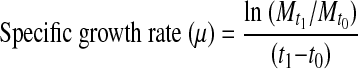 |
where 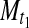 and
and 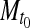 = cell mass (mg/ml) sampled at times t1 and t0, respectively (Fig. 8a).
= cell mass (mg/ml) sampled at times t1 and t0, respectively (Fig. 8a).
Fig. 8.
a Growth biomass (mg/ml) of P. luminescens during its exponential growth phase between 3.27 h and 7.07 h. b Bioluminescence during the exponential phase
Inoculum Preparation
The 10% starter volumes of luminescent cultures (>1.0 × 104 RLU), were inoculated into medium to obtain a high initial high bioluminescence in culture medium with pigmentation (Fig. 1).
Fig. 1.

Aninoculum starter culture of P. luminescens, as seen in NB medium with added lipids
Batch Growth in a Bioreactor
Batch runs in eNB containing 2% lipids and 0.2% glucose were conducted in a 2 l Sartorius-stedim A + fermentor until bioluminescence readings started to diminish or were no longer detectable. During the batch run, cell mass, bioluminescence, and pigmentation were measured and recorded. The process details are shown in Table 1. Batch growth was followed with all controls while sterility was maintained in the vessel as recommended [15].
Table 1.
Conditions of batch growth of the bioluminescent bacteria P. luminescens, in eNB medium for mass production
| No. | Batch | Details (inoculum and vessel) | Medium conditions |
|---|---|---|---|
| I. | Process scale: batch run 500 ml medium in bioreactor (Sartorius A + of 2 l capacity). | 10% Inoculum (15 h old) contained bioluminescence >1.0 × 104 RLU. | 500 ml NB (0.8%) (with 2% lipids and added glucose 0.2%. |
The run was conducted at a temperature of 29 ± 1°C with agitation of 200 rpm. Air flow was maintained at 0.6 vvm
Entomopathogenicity Test
To check for insect pathogenicity, larval instars of G. mellonella were injected with approximately 5 μl of juvenile nematodes with the bacterial symbiont [16]. The injected larvae were examined for mortality, pigmentation and bioluminescence up to 48 h post-injection. After 5 days, P. luminescens was recovered from dead larvae confirming bacterial infection. Larvaecidal activity of P. luminescens was indicated by the luminosity and pigmentation of dead larvae. The intense luminosity exceeded the measurable range of the luminometer (>1.0 × 107 RLUs).
Results and Discussion
Isolation and Confirmation of Phase I Cells
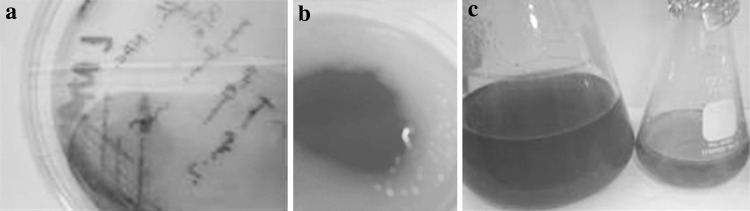
Initially the phase I cells for mass culture were confirmed on NBTA (Fig. 2) and MAC agar plates before being used as starter inoculums. However, confirmatory testing on NBTA was always reliable as compared to on MAC agar medium. The confirmed primary forms of P. luminescens were used for culture inoculation and nematode feeding. In the eNB, P. luminescens showed strong, visually detectable luminescence. Luminosity persisted up to 8 days at room temperature (Fig. 3a). Thus, the eNB medium could be an alternative media for phase I cells (Fig. 3b).
Fig. 2.

Pluminescens on NBTA medium after 48 h showing phase I colonies
Fig. 3.
