Abstract
Gene expression analysis by reverse transcriptase real-time or quantitative polymerase chain reaction (RT-qPCR) is becoming widely used for non-model plant species. Given the high sensitivity of this method, normalization using multiple housekeeping or reference genes is critical, and careful selection of these reference genes is one of the most important steps to obtain reliable results. In this study, reference genes commonly used for other plant species were investigated to identify genes displaying highly uniform expression patterns in different varieties, tissues, developmental stages, fungal infection, and osmotic stress conditions for the non-model crop Musa (banana and plantains). The expression stability of six candidate reference genes was tested on six different sample sets, and the results were analyzed using the publicly available algorithms geNorm and NormFinder. Our results show that variety, plant material, primer set, and gene identity can all influence the robustness and outcome of RT-qPCR analysis. In the case of Musa, a combination of three reference genes (EF1, TUB and ACT) can be used for normalization of gene expression data from greenhouse leaf samples. In the case of shoot meristem cultures, numerous combinations can be used because the investigated reference genes exhibited limited variability. In contrast, variability in expression of the reference genes was much larger among leaf samples from plants grown in vitro, for which the best combination of reference genes (L2 and ACT genes) is still suboptimal. Overall, our data confirm that the stability of candidate reference genes should be thoroughly investigated for each experimental condition under investigation.
Electronic supplementary material
The online version of this article (doi:10.1007/s11032-012-9711-1) contains supplementary material, which is available to authorized users.
Keywords: Musa, Banana, Reference genes, qPCR, Gene expression, Mycosphaerella
Introduction
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) is a routinely used technique for gene expression analysis because of its main advantages of relatively low cost, good speed, a wide dynamic range, and feasibility in non-model organisms (Thellin et al. 1999). However, extreme care needs to be exercised in the interpretation of RT-qPCR data and, in particular, normalization is crucial to control for experimental errors that can be introduced at a number of stages throughout the procedure (reviewed in Bustin 2002; Deepak et al. 2007; Gachon et al. 2004; Guenin et al. 2009; Huggett et al. 2005; Nolan et al. 2006; Radonic et al. 2004). The most reliable method of normalization involves the use of one or preferably more housekeeping or reference genes as internal standards. The expression of these reference genes is therefore expected to remain constant under different experimental conditions. Commonly used reference genes are cellular maintenance genes, which regulate basic and ubiquitous cellular functions such as components of the cytoskeleton, glycolytic pathway, protein folding, synthesis of ribosome subunits, electron transport, and protein degradation (Gachon et al. 2004; Huggett et al. 2005). Recent studies have shown that the transcriptional levels of these reference genes are not always stable, and that no single reference gene has a constant expression level under all experimental conditions (Dheda et al. 2005; Gutierrez et al. 2008a; Schmittgen et al. 2000; Thellin et al. 1999; Tricarico et al. 2002; Vandesompele et al. 2002). However, according to a recent metastudy, many of the published articles on plant gene expression still rely solely on one reference gene for normalization (Gutierrez et al. 2008b). Different statistical procedures or software packages have been reported to identify the best suitable reference gene(s) for a sample set, such as geNorm (Vandesompele et al. 2002), NormFinder (Andersen et al. 2004), ΔCt approach (Livak and Schmittgen 2001), Bestkeeper (Pfaffl et al. 2004), and “Stability index” (Brunner et al. 2004). For plants, multiple reference genes have been analyzed in the model plants Arabidopsis thaliana (Czechowski et al. 2005; Graeber et al. 2011; Hong et al. 2010; Lilly et al. 2011; Remans et al. 2008; Rieu et al. 2008), tobacco (Schmidt and Delaney 2010), and rice (Jain et al. 2006; Kim et al. 2003). Recently, studies have also been published on vegetables (Castro et al. 2011; Die et al. 2010; Expósito-Rodriguez et al. 2008; Garg et al. 2010; Gutierrez et al. 2011; Hu et al. 2009; Libault et al. 2008; Mascia et al. 2010; Migocka and Papierniak 2011; Nicot et al. 2005; Obrero et al. 2011; Wan et al. 2010), fruits (Reid et al. 2006; Tong et al. 2009), cereals and grasses (Dombrowski and Martin 2009; Hong et al. 2008; Jarosova and Kundu 2010; Lee et al. 2010; Paolacci et al. 2009; Silveira et al. 2009), trees (Brunner et al. 2004; Li et al. 2011; Goncalves et al. 2005), and a variety of other plant species (Artico et al. 2010; Cordoba et al. 2011; Cruz et al. 2009; Dong et al. 2011; Iskandar et al. 2004; Mallona et al. 2010; Maroufi et al. 2010; Tu et al. 2007; Yang et al. 2010). While this article was being reviewed, Chen et al. (2011) published the first report describing the validation of reference genes in dessert banana that mainly focuses on fruit tissues.
