Abstract
Mahua (Madhuca longfolia) extract and black grapes (Vitis vinifera) must samples 100:0 (grape:mahua), 95:5 (grape:mahua) and 90:10 (grape:mahua) were analyzed for quality characteristics. Samples were kept for fermentation and monitored for quality analysis for 15 days. 90:10 (grape:mahua) sample was found to be best on the basis of ranking test and subjected to clarification using bentonite and gelatine. Sample treated with a combination of 0.02 g/100 g bentonite and 0.04 g/100 g gelatin showed better results for anthocyanin (52.2 mg/100 g) and tannin (0.038%w/v). After ageing of 3 months TSS was found highest (2.7ºBx) in the non-clarified sample and lowest (2.1ºBx) in sample treated with 0.06 g/100 g bentonite and 0.03 g/100 g gelatine. pH was highest (3.29) in sample treated with 0.06 g/100 g bentonite and 0.03 g/100 g gelatine and lowest (3.16) in sample with 0.04 g/100 g bentonite and 0.03 g/100 g gelatine. Anthocyanin content was highest (56.1 mg/100 g) in control sample and lowest (29.22 mg/100 g) in sample treated with 0.04 g/100 g bentonite and 0.02 g/100 g gelatin. Tannin content was found to be highest (0.079%w/v) in control sample and lowest (0.03%w/v) in sample treated with 0.02 g/100 g bentonite and 0.04 g/100 g gelatine.
Keywords: Wine, Mahua, Gelatine, Quality, Ageing
Introduction
In India, out of total grape production, fresh table grapes accounts for 80% followed by raisin (18%), wine and juice (2%) [1]. Wine is the alcoholic beverage, made of fermented grape juice. Various varieties of grapes and strains of yeasts are used depending on the types of wine produced [2]. Alcohol strongly contributes to palate fullness in wine. Perceived density of a de-alcoholised wine generally increased with increasing alcohol over a 14%v/v range, while its perceived viscosity was highest at 10% ethanol [3]. Later work using model wines showed a positive monotonic effect of alcohol content on both perceived viscosity and density over the same alcohol range [4].
Mahua (Madhuca longfolia), is the succulent, cream-colored corollas are rich source of sugar and contain appreciable amount of calcium, vitamins, some other minerals, cellulose, traces of fat, proteins, and appreciable amount of vitamin C and vitamin B-complex [5]. Mahua is useful in arresting bleeding, smoothing effect of skin, taken in diabetes mellitus with beneficial results. Production of liquor from mahua is cheaper than sugar and easily available in bulk, it could prove to be a good substitute for the fermentation industries [6].
Therefore the present study was undertaken to study the effect of incorporation of mahua extract on the overall quality of red wine, effect of addition of fining agents (bentonite and gelatin) and to study the effect of ageing on the quality parameters of red wine.
Materials and Methods
Black grapes (Vitis vinifera L.) and compressed yeast (Saccharomyces cerevisiae var.) were purchased from local market of Sangrur, India. Mahua flowers (dry) were procured from Madhya Pradesh, India. All chemicals used in the study were either AR grade or extra pure.
Pretreatment, Fermentation and Ageing
Washed grapes were destemmed, crushed, sulfiting (7–150 ppm) was done, then pressed to separate the liquids from the solids [7, 8]. Dried mahua flowers were boiled in water (2:1, 100°C, 30 min). Three samples were prepared containing 0, 5, 10%v/v mahua extract. Yeast (S.cerevisiae var.) (5%w/v) was added and fermentation was carried out at room temperature (25 ± 2°C) in amber coloured bottles under anaerobic condition by cotton plugging the bottles. Fermentation was followed by measuring the drop in degree brix and was assumed to be completed after 15 days since the degree brix ceased to drop further. At this stage, sorbic acid was added to wine (200 ppm) to inhibit the fermentation process and to inactivate the yeasts. Waste metabolites were settled at the bottom of the bottle. Clear wine was separated by the process of racking and siphoning for 3–4 times. Samples were analyzed for TSS, pH, titratable acidity, specific gravity, anthocyanin, tannin, ethanol, reducing sugars and overall acceptability. The best selected sample on the basis of ranking test was further subjected to clarification using bentonite and gelatine which were added to give three realistic levels (0.02, 0.04, 0.06 g/100 g bentonite and 0.02, 0.03, 0.04 g/100 g gelatine). The nine treatment combinations (3bentonite × 3gelatin) and a control sample were analyzed for the study. Samples were further subjected to ageing for 3 months at ambient temperature (25 ± 2°C). Changes took place were recorded over an interval of 1 month.