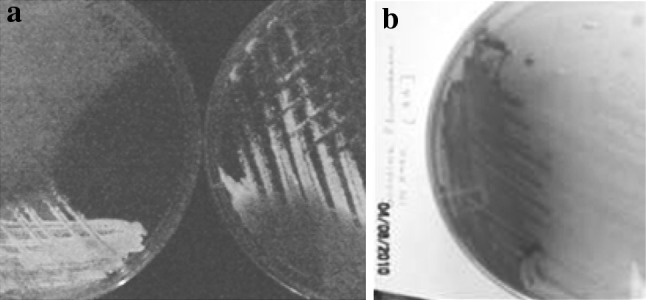
Phase I characteristics of P. luminescensa luminosity after 8 days, b pigment production on rich medium
Enriched NB Medium as a Growth Medium
To mass produce entomopathogenic nematode (EPNs), a batch of primary forms of the symbiotic bacteria P. luminescens are required. Their secretions may act as food or developmental signals that triggers or modulates the nematode life cycle, inducing the development of the reproductive adult stages [5]. The eNB is therefore used to mass produce Heterorhabditidae nematodes, by liquid suspension technology for large-scale production [21].
The typical characteristics of the primary forms (phase I) of P. luminescens include bioluminescence, pigmentation and antimicrobials. Also, phase I cells produce and secrete numerous lipases, proteases, and toxins that enables the bioconversion of the insect host into nutrition for both symbiotic partners [1]. In this study, we formulated a scale up medium (eNB) used for mass culturing P. luminescens under controlled conditions. The eNB did not have any visual effects on bioluminescence and pigmentation of the phase I cells. Therefore, the enriched NB (Fig. 4) was consistently used for starter cultures. Cultures that exhibited strong characteristics of phase I cells were stored frozen at −20°C in 15% glycerol for preservation. Phase II cells were never used for batch experiments. Visual comparisons of phase I cells on both solid and liquid media showed that the pigment coloration deepens due to increasing cell densities (Fig. 4b, c).
Fig. 4.
Pigmentation of P. luminescens in response to culturing conditions: a after 15 days under ambient conditions on NA (22°C), b on NA after 20 h of growth (27°C), c Uninoculated control (right) NB and inoculated NB (left)
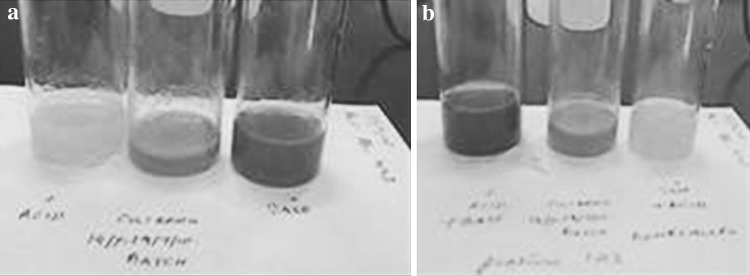
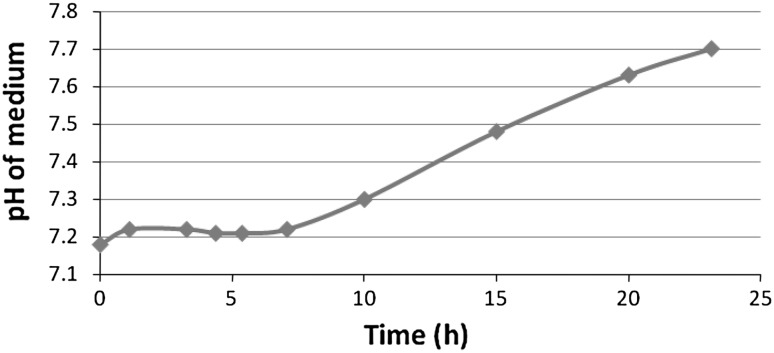
In the batch culture of P. luminescens, an increase in pigmentation can also be correlated with the increase of culture pH. At pH values less than 7.0 the medium would shift to a yellow color. Pigmentation that developed in primary forms in NB and eNB medium was observed to show that the pH of the culture increased, turning alkaline. A mere yellow medium in the lag phase of growth period may be indicative of the pH of the medium and not of the pigment produced. The color of secreted pigment was reversible in response to increasing pH (Fig. 5) but was nowhere an indication on how much pigment was produced by the cells during growth.
Fig. 5.
