Abstract
3-Hydroxypropionic acid (3-HP) is a commercially valuable platform compound. Klebsiella pneumoniae has been concerned as an appropriate host for 3-HP production because of its robust capacity to metabolize glycerol. Glycerol conversion to 3-HP in K. pneumoniae comprises two successive reactions: glycerol dehydratase catalyzes glycerol to 3-hydroxypropionaldehyde (3-HPA); aldehyde dehydrogenase catalyzes 3-HPA to 3-HP. Previous studies focusing on inducible expression of aldehyde dehydrogenase have shown defects of high cost of inducer and low catalytic activity due to inclusion body. Here we show a different strategy that a native promoter in the host K. pneumoniae was used to drive the heterologous expression of aldehyde dehydrogenase gene ald4 from Saccharomyces cerevisiae. The 3-HP yield of the recombinant reached a peak of 4.23 g/L at log phase, but it decreased during later period of fermentation. Except the validation of high activity of ald4, particularly, the 3-HP formation was uncovered to be closely coupled with cell division, and the lacking of NAD and ATP at latter fermentation phase became the bottleneck for cell growth and 3-HP accumulation. Furthermore, 3-HP is postulated to be converted to 3-HPA via feedback inhibition or other metabolite via unknown mechanism. Since glycerol dissimilation is a common mechanism in a variety of bacteria, the expression strategy using native promoter and implications may provide significant insight into the metabolic engineering for 3-HP production.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-012-0280-0) contains supplementary material, which is available to authorized users.
Keywords: 3-Hydroxypropionic acid, Klebsiellapneumoniae, Saccharomyces cerevisiae, Aldehyde dehydrogenase, Native promoter, Glycerol, Heterologous expression
Introduction
Diminishing oil reserves and increasing concerns with the deteriorated environment have fueled bio-refinery as a replacement for conventional petro-chemical route. 3-Hydroxypropionic acid (3-HP) holds the third place in the list of the US DOE’s top 12 value-added platform compounds among renewable biomass products [20]. 3-HP serves as the versatile precursor of several commercially important chemicals, such as 1,3-propanediol, acrylic acid, acrylamide, acrolein [7, 17], polymer [1], or as a potential nematicide [14]. Glycerol-based 3-HP biosynthesis through microbial metabolic engineering has been centered in recent years due to ample glycerol as a main by-product in the flourishing biodiesel industry [2, 10].
3-HP biosynthesis from glycerol generally undergoes two steps: first, glycerol dehydratase catalyzes glycerol to 3-hydroxypropionaldehyde (3-HPA) [5, 6]; second, aldehyde dehydrogenase catalyzes 3-HPA to 3-HP. Klebsiella pneumoniae has been recognized as a promising host for its powerful ability to utilize glycerol [21]. In K. pneumoniae and many other bacteria, glycerol dissimilation is the central carbon metabolism under anaerobic or microaerobic conditions when glycerol is sole carbon and energy sources. Four enzymes, termed dha regulon, govern glycerol dissimilation, including glycerol reduction by glycerol dehydratase and 1,3-propanediol oxidoreductase, along with glycerol oxidation by glycerol dehydrogenase and dihydroxyacetone (DHA) phosphate kinase [6, 11]. By coupling with glycerol oxidation, glycerol reduction confers NAD+ regeneration and cell growth [4].
Previous studies focused on inducible expression of key enzymes in prokaryotic host such as E. coli or K. pneumoniae. However, it posed problems of high cost of inducer (e.g., isopropyl β-d-thiogalactoside, IPTG) and low catalytic activity due to inclusion body. Hence, there is a demand for development of inexpensive and efficient expression approaches tailor-made for practical application. In this present work, a native promoter of glycerol dehydratase gene from K. pneumoniae was recruited to drive the heterologous expression of aldehyde dehydrogenase gene ald4 from Saccharomyces cerevisiae in K. pneumoniae. Based on detailed analysis on gene expression, glycerol consumption, cell growth, 3-HP accumulation, and by-products formation, this study was to (i) establish efficient expression method, and (ii) determine the bottleneck in the conversion of 3-HPA to 3-HP.