Physicochemical and Sensory Analysis
Wine was subjected to TSS, pH, titrable acidity (TA, expressed as g/L of tartaric acid) and specific gravity tests by standard methods [9]. Tannin content was determined by colorimetric estimation, based on measurement of blue color formed by reduction of phosphotungstomolybdic acid by tannin like compounds in alkaline solution [9]. Reducing sugar was estimated by determining the volume of unknown sugar solution required to completely reduce a measured volume of Fehling’s solution [9]. Alcohol content was determined by distillation [10]. Anthocyanin extraction was done with ethanolic HCl and measurement of color was carried out at the wavelength of maximum absorption at ambient temperature (25 ± 2°C) [9]. Ranking test was applied for sensory analysis for their color, taste, and overall acceptability. A panel of 20 semi-trained panelists rated the samples on the basis of ranking test. This test is used to determine how several samples differ on the basis of a single characteristic. Panelists were presented all samples simultaneously with code numbers and asked to rank samples according to the intensity of specific characteristic (color, flavor, taste etc.) [9]. The best sample was selected from the three samples on the basis of this test.
Statistical Analysis
Tukey test was used which compares the means of every treatment (3 determinations) to the means of every other treatment. Means that have no superscript in common (a, b, c etc.) are significantly different from each other (P < 0.05). Microsoft Excel (2007) was used for analysis.
Results and Discussion
The pH and TSS were initially adjusted to 3.3 and 22°Bx for all three samples as the wines having must TSS 22–24°Bx yielded wine with 8–10%v/v alcohol [11]. The higher anthocyanin content in 100:0 (grape:mahua) sample (79.2 mg/100 g) (Table 1) may be due to the high content of grape skin in contact with juice which resulted in higher extraction rate of anthocyanins from skin to the juice. Tukey’s test revealed that there was significant difference in TSS, reducing sugars and anthocyanin content on addition of mahua extract to samples 95:5 and 90:10 (grape:mahua) as compared to 100:0 (grape:mahua) sample. No significant difference in specific gravity was observed in all the three samples. Variation in parameters may be due to varietal difference, storage conditions, climatic conditions etc. [12].
Table 1.
Initial composition of grape-mahua must
| 100:0 (grape:mahua) | 95:5 (grape:mahua) | 90:10 (grape:mahua) | |
|---|---|---|---|
| TSS (°Bx) | 19.5a | 21.4bd | 23.5ce |
| pH | 3.46a | 3.6ac | 3.78bc |
| TA (g/L tartaric acid) | 0.83a | 0.77ac | 0.71bc |
| SG (20°C) | 1.089a | 1.096ab | 1.098ab |
| Anthocyanin (mg/100 g) | 79.2a | 62.5bc | 59.8bc |
| RS (%w/w) | 6.49a | 6.68bd | 7.1ce |
TSS total soluble solids, TA titratable acidity, SG specific gravity, RS reducing sugars
Composition of Wine After Fermentation
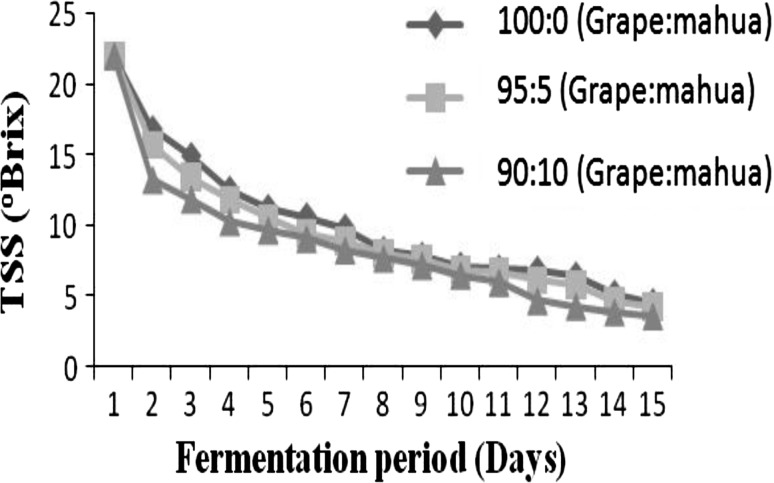
