Summary
We report results of a pilot study of high-dose vitamin D in sickle cell disease (SCD). Subjects were followed for 6 months after receiving a six-week course of oral high-dose cholecalciferol or placebo. Vitamin D insufficiency and deficiency was present at baseline in 82.5% and 52.5% of subjects, respectively. Subjects who received high-dose vitamin D achieved higher serum 25-hydroxyvitamin D, experienced fewer pain days per week, and had higher physical activity quality-of-life scores. These findings suggest a potential benefit of vitamin D in reducing the number of pain days in SCD. Larger prospective studies with longer duration are needed to confirm these effects.
Keywords: Vitamin D, Chronic Pain, Sickle Cell, Paediatrics, Quality of Life
Individuals with sickle cell disease (SCD) develop daily chronic pain with age (McClish et al, 2009). Its aetiology is multi-factorial and includes vaso-occlusive bone infarction, peripheral nerve injury, central pain sensitization, and hyperalgesia syndrome from prolonged high-dose opioid use (Smith & Scherer, 2010). Vitamin D deficiency (VDD) is emerging as a significant public health concern for individuals with SCD (Goodman et al, 2010), We recently reported an association between VDD and chronic pain in a cohort of severely affected children with SCD (Osunkwo et al, 2011). A case of dramatic improvement in sickle chronic pain following high-dose vitamin D supplementation has also been described (Osunkwo, 2011). This pilot study aimed to determine the correlation between VDD and chronic pain and evaluate the safety and efficacy of high-dose vitamin D in reducing chronic pain in paediatric SCD.
Design and Methods
In accordance with the Declaration of Helsinki and with Emory University Institutional Review Board approval, written informed consent/assent was obtained prior to any study procedures. Forty-six SCD subjects (7–21 years) were enrolled during steady state i.e. ≥30 days from blood transfusion, ≥14 days from any acute sickle complication. Exclusions included chronic transfusion therapy, serum creatinine >132μmol/l, alanine transaminase >3 × upper limit of normal, malabsorption, serum calcium <2.0 or >2.75 mmol/l, prior treatment for VDD, oral steroids, thiazide diuretics, depot medroxyprogesterone acetate, phenobarbital or phosphenytoin. A pain diary was completed daily for 30 days and continued for six-months (Dampier et al, 2002). Subjects had “chronic pain” if baseline pain diary documented scores ≥1 (out of a maximum of 10) on ≥50% days irrespective of numerical pain scores on any given day. Subjects were stratified by chronic pain status at randomization to receive either high-dose vitamin D (cholecalciferol, BTR Group Inc, Pittsfield IL, USA) or placebo, dosed according to weight (240,000–600,000 iu) and given over 6 weeks. To ensure no subject developed severe VDD during the trial, they received an oral daily supplement containing 500 mg calcium and 200 iu vitamin D (Lang Naturals Inc., Newport RI) for six months. Serial blood samples for serum 25-hydroxyvitamin D (25OHD) concentrations were obtained at baseline, weeks 8, 16 and 24, stored frozen at −80°C and analysed in one batch at the end of the study.
Serum 25OHD concentrations were determined using IDS-iDSYS immunoassay (IDS, Inc; Fountain Hills, AZ); intra- and inter-assay coefficient of variance was 1.8–4.0% and 10.1–13.0%, respectively. Participation in the vitamin D external quality assessment scheme (DEQAS, site #606) ensured assay quality control. Serum 25OHD <50 nmol/l indicated VDD and <75 nmol/L represented vitamin D insufficiency (Holick et al, 2011). The Pediatric Quality of Life Inventory (PedsQL™) version 4.0 was administered at each study visit with weekly phone contact to encourage compliance with pain diary completion and return. Adverse events were monitored under a Data Safety Monitoring Board. Specific questions were used to elicit symptoms of hypocalcaemia (peri-oral tingling, lip/extremity twitching, hands/large muscle group spasms). Statistical tests were performed using SAS 9.2 (Cary, NC) and significance was assessed at 5%. Due to the longitudinal nature of the data and presence of missing values, repeated measures analyses were conducted using the MIXED procedure in SAS. Spearman's rank correlation coefficient was used to quantify the relationship between PedsQL scores with 25OHD concentrations and pain days. Two-way analysis of variance models compared PedsQL scores between treatment groups over time.