Materials and Methods
Plasmids, Strains and Cultivation
Plasmid pET-28a was purchased from Novagen. K. pneumoniae DSM 2026 was strain from DSMZ GmbH, Germany. S. cerevisiae (baker’s yeast) were purchased from ATCC. S. cerevisiae was cultivated in yeast extract peptone dextrose medium (g L−1): glucose, 20; yeast extract, 10; peptone, 20; The medium (per liter) of K. pneumoniae comprised the following ingredients: K2HPO4·3H2O, 3.4 g; KH2PO4, 1.3 g; (NH4)2SO4, 4 g; MgSO4·7H2O, 0.5 g; CaCO3, 0.1 g; yeast extract, 3 g; glycerol, 20 g; and 1.25 mL of trace element solution. The trace element solution contained (per liter): FeSO4, 32 g; ZnCl2·6H2O, 2.72 g; MnCl2·4H2O, 0.68 g; CoCl2·6H2O, 1.88 g; H3BO3, 0.24 g; Na2MoO4, 0.02 g; CuCl2·2H2O, 1.88 g; and 40 mL of concentrated HCl. The recombinant was microaerobically cultivated in 50 mL Erlenmeyer’s flask containing 25 mL medium with 50 μg/mL kanamycin at 37 °C and 120 rpm continuous shaking. The microaerobic condition was plugged by foam stopper.
Chemicals
3-HP was purchased from Tokyo Chemical Industry (TCI) Co. Ltd. (Tokyo, Japan). Ex Taq DNA polymerase, restriction and DNA modifying enzymes were purchased from TaKaRa (Dalian, China). DNA synthesis and sequencing were performed by Beijing Sunbiotech Co. Ltd., China.
Construction of the Recombinant
The gene ald4 were cloned by PCR from the genomic DNA of S. cerevisiae (baker’s yeast). Below demonstrates the primer sequences for amplification of ald4 (1,560 bp, GenBank 854556): 5′-CCGGAATTCATGTTCAGTAGATCTACGCTCTG-3′ (forward primer); 5′-CTCAAGCTTTTACTCGTCCAATTTGGCAC-3′ (reverse primer).
Restriction sites of EcoRI and HinDIII were flanked ald4 gene. Below are the PCR parameters: 94 °C, 3 min; 94 °C, 1 min; 55 °C, 40 s; 72 °C, 1.5 min; 30 cycles; 72 °C, 8 min, 16 °C, holding.
The T7 promoter sequence in plasmid pET-28a backbone was replaced by the native promoter of dhaB in dha regulon of K. pneumoniae DSM 2026. This promoter named Pk is the nucleotide sequence from the immediate downstream of termination codon of prior adjacent gene to the initiation codon of dhaB1, the first subunit of glycerol dehydratase gene (GenBank U30903). Other molecular manipulations were subjected to standard protocol [13]. The recombinant plasmid was transformed into K. pneumoniae, and the positive recombinant was screened by LB kanamycin plate and further confirmed by sequencing.
SDS-PAGE Analysis
The ald4 expression in recombinant K. pneumoniae was analyzed by 12 % (v/w) polyacrylamide gel electrophoresis (PAGE) with cell-free extract under denaturing condition. Mini-Protein III Electrophoresis System (Bio-Rad, USA) was used to perform the operation. Coomassie Brilliant Blue R-250 (0.2 %, w/v) was applied to stain protein on the gel and the concentration of protein was measured by Bradford method with bovine serum albumin (BSA) as standard protein.
By recruitment of native promoter Pk, the protein of ald4 was induced by DHA, the metabolite in glycerol oxidative pathway. The generation of enzyme Ald4 needs not any additional inducer, it can be continuously expressed by recombinant K. pneumoniae (kp-pET-28a-ald4) during fermentation.
Metabolite Assay
Glycerol concentration in fermentation broth was determined by sodium periodate oxidation [3, 19]. 3-HP, lactic acid and acetic acid present in fermentation broth were determined by high-performance liquid chromatography (HPLC) (Shimazu SCL-10A, Japan) equipped by ultraviolet detector and a Diamonsil C18 column (5 μm, 250 mm × 4.6 mm). The mobile phase was methanol: water = 5:95 (v/v) with adding H3PO4 until final concentration of 0.05 % (v/v). The flow rate was 0.8 mL min−1 and the column temperature was 30 °C.
Results
Characterization of ald4 Recombinant
For conversion of 3-HPA to 3-HP, aldehyde dehydrogenase gene is required to incorporate into K. pneumoniae. Because of no intron in the sequence of ald4, the genomic DNA was used as template. The ald4 gene was cloned by PCR, ligated to expression vector pET-28a. The resulting recombinant vector was transformed into K. pneumoniae and cultivated on LB kanamycin plate. The positive clones were selected and grown at 37 °C till final cell density ranging from 1.0 to 1.5. The extracted plasmid was digested by restriction enzymes and run on 1 % agarose gel. The size of electrophoresis band was revealed to be in accordance with that reported in GenBank (Fig. 1). And sequencing report also confirmed this gene.