Anthocyanin content of the must was 79.2, 62.5 and 59.8 mg/100 g for the samples 100:0, 95:5 and 90:10 (grape:mahua) which was reduced to 74.6, 58.9 and 57.5 mg/100 g respectively in finished wine (Table 2). The TA of 100:0 (grape:mahua) sample decreased from 0.83 in must to 0.78 g/L in wine, whereas, the TA of samples 95:5 and 90:10 (grape:mahua) increased from 0.77 and 0.71 to 0.81 and 0.74 g/L respectively. The increase in TA was concomitant with decrease in pH from 3.6 and 3.78 in must to 3.54 and 3.65 in samples 95:5 and 90:10 (grape:mahua) respectively. It was observed that as the content of mahua extract was increased in grape must, there was an increase in the pH value. Following fermentation of tartaric-dominant grape wines, an increase in pH of 0.1 units may be expected in others a significant increase in TA and a corresponding decrease in pH may be expected during fermentation [13].The reducing sugar content decreased from 6.49, 6.68 and 7.1 in must to 0.67, 0.49 and 0.26%w/w in wine. Specific gravity was found to be least (0.9948) in 90:10 (grape:mahua) sample and highest (0.9963) in 100:0 (grape:mahua) sample. The low specific gravity indicated that as the rate of addition of mahua extract is increased, there is an increased rate of reducing sugars available for conversion to alcohol and increase in alcohol content was subjected to a decrease in specific gravity of the wine. Ethanol was found to be least in 100:0 (grape:mahua) sample (8.8% v/v) and highest in 90:10 (grape:mahua) sample (10.2% v/v) indicating that ethanol content increased with an increase in the mahua extract. Tannins were found to be least in 90:10 (grape:mahua) sample (0.064%w/v) and highest in 100:0 (grape:mahua) sample (0.078%w/v) since the grape skin contains higher amount of tannins than mahua flowers. The TSS was finally reduced to 4.5, 4.2 and 3.5°Bx in samples 100:0, 95:5 and 90:10 (grape:mahua) respectively. Rate of decrease in TSS was highest during the first 2 days of fermentation (Fig. 1). This may be due to the higher concentration of substrate available for the activity of yeast during the initial period of fermentation during which maximum amount of sugars was reduced to alcohol and CO2 90:10 (grape:mahua) sample was found to have least TSS (3.5) indicating that it was best fermented. Alobo and Offonry [14] reported a gradual decrease in TSS with a corresponding increase in alcohol content.
Table 2.
Composition of wine after fermentation
| 100:0 (grape:mahua) | 95:5 (grape:mahua) | 90:10 (grape:mahua) | |
|---|---|---|---|
| TSS | 4.5a | 4.2ac | 3.5bc |
| pH | 3.57a | 3.54ab | 3.65ab |
| TA (g/L tartaric acid) | 0.78a | 0.81ab | 0.74ab |
| SG (20°C) | 0.9963a | 0.9953ab | 0.9948ab |
| Anthocyanin (mg/100 g) | 74.6a | 58.9bc | 57.5bc |
| Tannin (% w/v tannic acid) | 0.078a | 0.069ac | 0.064bc |
| Ethanol % (v/v) | 8.8a | 9.4ac | 10.2bc |
| RS (%w/w) | 0.67a | 0.49bc | 0.26bd |
TSS total soluble solids, TA titratable acidity, SG specific gravity, RS reducing sugars
Fig. 1.