Results
Thirty-nine randomized subjects were evaluable (59% female); 7 withdrew before randomization (inconvenience (2), unable to swallow pills (1), relocation (3), medical exclusion (1)). Twenty-five subjects completed the full 6-months of follow-up. Table SI and Figure S1 detail the study design and vitamin D dosing. Four pain diaries were administered: Diary 1 collected data for the first 30 days; Diaries 2–4 each collected data for 60 days. All subjects returned Diary 1, 74% returned Diaries 1 and 2 while 54% returned Diaries 1, 2 and 3. Two subjects returned empty diaries. Compliance with pain diary completion was 82.4±18.1% and comparable to previous reports (Dampier et al, 2002). Table I summarizes baseline characteristics of all participants and each treatment group. Seventeen percent of subjects were vitamin D sufficient at baseline (serum 25OHD ≥75 nmol/l). Subjects experienced 8.6±8.5 pain days in the 30 days prior to randomization; 25% reported pain on ≥50% of diary days and were classified as having “chronic pain”. As expected, subjects with “chronic pain” spent more time in the hospital and had more emergency department (ED) visits for pain compared to those with “no chronic pain”. There was a modest trend towards lower baseline physical activity PedsQL scores among those with “chronic pain” (p=0.11).
Table I.
Baseline characteristics of total study population and by randomization group (vitamin D/placebo). 46 subjects were enrolled: 39 randomized subjects had SS homozygous sickle cell disease.
| Baseline Characteristics | Enrolled Subjects (n = 46) | Randomized |
P value | |
|---|---|---|---|---|
| Vitamin D (n = 20) | Placebo (n = 19) | |||
| Age (years) | 13.2 ± 3.1 | 12.9 ± 0.6 | 13.2 ± 0.8 | 0.722 |
| Gender (Male: Female) | 19:27 | 9:11 | 7:12 | 0.616 |
| Genotype | ||||
| SS | 29 | 12 | 12 | ns |
| Sß0 - thal | 2 | 1 | 1 | |
| Sß+ - thal | 10 | 4 | 5 | |
| SC | 5 | 3 | 1 | |
| Pain Status | ||||
| Chronic pain (n =11) | 11 | 6 | 5 | ns |
| No chronic pain (n = 28) | 29 | 14 | 14 | |
| Pain Days at Randomization | 8.6 ± 8.5 | 7.7 ± 7.2 | 10.1 ± 9.7 | 0.379 |
| Complications | ||||
| Number of ED visits for pain in prior 12 months | 0.68 ± 0.94 | 0.59 ± 0.94 | 0.67 ± 0.67 | 0.816 |
| Number of hospitalized days with pain in prior 12 months | 6.5 ± 19.6 | 5.7 ± 2.1 | 6.2 ± 2.3 | 0.870 |
| Baseline laboratory indices | ||||
| 25 hydroxyvitamin D (nmol/l) | 51.4 ± 19.7 | 55.2 ± 19 | 50.4 ± 20.5 | 0.476 |
| Calcium (mmol/l) | 2.325 ± 0.03 | 2.325 ± 0.03 | 2.325 ± 0.03 | 0.958 |
| Alkaline phosphatase (u/l) | 177 ± 72 | 182.5 ± 17.5 | 185.6 ± 18.6 | 0.907 |
| Health Related QOL | ||||
| Physical Activity | 71.9 ± 18.3 | -- | -- | -- |
| School Functioning | 55 ± 19.1 | -- | -- | -- |
Sß0 – thal, sickle beta zero thalassaemia; Sß+ - thai, sickle beta plus thalassaemia; SC, haemoglobin SC disease; AVN, avascular necrosis; ED, emergency department; QOL, quality of life.