Fig. 1.
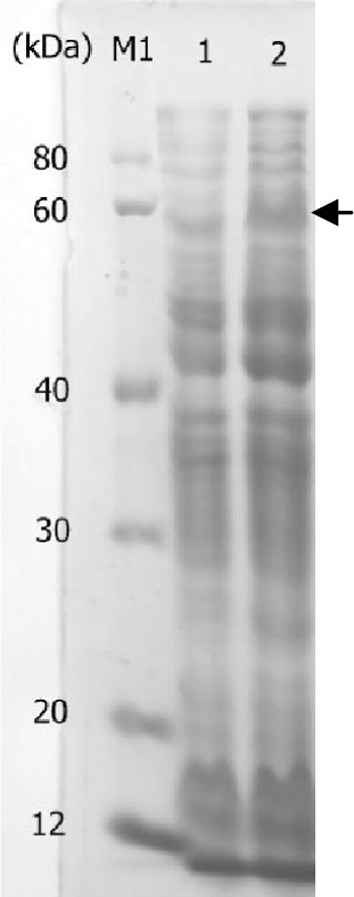
SDS-PAGE analysis of aldehyde dehydrogenase ald4 from Saccharomyces cerevisiae. M1 protein marker I; lane 1 k.p(pET-28a, native PK promoter) at 4 h; lane 2 k.p(pET-28a-ald4, native PK promoter) at 4 h; arrow head indicates the expressed Ald4
To investigate the expression of ald4, we performed flask batch fermentation followed by SDS-PAGE analysis of cell-free extract. A protein of approximately 57 kDa was observed, which was not present in the control (wild type K. pneumoniae harboring blank plasmid pET28a) (Fig. 1). Moreover, this ald4 protein was present during the entire fermentation period, which implying the continuous conversion of 3-HPA to 3-HP. Interestingly, a protein of approximately 55 kDa was present in the control under identical culture conditions, which is estimated to be the native aldehyde dehydrogenase. In fact, as deposited in GenBank, there exist total 20 copies of identified or putative aldehyde dehydrogenase genes in sequenced K. pneumoniae subsp. pneumoniae MGH 78578. We thereby postulated the expression of native aldehyde dehydrogenase genes and accordingly the 3-HP biosynthesis in the control. One such evidence is PuuC, an inherent gene in K. pneumoniae enabling 3-HP formation [2]. Collectively, the above results have verified the expression of aldehyde dehydrogenase which is the premise for conversion of 3-HPA to 3-HP.
Production of 3-HP by Recombinant K. pneumoniae
Growth Rate and Glycerol Consumption
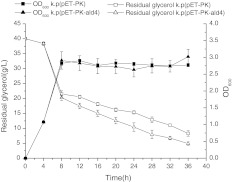
Glycerol can be the sole carbon and energy sources for maintaining central metabolism based upon the evolved dha regulon in K. pneumoniae [5, 6]. To explore the influence of glycerol on cell growth, we investigated the growth rate and glycerol consumption in a total 36 h of batch fermentation. Both the recombinant and the control showed similar metabolism characteristics (Fig. 2). The lag phase was ranging from 0 to 4 h, and the log phase was from 4 to 8 h. Since 8 h, the cell density (OD600) stopped to increase, meaning that the recombinant entered stationary phase and cell division ceased. Rapid glycerol consumption occurred during 0–8 h. Since 8 h, there existed a steep decrease of glycerol consumption. As a consequence, the recombinant presented a low cell concentration (approximately OD600, 2.5) as well as certain glycerol residue. We thereby deduced the presence of limiting factors in late period of fermentation. The major cause may be the lacking of NAD+ and ATP.
Fig. 2.