Changes in TSS during fermentation
The significance of difference between sample 100:0, 95:5 and 90:10 (grape:mahua) was assessed using Tukey’s test. Sample 90:10 (grape:mahua) showed significant difference in pH as compared to 100:0 and 95:5 (grape:mahua) sample. The ethanol content of 90:10 (grape:mahua) sample was also significantly higher than 100:0 and 95:5 (grape:mahua) sample. Overall acceptability of the three wine samples was assessed using Ranking test. 90:10 (grape:mahua) sample containing 10% mahua extract was found to be best among all three samples followed by 100:0 and then 95:5 (grape:mahua) sample. 90:10 (grape:mahua) sample was found to contain TSS (3.5), pH (3.65), TA (0.74 g/L), SG (0.9948), anthocyanin (57.5 mg/100 g), tannin (0.064%w/v), ethanol (10.2%v/v) and reducing sugars (0.26%w/w) respectively. Hence, 90:10 (grape:mahua) sample was found to be best and was selected for further studies.
Changes in Anthocyanin and Tannin After Clarification
It was found that sample no. 3 (0.02B+0.04G) showed better results for anthocyanin (52.2 mg/100 g) and tannin (0.038%w/v) (Table 3). The significance of differences between anthocyanin and tannin content was assessed using Tukey’s test. The anthocyanin content of sample 3 was found to be significantly higher than samples 4, 7, 8, and 9 whereas tannin content of sample 3 was significantly lower than samples 4, 7, 8 and 10 (Table 3). Use of bentonite and gelatin at a concentration of 0.02/100 and 0.04 g/100 g was found to be best suitable for clarification of wine. With increase in the concentration of bentonite, there was a significant loss of anthocyanins (Table 3). Clarification reduces the content of both extractive and volatile substances including anthocyanins [12].
Table 3.
Effect of various concentrations of bentonite and gelatin on the anthocyanin and tannin content of wine
| Sample | After clarification | |
|---|---|---|
| Anthocyanin (mg/100 g) | Tannin (% w/v tannic acid) | |
| 1 | 55.3a | 0.041a |
| 2 | 54.4ac | 0.039ac |
| 3 | 52.2ace | 0.038ace |
| 4 | 47.6bcef | 0.055bdfg |
| 5 | 51.2acefi | 0.046adegi |
| 6 | 48.4acefil | 0.04acehik |
| 7 | 43.9bdefilo | 0.061bdfgjlm |
| 8 | 42.5bdfgjmoq | 0.053bdfgikmo |
| 9 | 43.4bdefkloqs | 0.044acehiknpq |
| 10 | 57.5acehjnprt | 0.064bdfhjlmor |
1 (0.02B+0.02G), 2 (0.02B+0.03G), 3 (0.02B+0.04G), 4 (0.04B+0.02G), 5 (0.04B+0.03G), 6 (0.04B+0.04G), 7 (0.06B+0.02G), 8 (0.06B+0.03G), 9 (0.06B+0.04G), 10 (control sample with no bentonite and gelatin treatment) where B bentonite and G gelatin (g/100 g)
Tannins contribute to astringency in wine and need to be reduced during clarification. Tannin content was found to be maximum (0.061%w/v) in sample containing a combination of 0.06 g/100 g bentonite and 0.02 g/100 g gelatin, whereas, it was found to be minimum (0.038%w/v) in sample containing combination of 0.02 g/100 g bentonite and 0.04 g/100 g gelatin respectively (Table 3). This indicates that gelatin had more effect on reducing the tannin content of wine than bentonite. The reaction between tannins and proteins increases astringency in wine [15]. The perception of astringency in wine could be influenced by ethanol [16] acidity [16, 17] viscosity [18] simple sugars [19] polysaccharides [20, 21] and anthocyanins [20].