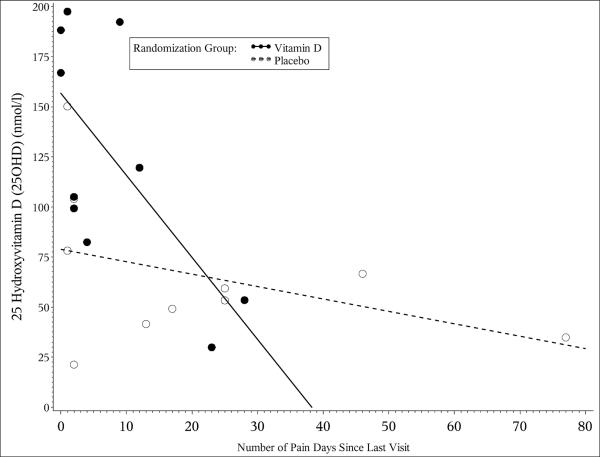
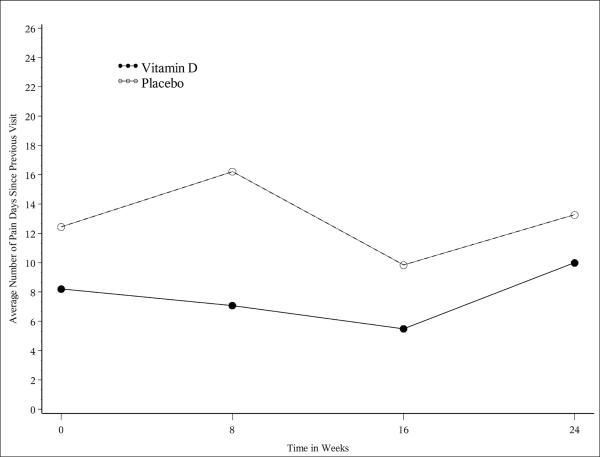
Mean serum 25OHD concentrations increased significantly in the vitamin D group at weeks 8 and 16 (p=0.012 and 0.03, respectively) with a trend towards significance at week 24 (p=0.057). There was a significant negative correlation (r=−0.68, 95% CI [−0.9, 0.1]) between serum 25OHD concentrations and the number of pain days per week in the vitamin D group at week 8 with a trend towards significance at week 16 (r=−0.54, 95% CI [−0.9,0.3], Figure 1a). Over the first 16 weeks of the study, there was a distinct trend towards fewer pain days between visits in the vitamin D group that failed to reach statistical significance due to the small sample size (Figure 1b). Serum 25OHD concentrations correlated positively with improved physical functioning PedsQL scores (r=0.219, 95% CI [0.01,0.4]). In contrast, the number of pain days correlated negatively with physical functioning PedsQL (r=−0.246 95% CI [−0.4,−0.04]) as subjects who experienced more days with pain demonstrated reduced physical functioning. There were no significant differences in mean PedsQL component scores between treatment groups over time for any other subscale measured.
Figure 1.
[a]: The scatter plot depicts the relationship between serum 25OHD concentrations (nmol/l) with the number of pain days for the vitamin D group (dark circles, bold line) and placebo group (open circles, dotted line) at week 8. The slope of the regression lines representing the strength of the relationship between 25OHD and number of pain days varied at each time point in the study. Serum 25OHD concentrations correlated negatively with the number of pain days at week 8 (r = −0.680 95% CI [−0.903, −0.102], p=0.019) for vitamin D group compared to the placebo group (r = −0.403 95% CI [−0.816, −0.324], p=0.258)
[b]: There was a distinct trend towards fewer pain days between visits over the first 16 weeks of the study in the vitamin D group. Although this change did not reach statistical significance due to the small sample size, it is in contrast to the sporadic fluctuation in the number of pain days seen in the placebo group over the same time period.
Discussion
Pain remains the leading cause of morbidity and healthcare utilization for individuals with SCD (Smith et al, 2010). A quantitative reduction in the daily pain experience is urgently needed to significantly improve quality of life for these patients (Dampier et al, 2010; van Tuijn et al, 2010). Approximately 82.5% of subjects study were vitamin D insufficient and >52% were vitamin D deficient. Contrary to our expectation, 25OHD concentrations did not correlate significantly with chronic pain status or PedsQL scores at baseline in this cohort.