The reversibility of the pigmentation by the addition of: a 0.1 M HCl (left) and 0.1 M NaOH (right) and b reversed where 0.1 M NaOH (left) and 0.1 M HCl (right) added to the same
Also, cultures that grew in NB and sub-cultured into Rich medium produced a high luminescence (2 × 105 RLUs) which persisted up to 1 week. Rich medium with peptone, YE, glycerol and salt remained at 104 RLUs after 7–8 days (Table 2).
Table 2.
Batch trials with different media NB/Rich to check bioluminescence and pigmentation (Fig. 7)
| Days after inoculation | NB with salt, peptone and glycerol | Rich medium with peptone, YE, glycerol and salt |
|---|---|---|
| (I) | (II) | |
| 3 | 4.2 × 103 | 2.0 × 104 |
| 7 | 2.1 × 105 | 4.4 × 104 |
| 8 | 2.2 × 105 | 8.8 × 104 |
| Pigmentation | Red | Red |
The red pigmentation was seen in both media (NB and eNB media), as result of production by phase I cells. Based upon high luminosity and pigmentation, the eNB medium was thus used for culturing the phase I cells of P. luminescens (Fig. 6).
Fig. 6.

Pigmentation in P. luminescens in NB only, NB with glucose and NB with lipid only (Left to Right)
In contrast, if the phase I cells were cultivated in Rich medium for 7 days and then inoculated into NB medium, bioluminescence was only 1.2 × 104 RLU after 5 days (Fig. 7). The cell suspensions also displayed viscous, butyrous growth which may have been indicative of extracellular polysaccharide production. It was reported that phase I cells of P. luminescens (formerly Xenorhabdus luminescens) produced capsular material [17]. Hence, we looked for pigmentation and high luminescence within the exponential growth period (Fig. 8), to determine bacterial harvesting times based upon cell mass and feeding times of H. bacteriophora.
Fig. 7.

NB (left) and eNB media (right)
Growth Characteristics of P. luminescens in Enriched NB Medium
Growth of P. luminescens in NB medium showed high bioluminescence during the exponential growth period, but did not decline sharply thereafter. The culture yielded 37 mg/ml of bioluminescent (>1 × 106 RLUs) cells, that were obtained after 7 h growth in 500 ml working volume in the bioreactor (Fig. 8). Under experimental conditions, doubling occurred in 2.1 h. A study on fed batch processing reported 0.39 gl−1 h−1 of biomass between 16 and 18 h after inoculation [18]. Since the growth period after 7 h to 24 h could not be monitored in this study for fresh biomass, we have not expanded the growth curve for that period. We preferred to consider the earliest growth phase that could develop bioluminescence to be used to induce development of H. bacterophora for mass production. Since luminosity was observed to increase within the first 7 h of growth, we decided to harvest the cells at the 7th hour after media inoculation for nematode feedings.
In the batch culture, the cell biomass (mg/ml) sampled was M0 = 7.55 mg/ml and M1 = 16.2 mg ml−1 at times t0 (3.27 h) and t1 (5.37 h), respectively. The relationship between biomass and time approximated a straight line that indicated the exponential phase [14]. The specific growth rate (μ) is estimated from the slope of exponential growth [19]. The specific growth rate hence was determined to be 0.36 h−1 as observed between 3.27 and 5.37 h (Fig. 8a). The mean generation time of the culture was 2.1 h.
Observed deviations from the expected linear growth rate might be explained by changes in culture pH which was monitored during the exponential phase [20]. The pH of the medium increased during the growth (Fig. 9). Studies such as this that monitor cell mass, pH and bioluminescence can be useful for determining bacterial feeding times for H. bacteriophora in liquid culturing [4, 5].
Fig. 9.
Changes in pH of NB medium during growth of P. luminescens
Entomopathogenicity of P. luminescens towards G. mellonella
During entomopathogenicity testing of Galleria mellonella larvae injected with the nematode-bacterial suspension, 4 out of 5 infected larvae died within 24 h (Fig. 10). Larvaecidal activity was indicative through expression of luminosity that exceeded the measurable range of the luminometer (>1.0 × 107 RLUs) and red pigmentation of the insect carcasses. The cellular fraction of the injection volume contained 3–4 pigmented, luminescent cells per microliter. This confirms an earlier report suggesting that the LD50 of P. luminescens was 4–5 cells per microliter and proves that the use of the bacteria-nematode complex can be an effective biocontrol mechanism [22]. This insect mortality demonstrates the pathogenicity of the bacterial fed nematode suspension. After 2 days, the insect cadavers were used to isolate P. luminescens. This study demonstrates that using phase I cells in the late exponential or early stationary phase may benefit the mass production of H. bacteriophora.