Growth rate and glycerol consumption. k.p(pET-PK): Klebsiella pneumoniae harboring blank plasmid pET-28a, native PK promoter; k.p(pET-PK-ald4): K. pneumoniae harboring plasmid pET-28a, native PK promoter, ald4 gene
3-HP Formation
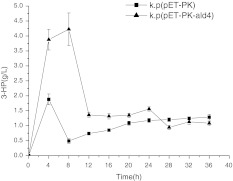
To investigate the influence of native promoter Pk on 3-HP biosynthesis, we assayed 3-HP yield present in flask batch fermentation process under micro-aerobic condition. Compared with the control, Kp-pET28a-ald4 presented more 3-HP yield at 8 h. During log phase (4–8 h), Kp-pET28a-alh4 rapidly accumulated 3-HP (Fig. 3). Hence, a synchronized 3-HP formation and glycerol consumption occurred during log phase, revealing the close coupling between 3-HP formation and cell division. More interestingly, the recombinant demonstrated a steep decrease of 3-HP formation after log phase. The underlying mechanism is unclear. The plausible reasons may be the exhaustion of NAD, as well as the feedback inhibition of dha regulon, because the conversion of 3-HPA to 3-HP is reversible. All these observations may be explained by the evolved metabolic rigidity of dha regulon [15].
Fig. 3.
Batch fermentation for 3-HP production. Filled square k.p(pET-PK): Klebsiella pneumoniae harboring blank plasmid pET-28a, native PK promoter; filled triangle k.p(pET-PK-ald4): K. pneumoniae harboring plasmid pET-28a, native PK promoter, ald4 gene
By-Products
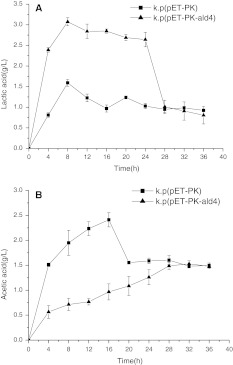
Considering the by-products formed in glycerol oxidative pathway can attenuate the carbon flux to reductive pathway, we investigated the yields of lactic acid and acetic acid, the two major by-products responsible for NAD and ATP regeneration. As shown in Fig. 4, during the first 28 h, the recombinant yielded more lactic acid but less acetic acid than the control strain. This result may be ascribed to the different distances, that from the substrate glycerol to lactic acid or acetic acid. Another common phenomenon of two strains is, during 4–8 h, they showed apparent increase of lactic acid and acetic acid. Easy to understand, biosynthesis of these two by-products is responsible for NAD and ATP regeneration, and the increased NAD and ATP were applied for cell growth and 3-HP formation (Figs. 3, 4).
Fig. 4.
A Time course of lactic acid formation in batch fermentation. B Time course of acetic acid formation in batch fermentation. Filled square k.p(pET-PK): Klebsiella pneumoniae harboring blank plasmid pET-28a, native PK promoter; filled triangle k.p(pET-PK-ald4): K. pneumoniae harboring plasmid pET-28a, native PK promoter, ald4 gene
In principle, bacterial growth is a crucial parameter for gene expression and metabolite formation [9]. Biosynthesis of desired cellular metabolite from central pathway is a feasible strategy because of close coupling with cell growth. For example, starting from pyruvate or acetyl-CoA, the noticeable hub of the cell network, many economically important target metabolites can be readily accumulated, such as ethanol and lactic acid through high density fermentation. By contrast, butanol is hard to accumulate. One such reason may be the long distance away from cellular central metabolism. In this present study, 3-HP formation was closely coupled with cell division. Therefore, measures associated with cell growth could be beneficial to 3-HP formation. Once biomass was enhanced upon further study, e.g. optimization of cultivation condition, attenuation of toxic metabolite, or reconstruction of regulatory circuits, 3-HP could be significantly accumulated. Admittedly, it is a daunting task to accumulate biomass due to myriads of limiting factors and unknown mechanisms such as cell division, cell-to-cell communication, as well as metabolite transportation [18]. Upon better understanding of the underlying mechanism, along with technical advances in metabolic engineering, the barriers could be overcome and 3-HP could be highly accumulated.
Metabolic Analysis
To evaluate the capacity of the recombinant to generate 3-HP, we calculated glycerol conversion ratio and 3-HP productivity. As reported in Table 1, the control strain yielded 3-HP of 1.88 g/L. By contrast, the recombinant k.p(pET-PK-ald4) presented more 3-HP, higher glycerol conversion ratio and higher productivity. During the first 4 h of batch fermentation, it produced 3-HP with productivity (g L−1 h−1) of 0.97. Unfortunately, during 4–8 h, the productivity decreased suddenly. This striking phenomenon implied the bottleneck for 3-HP accumulation.
Table 1.