Though the evidence is somewhat limited, several studies showed an association between VDD and chronic pain syndromes as well as reports of improved pain symptoms and decreased analgesic use with vitamin D supplementation (Atherton et al, 2009; Gloth & Greenough, 2004; Holick, 2006; Lofti et al, 2007; Osunkwo, 2011; Straube et al, 2010). This pilot study aimed to evaluate the clinical efficacy of high-dose vitamin D in improving chronic pain in paediatric SCD. There was a notable reduction in the number of pain days during the period of peak serum 25OHD concentrations that was supported by evidence of improved physical functioning. These results provide preliminary evidence for fewer days with pain, concurrent with a rise in serum 25OHD concentrations in SCD with the peak effect occurring when serum 25OHD concentrations exceeded 75 nmol/l. However, the effect of vitamin D on the number of pain days was not dependent on baseline vitamin D status. This implies that vitamin D therapy may still be beneficial even in chronic pain patients who have adequate serum 25OHD concentrations (up to 75 nmol/l).
The mechanisms by which vitamin D modulates pain remain unclear and may be a result of direct nervous system effects or indirectly from improved bone health (Gloth& Greenough, 2004; Holick, 2006). Impaired bone mineralization allows the osteoid matrix to absorb fluid and expand, causing outward pressure on the highly innervated periosteal tissues resulting in pain syndromes (Reginato et al, 1999). While the optimal dose of vitamin D for chronic pain is unknown, higher doses (up to 600,000 iu) over 6 weeks were safe and necessary to restore vitamin D status in this pilot. Our results also suggest the need for either pulsed dosing of vitamin D therapy or higher maintenance doses to sustain optimal serum 25OHD concentrations above 75nmol/L. Incorporating regular weekly or monthly doses under healthcare supervision may be an effective strategy to sustain adequate vitamin D status in SCD patients throughout the year while ensuring reasonable adherence and should be the subject of future studies.
Adolescents with SCD experience more disease complications with age that is often associated with declining PedsQL scores with age (Dampier et al, 2010; Smith et al, 2010). Physical activity PedsQL scores increased in response to reduced pain in the vitamin D group, supporting vitamin D's role in musculoskeletal health. The small sample size and short study duration were limitations of this study; however, our results support the rationale for larger longitudinal studies to determine whether maintenance of optimal vitamin D status for prolonged periods will improve SCD pain. The use of bound paper diaries was quite cumbersome for study participants and partly accounted for the high dropout rate. Emerging technology using electronic diary capture tools will be essential to accurately assess daily pain with more ease in a longitudinal study. The reasons for more dropouts in the placebo arm remain elusive and should be addressed in future studies.
In summary, these results suggest an improvement in both pain (clinical outcome) and quality of life (functional outcome) in response to a short pulse of high dose vitamin D in children and adolescents with SCD and the need to maintain optimal 25OHD concentrations over time to sustain this benefit. Larger longitudinal randomized controlled studies are needed to confirm the efficacy of optimizing vitamin D status on reducing SCD pain while improving physical functioning and will help guide the role for vitamin D as a component of chronic pain therapy in SCD.
Supplementary Material
Acknowledgements
This study was supported by grant funding from the Aflac Cancer Center and Blood Disorders Services Research Grant (IO), National Institute of Health grant K24 RR 023356 (TRZ) and K23 AR054334 (VT). We received cholecalciferol and placebo caplets from Joanne and James Grote as a gift from the BTR Group Inc., Pittsfield IL, USA.