Effect of Ageing
Ageing was carried out for all the samples for 3 months. There was a significant decrease in TSS of all the samples except sample 10 (containing no bentonite or gelatin). It was found that TSS was highest (2.7°Bx) in the non-clarified sample and lowest (2.1°Bx) in sample treated with 0.06 g/100 g bentonite and 0.03 g/100 g gelatin after 3 months respectively (Table 4). Similarly, pH of all the samples decreased significantly except sample 4 (0.04B+0.02G). The minimum pH value (3.16) was found in sample 5 (0.04B+0.03G). Sims and Morris [22] reported that higher pH resulted in a loss of color intensity and redness and increased browning during storage for 9 months. The anthocyanin content in all samples of wine decreased on ageing which was found to be significant for all the samples except sample 2 (0.02B+0.03G) and sample 9 (0.06B+0.04G) (Table 4). The loss of anthocyanins was more in sample treated with a higher concentration of bentonite. The concentration of total anthocyanins and total phenols in wines treated with bentonite and gelatin declined with ageing [23].
Table 4.
Changes in TSS, pH, specific gravity, anthocyanin content, tannin content and titratable acidity of wine during ageing
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| TSS (Bx) | ||||||||||
| 1 M | 2.7a | 2.7a | 2.8a | 2.8a | 2.9a | 2.8a | 2.4a | 2.5a | 2.8a | 2.9a |
| 2 M | 2.6ac | 2.5ab | 2.6ac | 2.5ac | 2.8ac | 2.6ac | 2.3ac | 2.4ac | 2.6ac | 2.8ab |
| 3 M | 2.3bc | 2.3bb | 2.4bc | 2.4bc | 2.2bd | 2.2bd | 2.2bc | 2.1bc | 2.3bc | 2.7ab |
| pH | ||||||||||
| 1 M | 3.37a | 3.37a | 3.38a | 3.39a | 3.4a | 3.38a | 3.39a | 3.38a | 3.39a | 3.4a |
| 2 M | 3.31ac | 3.3ac | 3.34ac | 3.32ab | 3.28bc | 3.36ac | 3.34ac | 3.33ac | 3.35ac | 3.34ac |
| 3 M | 3.25bc | 3.22bc | 3.28bc | 3.27ab | 3.16bd | 3.22bd | 3.24bc | 3.22bd | 3.29bc | 3.27bd |
| SG (20°C) | ||||||||||
| 1 M | 0.999a | 0.996a | 0.997a | 0.999a | 0.997a | 0.999a | 0.996a | 0.997a | 0.999a | 0.997a |
| 2 M | 0.994ab | 0.994ab | 0.994ab | 0.996ab | 0.995ab | 0.997ab | 0.994ab | 0.994ab | 0.997ab | 0.995ab |
| 3 M | 0.993ab | 0.993ab | 0.992ab | 0.991ab | 0.993ab | 0.993ab | 0.993ab | 0.992ab | 0.993ab | 0.994ab |
| Anthocyanin (mg/100 g) | ||||||||||
| 1 M | 40.56a | 39.26a | 36.84a | 35.54a | 36.81a | 36.3a | 35.28a | 35.03a | 30.19a | 56.43a |
| 2 M | 38.56ac | 37.96ab | 36.5ac | 33.43ac | 35.7ac | 33.9ac | 32.34ac | 31.64bc | 30.03ab | 56.17bc |
| 3 M | 37.57bc | 37.32ab | 35.82bc | 29.22bd | 33.48bc | 29.3bd | 30.96bc | 30.56bc | 29.72ab | 56.1bc |
| Tannin (%w/v tannic acid) | ||||||||||
| 1 M | 0.039a | 0.038a | 0.036a | 0.053a | 0.043a | 0.036a | 0.056a | 0.049a | 0.04a | 0.06a |
| 2 M | 0.038ab | 0.035ac | 0.031bc | 0.046ac | 0.039bc | 0.034bc | 0.05ac | 0.044ac | 0.035ac | 0.072bc |
| 3 M | 0.037ab | 0.033bc | 0.03bc | 0.04bc | 0.035bd | 0.033bc | 0.045bc | 0.039bc | 0.031bc | 0.079bd |
| TA (g/L tartaric acid) | ||||||||||
| 1 M | 1.4a | 1.7a | 1.8a | 1.8a | 1.7a | 1.6a | 1.7a | 1.7a | 1.6a | 1.7a |
| 2 M | 1.5ac | 1.8ac | 1.9ac | 2.0ac | 1.9ac | 1.9bc | 1.9ac | 1.8ac | 2bc | 1.8ab |
| 3 M | 2.1bd | 1.9bc | 2.1bc | 2.2bc | 2.0bc | 2.2bd | 2.1bc | 2.0bc | 2bc | 1.9ab |
1 (0.02B+0.02G), 2 (0.02B+0.03G), 3 (0.02B+0.04G), 4 (0.04B+0.02G), 5 (0.04B+0.03G), 6 (0.04B+0.04G), 7 (0.06B+0.02G), 8 (0.06B+0.03G), 9 (0.06B+0.04G), 10 (control sample with no bentonite and gelatin treatment) where B bentonite and G gelatin (g/100 g), TA titratable acidity, SG specific gravity, TSS total soluble solids, M month