Musa (bananas and plantains, collectively referred to as banana) species provide a staple food in many developing countries and with an annual production of more than 130 million tons per year it is the fourth most important food crop worldwide (FAO 2009). Diseases and pests (Jones 2009) as well as abiotic stresses including drought and temperature changes (Israeli and Lahav 2000; van Asten et al. 2011) are amongst the major and increasingly damaging constraints on banana production. Our aim is to provide tools for investigating the expression of genes involved in stress responses of non-fruit tissues of banana, with the ultimate goal of gaining further insight in the molecular mechanisms underlying the interactions between banana plants and their environment.
Banana is a typical non-model crop with limited genomic and cDNA/expressed sequence tag (EST) sequences available. The Global Musa Genome Consortium (GMGC) (Global Musa Genomics Consortium 2011) reports that currently less than 1 % of the Musa genome is sequenced (Carpentier et al. 2008). Therefore, as for most non-model crops, the possibilities for gene expression analyses in banana species are limited. For example, no microarray slides are available and the lack of a reference sequence makes next-generation RNA-Seq analyses difficult. There is a need for alternative techniques such as SuperSAGE (Coemans et al. 2005) and RT-qPCR. For studies on banana, an actin, a 25S ribosomal protein, a pectate lyase and GAPDH have been used as unique reference genes for expression experiments (Elitzur et al. 2010; Mbeguie-Mbeguie et al. 2007; Shekhawat et al. 2011; Thomas-Hall et al. 2007; van den Berg et al. 2007; Wang et al. 2010). In this study, we validated candidate reference genes for expression studies in banana plants by evaluating their robustness under different conditions, and in different tissues and varieties.
Materials and methods
Plant material and growth conditions
A summary of the different types of cultures, tissues, and varieties used is provided in Table 1.
Table 1.
Summary of the experiments, varieties, cultures/tissues, and experimental treatments
| Experiment | Variety (genomic group) | Culture type/tissue | Experimental treatment |
|---|---|---|---|
| In vitro | Grand Nain (AAA) | In vitro plants/pooled leaves | Effect of acetone |
| GHa development | Tuu Gia (AA) | Greenhouse plants/leaf | Gene expression at different time points |
| GHa varieties | Tuu Gia (AA) | Greenhouse plants/leaf | Variation in gene expression among varieties |
| Yangambi Km5 (AA) | |||
| Leaf disc | Tuu Gia (AA) | Leaf discs | Effect of Mycosphaerella fijiensis inoculation |
| Meristem sucrose | Cachaco (ABB) | In vitro meristem cultures | Effect of sucrose-induced osmotic stress |
| Meristem varieties | Cachaco (ABB) | In vitro meristem cultures | Variation in gene expression among varieties |
| Mbwazirume (AAAhb) | |||
| Williams (AAA) |
a GH greenhouse
bHighland banana
In vitro plantlets
Plants of the variety Grand Nain [AAA Cavendish sub-group; International Transit Centre (ITC accession number 0180)], were grown on semi-solid regeneration medium [REG: MS medium supplemented with vitamins (Murashige and Skoog 1962), 1 μM benzyladenine, 1 μM indole acetic acid, 10 mg l–1 ascorbic acid, 0.09 M sucrose, and 3 g l−1 Gelrite®] at 26 ± 2 °C under a 16-h photoperiod with a photosynthetic photon flux density of 50 μE m−2 s−1 provided by Cool White fluorescent lamps (TLD 58 W/33; Philips, France). After 5.5 weeks of growth, the plants were transferred to a liquid REG medium. After 2 months, fresh liquid REG medium was added, and to half of the plants, acetone was supplemented to a final concentration of 0.5 % (v/v). Acetone treatment was tested since acetone is used to dissolve certain biologically active compounds in the author’s laboratory. Leaves were harvested 2 days after the addition of acetone from six and seven plants grown on the REG medium without and with 0.5 % (v/v) acetone, respectively.