Fig. 10.
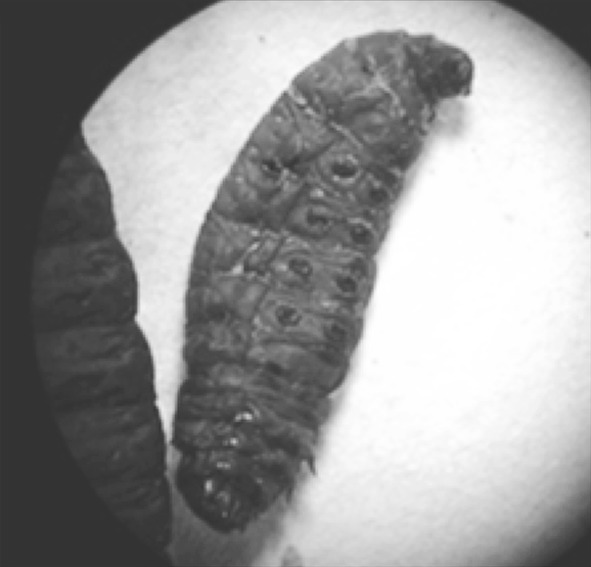
A dead and luminescent G. mellonella larvae after 48 h
In conclusion, this study is essential to address one of the main barriers in mass producing EPNs, batch consistency for quantity and quality. The rearing of beneficial nematodes is challenging because the symbiotic relationship between the nematode and the bacteria makes it difficult to study the biology on the nematode-bacteria-insect triad. Hence, the present study is an effort to investigate how P. luminescens can be used as a food source and a source of signals needed by the nematode for growth and development for their mass production.
Acknowledgment
The authors thank the National Coordinator, NAIP (ICAR, N Delhi) for funding. We also are grateful to the University of North Carolina at Pembroke and the UNCP Biotechnology Research and Training Center for the use of their facilities to conduct this research. The senior author is also thankful to the Director, CIAE, Bhopal.
References
- 1.Boemare NE, Givaudan A, Brehelin M, Laumond C. Symbiosis and pathogenicity of nematode-bacterium complexes. Symbiosis. 1997;22:21–45. [Google Scholar]
- 2.Susan F, Michael A, Nealson KH. Cloning, organization, and expression of the bioluminescence genes of Xenorhabdus luminescens. J Bacteriol. 1990;172(10):5767–5773. doi: 10.1128/jb.172.10.5767-5773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frackman S and Nielson KH (1990) The molecular genetics of Xenorhabdus. In: Gaugler R and Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Boca Raton Press, Florida, pp 285–300
- 4.Johnigk S-A, Ecke F, Poehling M, Ehlers R-U. Liquid culture mass production of biocontrol nematodes, Heterorhabditis bacteriophora (Nematoda: Rhabditida): improved timing of dauer juvenile inoculation. Appl Microbiol Biotechnol. 2004;64:651–658. doi: 10.1007/s00253-003-1519-9. [DOI] [PubMed] [Google Scholar]
- 5.Strauch O, Ehlers R-U. Food signal production of Photorhabdus luminescens inducing the recovery of entomopathogenic nematodes Heterorhabditis spp. in liquid culture. Appl Microbiol Biotechnol. 1998;50:369–374. doi: 10.1007/s002530051306. [DOI] [Google Scholar]
- 6.Ehlers R-U, Lunau S, Krasomil-Osterfeld K, Osterfeld KH. Liquid culture of the entomopathogenic nematode–bacterium complex Heterorhabditis megidis/Photorhabdus luminescens. Biocontrol. 1998;43:77–86. doi: 10.1023/A:1009965922794. [DOI] [Google Scholar]
- 7.Ehlers R-U, Stoessel S, Wyss U. The influence of phase variants of Xenorhabdus spp.and Escherichia coli (Enterobacteriaceae) on the propagation of entomopathogenic nematodes of the genera Steinernema and Heterorhabditis. Rev Nematol. 1990;13:417–424. [Google Scholar]
- 8.Han R, Ehlers R-U. Effect of Photorhabdus luminescens phase variants on the in vivo and in vitro development and reproduction of Heterorhabditis bacteriophora and Steinernema carpocapsae. FEMS Microbiol Ecol. 2001;35:239–247. doi: 10.1111/j.1574-6941.2001.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 9.Inman Floyd III and Holmes Leonard D (2010) Mass production of the beneficial nematode, Heterorhabditis bacteriophora, in submerged culture. http://www.uncp.edu/biotech/research/Nematology/index.htm. Accessed 14 April 2010