Footnotes
Author Contributions: Study concept: Osunkwo I, Tangpricha, Ofori-Acquah; Study design: Osunkwo I, Tangpricha, Dampier, Eckman. Acquisition of data: Osunkwo I, Cherry, Tangpricha, Ofori-Acquah, Ghosh, Alvarez, Rhodes; Analysis and interpretation of data: Osunkwo I, McCracken, Osunkwo CE, Tangpricha, Ziegler, Dampier; Drafting of the manuscript: Osunkwo I, Ogunbodede. Critical revision of the manuscript for important intellectual content: Dampier, Eckman, Tangpricha, Ziegler; Statistical analysis: McCracken. Study supervision: Osunkwo I, Tangpricha. Manuscript editing: Cowan D
References
- Atherton K, Berry D, Parsons T, GJ M, Power C, Hypponen E. Vitamin D and widespread pain in a white middle aged British population: evidence from a cross sectional population survey. Annals of the Rheumatic Diseases. 2009;68(6):817–822. doi: 10.1136/ard.2008.090456. [DOI] [PubMed] [Google Scholar]
- Dampier C, Ely E, Brodecki D, O'Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Pediatr Hematol Oncol. 2002;24(8):643–647. doi: 10.1097/00043426-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Z, Smith-Whitley K, Wang W. Health-related quality of life in children with sickle cell disease: a report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatr Blood Cancer. 2010;55(3):485–494. doi: 10.1002/pbc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman B, Artz N, Radford B, Chen I. Prevalence of vitamin D deficiency in adults with sickle cell disease. J Natl Med Assoc. 2010;102(4):332–335. doi: 10.1016/s0027-9684(15)30605-2. [DOI] [PubMed] [Google Scholar]
- Gloth F, Greenough W. Vitamin D deficiency as a contributor to multiple forms of chronic pain. Mayo Clin Proc. 2004;79(5):696, 699. doi: 10.1016/S0025-6196(11)62303-3. [DOI] [PubMed] [Google Scholar]
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon C, Hanley D, Heaney R, Murad M, Weaver C. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Lofti A, Abdel-Asser A, Hamdy A, Omran A, El-Rehany M. Hypovitaminosis D in female patients with chronic low back pain. Clinical Rheumatology. 2007;26(11):1895–1901. doi: 10.1007/s10067-007-0603-4. [DOI] [PubMed] [Google Scholar]
- McClish D, Smith W, Dahman B, Levenson J, Roverts J, Penberthy L, Aisiku I, Roseff S, Bovjerg V. Pain site frequency and location in sickle cell disease: the PiSCES project. Pain. 2009;145(1-2):246–251. doi: 10.1016/j.pain.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osunkwo I. Complete resolution of sickle cell chronic pain with high dose vitamin D therapy: a case report and review of the literature. J Pediatr Hematol Oncol. 2011;33(7):549–551. doi: 10.1097/MPH.0b013e31821ed3ea. [DOI] [PubMed] [Google Scholar]
- Osunkwo I, Hodgman EI, Cherry K, Dampier C, Eckman J, Ziegler T, Ofori-Acquah S, Tangrpicha V. Vitamin D deficiency and chronic pain in sickle cell disease. Br J Haematol. 2011;153(4):538–540. doi: 10.1111/j.1365-2141.2010.08458.x. [DOI] [PubMed] [Google Scholar]
- Reginato AJ, Falasca GF, Pappu R, McKnight B, Agha A. Musculoskeletal manifestations of osteomalacia: report of 26 cases and literature review. Semin Arthritis Rheum. 1999;28(5):287–304. doi: 10.1016/s0049-0172(99)80013-4. [DOI] [PubMed] [Google Scholar]
- Smith WR, Scherer M. Sickle-cell pain: advances in epidemiology and etiology. Hematology Am Soc Hematol Educ Program. 2010:409–415. doi: 10.1182/asheducation-2010.1.409. [DOI] [PubMed] [Google Scholar]
- Straube S, Derry S, Moore R, McQuay H. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2010;2010(1):CD007771. doi: 10.1002/14651858.CD007771.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tuijn CF, van Beers EJ, Schnog JJ, Biemond BJ. Pain rate and social circumstances rather than cumulative organ damage determine the quality of life in adults with sickle cell disease. Am J Hematol. 2010;85(7):532–535. doi: 10.1002/ajh.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.