Tannin content also showed a significant decrease in all the samples except sample 1 (0.02B+0.02G) (Table 4). The tannin was found to be least in sample containing 0.02 g/100 g bentonite and 0.04 g/100 g gelatin respectively. Usually the tannin content of non-clarified wine increases with storage resulting in the increased astringency of wine. But treatment of wine with gelatin significantly reduces the tannin content of wine [24]. Similarly control sample showed an increase in the tannin content on storage, while all other nine samples showed a decrease in tannin content with ageing. The titratable acidity was found to be highest in sample containing a combination of 0.04 g/100 g bentonite and 0.02 g/100 g gelatin and 0.04 g/100 g bentonite and 0.04 g/100 g gelatin respectively (Table 4). Chung et al. [25] found slight increase in titratable acidity of wine upon ageing for 9 months.
Conclusions
Fortification of mahua extract in the proportion of 10%v/v to red grape wine was the best and found to increase the overall acceptability of wine. After fermentation 90:10 (grape:mahua) sample showed significantly lower pH (3.54) and higher ethanol (10.2%v/v) as compared to other samples. Use of bentonite and gelatin at a concentration of 0.02/100 and 0.04 g/100 g was found to be best suitable combination for the clarification of wine. With the increase in the concentration of bentonite, there was a significant loss of anthocyanins. Gelatin had more effect on reducing the tannin content of wine than bentonite. After ageing anthocyanin content was lowest in sample treated with 0.04 g/100 g bentonite and 0.02 g/100 g gelatin (29.22 mg/100 g). Tannin content was found to be lowest (0.03%w/v) in sample treated with 0.02 g/100 g bentonite and 0.04 g/100 g gelatine.
References
- 1.Shikhamany SD. Grape production in India. In: Papademetriou MK, Dent FJ, editors. Grape production in the Asia-Pacific region. Bangkok: FAO Regional Office for Asia and the Pacific (RAP Publication 2000/13); 2001. [Google Scholar]
- 2.Johnson H (1989) Vintage: the story of wine. Simon & Schuster.11–6. ISBN 0671791826
- 3.Pickering GJ, Heatherbell DA, Vanhanen LP, Barnes MF. The effect of ethanol concentration on the temporal perception of viscosity and density in white wine. Am J Enol Vitic. 1998;49:306–318. [Google Scholar]
- 4.Nurgel C, Pickering G. Contribution of glycerol, ethanol and sugar to the perception of viscosity and density elicited by model white wines. J Texture Stud. 2005;36:303–325. doi: 10.1111/j.1745-4603.2005.00018.x. [DOI] [Google Scholar]
- 5.Mande BA, Andreasen AA, Sreenivasaya M, Kolachov P. Fermentation of Bassia flowers. Ind Eng Chem. 1949;41:1451–1453. doi: 10.1021/ie50475a037. [DOI] [Google Scholar]
- 6.Baisya RK. India fast emerging as a major market for wine. Process Food Ind. 2006;9:10–12. [Google Scholar]
- 7.Benda I. Wine and brandy. In: Reed G, editor. Prescott and Dunn’s industrial microbiology. Westport: AVI Technical 2 Books Inc; 1981. pp. 293–402. [Google Scholar]
- 8.Gayon PR, Glories Y, Maujean A, Dubourdieu D (2006) The microbiology of wine and vinification In: Handbook of enology, 2nd edn. Wiley, England