Batch fermentation parameters for 3-HP production
| Strains | 3-HP (g L−1) | Glycerol conversion ratio (mol mol−1 glycerol) | Productivity (g L−1 h−1) | Lactic acid (g L−1) | Acetic acid (g L−1) |
|---|---|---|---|---|---|
| k.p(pET-PK) | 1.88 ± 0.18 | 1.28 ± 0.43 | 0.47 ± 0.05 | 1.59 ± 0.08 | 2.41 ± 0.14 |
| k.p(pET-PK-ald4)/4 h | 3.88 ± 0.35 | 2.59 ± 0.72 | 0.97 ± 0.09 | ||
| k.p(pET-PK-ald4)/8 h | 4.23 ± 0.54 | 0.22 ± 0.02 | 0.53 ± 0.07 | 3.07 ± 0.09 | 1.53 ± 0.06 |
k.p(pET-PK): Klebsiella pneumoniae harboring blank plasmid pET-28a, native PK promoter; k.p(pET-PK-ald4): K. pneumoniae harboring plasmid pET-28a, native PK promoter, ald4 gene
Despite pET-28a-ald4 presented 3-HP of 4.23 g/L in flask batch fermentation, it is far from the requirement of industrial production. Guided by metabolic analysis, strategies for further enhancement of 3-HP are suggested below. First, because the over-expression of ald4 consumed NAD, the resulting shortage could hinder the further formation of 3-HP. Since lactic acid and acetic acid in glycerol oxidative pathway rub carbon flux towards reductive pathway [4], we propose their biosynthesis genes being replaced by NAD regeneration gene via homologous recombination manipulation. Second, since the remarkable tolerance of K. pneumoniae to high concentration glycerol, ample supply of glycerol during fermentation is likely able to enhance 3-HP yield. Simultaneously, high concentration of glycerol could circumvent feedback inhibition. Hence, sufficient provision of glycerol is necessary to ensure 3-HP biosynthesis. Apart from strategies mentioned above, a more efficient strategy to alleviate feedback inhibition may be the employment of isoenzyme which can convert 3-HPA to 3-HP, but cannot be inhibited by 3-HP. Hence, a parallel catalysis pathway could be engineered in K. pneumoniae. With the accumulation of 3-HP during fermentation, this parallel pathway could be timely initiated so as to maintain continuous catalysis [8].
Conclusion
In this present study, by recruitment of native promoter of dhaB gene in K. pneumoniae, the aldehyde dehydrogenase gene ald4 from S. cerevisiae was heterologously expressed. Two major conclusions could be drawn from this study. (i) The ald4 gene from S. cerevisiae is efficient for converting 3-HPA to 3-HP in K. pneumoniae; (ii) the recombinant rapidly generated 3-HP at log phase, revealing the close coupling of 3-HP formation with cell division; (iii) during late phase of fermentation, 3-HP may be converted into 3-HPA or other unknown metabolite. Compared with previous inducible expression, this protocol with native promoter possesses advantages below. (i) The close coupling between 3-HP formation and cell growth has suggested that the major goal of forthcoming research is biomass enhancement. (ii) The dual roles of glycerol as both substrate and inducer (instead of IPTG) clearly indicate the low cost in practical application. (iii) Compared with promoters in commercialized vectors, this native promoter is postulated to be more compatible with RNA polymerase, and therefore leads to higher catalytic activity. In short, we report here a different expression strategy for engineering 3-HP-producing strain. Considering the ubiquity of dha regulon in bacteria [12, 16], we propose this strategy to be general and the implications to be insightful.
Electronic supplementary material
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 20876009).