- 10.Hu K, Webster JM. Antibiotic production in relation to bacterial growth and nematode development in Photorhabdus- Heterorhabditis infected Galleria mellonella larvae. FEMS Microbiol Lett. 2000;189:219–223. doi: 10.1111/j.1574-6968.2000.tb09234.x. [DOI] [PubMed] [Google Scholar]
- 11.Daborn Phillip J, Nicholas W, Blight Mark A, Ffrench-Constant Richard H. Measuring virulence factor expression by the pathogenic bacterium Photorhabdus luminescens in culture and during insect infection. J Bacteriol. 2001;183(20):5834–5839. doi: 10.1128/JB.183.20.5834-5839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn Michael B, Domek John M, Gelman Dale B, Hu Jing S. The broadly insecticidal Photorhabdus luminescens toxin complex a (Tca): activity against the Colorado potato beetle, Leptinotarsa decemlineata, and sweet potato whitefly, Bemisia tabaci. J Insect Sci. 2005;5:32. doi: 10.1093/jis/5.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boemare NE, Ackhurst RJ. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp (Enterobacteriaceae) J Gen Microbiol. 1988;134:751–761. [Google Scholar]
- 14.Stanier RY, Adelberg EA and Ingraham JL (1985) Microbial growth. In: General microbiology. MacMillan Pub Ltd. Houndmill, Basingstoke, pp 275–279
- 15.Sartorious (2009). Manual of the fermenter-Sartorious A+
- 16.Shlomit Zioni (Cohen Nissan), Shlomit Glazer I & Segal D (1992) Life cycle and reproductive potential of the nematode Heterorhabditis bacteriophora strain HP 88. J Nematology 24(3):352–358 [PMC free article] [PubMed]
- 17.Michel B, Cherqui A, Drif L, Luciani J, Akhurst R, Boemare N. Ultrastructural study of surface components of Xenorhabdus sp. in different cell phases and culture conditions. J Invertebr Pathol. 1993;61(2):188–191. doi: 10.1006/jipa.1993.1033. [DOI] [Google Scholar]
- 18.Jeffke T, Jende D, Matje C, Ehlers RU, Berthe Corti L. Growth of Photorhabdus luminescens in batch and glucose fed-batch culture. Appl Microbiol Biotechnol. 2000;54:326–330. doi: 10.1007/s002530000399. [DOI] [PubMed] [Google Scholar]
- 19.Zwietering MH, Jongenburger I, Rombouts FM, Van’t RK. Modeling of the bacterial growth curve. Appl Environ Microbiol. 1990;56(6):1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contois DE. Kinetics of bacterial growth: relationship between population density and specific growth rate of continuous cultures. J Gen Microbiol. 1959;21:40–50. doi: 10.1099/00221287-21-1-40. [DOI] [PubMed] [Google Scholar]
- 21.Friedman M, Langston SE and Pollitt S (1991) Mass production in liquid culture of insect-killing nematodes. United States Patent, 5,023,183
- 22.Milstead JE. Heterorhabditis bacteriophora as a vector for introducing its associated bacterium into the hemocoel of Galleria mellonella larvae. J Invertebr Pathol. 1979;33:324–327. doi: 10.1016/0022-2011(79)90033-8. [DOI] [Google Scholar]