- 9.Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. 2. New Delhi: Tata McGraw-Hill Publishing Company Limited; 2007. [Google Scholar]
- 10.Compendium of international methods of analysis of wine and musts. Paris: OIV; 1990. Compendium of international methods of analysis of wine and musts. [Google Scholar]
- 11.Ethiraj S, Suresh ER. Fruit wines. In: Chadha KL, Pareek OP, editors. Advances in horticulture. New Delhi: Malhotra Publishing House; 1993. pp. 1957–1966. [Google Scholar]
- 12.Wurdig G, Woller R. Chemie des Weines. Stuttgart: Eugen Ulmer: 926; 1989. [Google Scholar]
- 13.Darias-Martín J, Socas-Hernández, Díaz-Romero, Díaz-Díaz Comparative study of methods for determination of titrable acidity in wine. J Food Comp Anal. 2003;16:555–562. doi: 10.1016/S0889-1575(03)00032-2. [DOI] [Google Scholar]
- 14.Alobo AP, Offonry SU. Characteristics of colored wine produced from Roselle (Hibiscus sabdariffa) Calyx extract. J Inst Brew. 2009;115:91–94. doi: 10.1002/j.2050-0416.2009.tb00351.x. [DOI] [Google Scholar]
- 15.Lea AGH. Flavour, colour, and stability in fruit products: the effect of polyphenols. In: Hemingway RW, Laks PE, editors. Plant polyphenols. New York: Plenum Press; 1992. pp. 827–837. [Google Scholar]
- 16.Fischer U, Noble AC. The effect of ethanol, catechin concentration and pH on sourness and bitterness of wine. Am J Enol Vitic. 1994;45:6–10. [Google Scholar]
- 17.Peleg H, Bodine KK, Noble AC. The influence of acid on astringency of alum and phenolic compounds. Chem Senses. 1998;23:371–378. doi: 10.1093/chemse/23.3.371. [DOI] [PubMed] [Google Scholar]
- 18.Smith AK, June H, Noble AC. Effects of viscosity on the bitterness and astringency of grape seed tannin. Food Qual Prefer. 1996;7:161–179. doi: 10.1016/S0950-3293(96)00028-6. [DOI] [Google Scholar]
- 19.Boselli E, Boulton RB, Thorngate JH, Frega NG. Chemical and sensory characterization of DOC red wines from Marche (Italy) related to vintage and grape cultivars. J Agric Food Chem. 2004;52:3843–3854. doi: 10.1021/jf035457h. [DOI] [PubMed] [Google Scholar]
- 20.Vidal S, Courcoux P, Francis L, Kwiatkowski M, Gawel R, Williams P, Waters E, Cheynier V. Use of an experimental design approach for evaluation of key wine components on mouth-feel perception. Food Qual Prefer. 2004;15:209–217. doi: 10.1016/S0950-3293(03)00059-4. [DOI] [Google Scholar]
- 21.Riou V, Vernhet A, Doco T, Moutounet M. Aggregation of grape seed tannins in model wine-effect of wine polysaccharides. Food Hydrocoll. 2001;16:17–23. doi: 10.1016/S0268-005X(01)00034-0. [DOI] [Google Scholar]
- 22.Sims CA, Morris JR. Effects of pH, sulfur dioxide, storage time, and temperature on the color and stability of red muscadine grape wine. Am J Enol Vitic. 1984;35:35–39. [Google Scholar]
- 23.Renata R, Downey MO, Iland PG, Keren B, Francis IL, Herderich Markus, Robinson SP. Exclusion of sunlight from Shiraz grapes alters wine colour, tannin and sensory properties. Aust J Grape Wine Res. 2007;13:53–65. doi: 10.1111/j.1755-0238.2007.tb00235.x. [DOI] [Google Scholar]
- 24.Saywell LG. Clarification of wines. Ind Eng Chem. 1934;26:981–982. doi: 10.1021/ie50297a018. [DOI] [Google Scholar]
- 25.Chung HJ, Son JH, Park EY, Kim EJ, Lim ST. Effect of vibration and storage on some physico-chemical properties of a commercial red wine. J Food Comp Anal. 2008;21:655–659. doi: 10.1016/j.jfca.2008.07.004. [DOI] [Google Scholar]