References
- 1.Andreessen B, Lange AB, Robenek H, Steinbüchel A. Conversion of glycerol to poly(3-hydroxypropionate) in recombinant Escherichia coli. Appl Environ Microbiol. 2010;76(2):622–626. doi: 10.1128/AEM.02097-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashok S, Raj S, Rathnasingh C, Park S. Development of recombinant Klebsiella pneumonia ΔdhaT strain for the co-production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol. Appl Microbiol Biotechnol. 2011;90:1253–1265. doi: 10.1007/s00253-011-3148-z. [DOI] [PubMed] [Google Scholar]
- 3.Bennett HC, Boley EL, Clark WC, Parsons LB, Segur JB, Troy A, Andrews JTR, Pohle WD. Report of the glycerin analysis committee. J Am Oil Chem Soc. 1950;27:412–413. doi: 10.1007/BF02637753. [DOI] [Google Scholar]
- 4.Celinska E. Debottlenecking the 1,3-propanediol pathway by metabolic engineering. Biotechnol Adv. 2010;28:519–530. doi: 10.1016/j.biotechadv.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Forage RG, Foster MA. Glycerol fermentation in Klebsiella pneumoniae: functions of the coenzyme B12-dependent glycerol and diol dehydratases. J Bacteriol. 1982;149:413–419. doi: 10.1128/jb.149.2.413-419.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forage RG, Lin EC. DHA system mediating aerobic and anaerobic dissimilation of glycerol in Klebsiella pneumoniae NCIB 418. J Bacteriol. 1982;151:591–599. doi: 10.1128/jb.151.2.591-599.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishii M, Chuakrut S, Arai H, Igarashi Y. Occurrence, biochemistry and possible biotechnological application of the 3-hydroxypropionate cycle. Appl Microbiol Biotechnol. 2004;64(5):605–610. doi: 10.1007/s00253-003-1540-z. [DOI] [PubMed] [Google Scholar]
- 8.Kleeb AC, Edalat MH, Gamper M, Haugstetter J, Giger L, Neuenschwander M, Kast P, Hilvert D. Metabolic engineering of a genetic selection system with tunable stringency. Proc Natl Acad Sci USA. 2007;104(35):13907–13912. doi: 10.1073/pnas.0705379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klumpp S, Zhang ZG, Hwa T. Growth rate-dependent global effects on gene expression in bacteria. Cell. 2009;139(7):1366–1375. doi: 10.1016/j.cell.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo L, Seo JW, Baek JO, Oh BR, Heo SY, Hong WK, Kim DH, Kim C. Identification and characterization of the propanediol utilization protein PduP of Lactobacillus reuteri for 3-hydroxypropionic acid production from glycerol. Appl Microbiol Biotechnol. 2011;89:697–703. doi: 10.1007/s00253-010-2887-6. [DOI] [PubMed] [Google Scholar]
- 11.Raj SM, Rathnasingh C, Jo JE, Park S. Production of 3-hydroxypropionic acid from glycerol by a novel recombinant Escherichia coli BL21 strain. Process Biochem. 2008;43:1440–1446. doi: 10.1016/j.procbio.2008.04.027. [DOI] [Google Scholar]
- 12.Raynaud C, Sarcabal P, Meynial-Salles I, Croux C, Soucaille P. Molecular characterization of the 1,3-propanediol (1,3-PD) operon of Clostridium butyricum. Proc Natl Acad Sci USA. 2003;100(9):5010–5015. doi: 10.1073/pnas.0734105100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sambrook J, Russel DW. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 14.Schwarz M, Kopcke B, Weber RW, Sterner O, Anke H. 3-Hydroxypropionic acid as a nematicidal principle in endophytic fungi. Phytochemistry. 2004;65(15):2239–2245. doi: 10.1016/j.phytochem.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 15.Stephanopoulos G, Vallino JJ. Network rigidity and metabolic engineering in metabolite overproduction. Science. 1991;252:1675–1681. doi: 10.1126/science.1904627. [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Heuvel J, Soucaille P, Qu Y, Zeng AP. Comparative genomic analysis of dha regulon and related genes for anaerobic glycerol metabolism in bacteria. Biotechnol Prog. 2003;19(2):263–272. doi: 10.1021/bp025739m. [DOI] [PubMed] [Google Scholar]
- 17.Suthers PF, Cameron DC (2001) Production of 3-hydroxypropionic acid in recombinant organisms. PCT Patent WO 2001016346A1
- 18.Maris AJ, Konings WN, Dijken JP, Pronk JT. Microbial export of lactic and 3-hydroxypropanoic acid: implications for industrial fermentation processes. Metab Eng. 2004;6(4):245–255. doi: 10.1016/j.ymben.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Wang JF, Xiu ZL, Fang SD. Determination of glycerin concentration during the fermentation of glycerin to 1,3-propanediol. Ind Microbiol. 2001;31:33–35. [Google Scholar]
- 20.Werpy T, Petersen G. Top value added chemicals from biomass. Washington, DC: U.S. DOE; 2004. [Google Scholar]
- 21.Zhu JG, Ji XJ, Huang H, Du J, Li S, Ding YY. Production of 3-hydroxypropionic acid by recombinant Klebsiella pneumoniae based on aeration and ORP controlled strategy. Korean J Chem Eng. 2009;26:1679–1685. doi: 10.1007/s11814-009-0240-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.