Greenhouse plants
Plants of the varieties Tuu Gia (AA, ITC.0610) and Yangambi Km5 (AAA Ibota sub-group, ITC.1123) were grown in pots in the greenhouse where the photoperiod was extended to 12 h by artificial light, if required. The temperature reached 26 °C in the day and 18 °C in the night, and the relative humidity ranged between 70 and 90 %. For the “development” experiment, the first sampling was performed using the second unfolded leaf of each of the 6 Tuu Gia plants (age, 6 months; time point, Ta) and the same leaf was sampled 16 (Tb) and 26 (Tc) days later. For the “variety” experiment, the leaf tissue of Tuu Gia at the Tc stage was compared to the leaf tissue of the age-matched Yangambi Km5 (Tc).
Leaf disc
Leaf disc infection was done essentially as described previously (Abadie et al. 2008). Briefly, 5 × 5 cm discs of the first unfolded leaf of 5- to 6-month-old greenhouse Tuu Gia plants were excised, rinsed multiple times with sterile water, and placed with the adaxial side onto 0.4 % (w/v) agar medium containing 8 mg l−1 gibberellic acid. The leaf discs were sprayed with a solution containing 2 × 104 Mycosphaerella fijiensis conidia in sterile water or with sterile water alone. The leaf discs were incubated at 26 °C under a 12-h photoperiod for 2 weeks. Eight whole leaf discs were sampled for each group 15 days after incubation of the leaf discs, i.e., at the time that the first symptoms of infection appeared in the sprayed group.
Meristems
Multiple shoot meristem cultures of Cachaco (ABB, cooking banana, ITC.0643), Mbwazirume (AAAh, East African highland banana, ITC.0084), and Williams (AAA Cavendish sub-group, ITC.0365) were initiated as previously described (Strosse et al. 2006) and maintained in the dark on a proliferation medium (P4; MS medium supplemented with vitamins (Murashige and Skoog 1962), 100 μM 6-benzylaminopurine, 1 μM indole acetic acid, 10 mg l−1 ascorbic acid, 0.09 M sucrose, and 2.5 g l−1 Gelrite®). For the “variety” experiments, meristems were harvested 6 days after subculture. For the “sucrose” experiment, Cachaco meristems were divided into three groups. Samples from all groups were subcultured on day 0 and placed back onto their growth medium. Samples from the cutting control group were harvested 24 h later. The plants in the “control” and “sucrose” groups were transferred to a fresh P4 medium (0.09 M sucrose) and P4 medium containing 0.4 M sucrose, respectively, on day 4; meristems were harvested 2 days later (day 6). Samples from five meristems were collected for each group.
In silico identification of candidate reference genes
Candidate reference genes were identified by literature search, with emphasis on reference genes previously used in plants. As indicated in Electronic Supplementary Material 1, the genes included in this study have different cellular functions. A BlastX similarity search (Altschul et al. 1990) was performed against the Musa 3′ EST database (donated to the GMGC by Syngenta) as well as all publicly available sequences in GenBank. One or more primer pairs were designed for each sequence using the Primer3 program (Primer3 2011) and the following parameters: length, 19–25 bp; optimal T m, 57–61 °C; GC %, 45–60 % and amplicon length, 75–200 bp. Subsequently, primer pairs were tested for heteroduplex formation using the OligoAnalyzer 3.1 program (OligoAnalyzer 2011). Before RT-qPCR, the primer pairs were tested by gradient RT-PCR using the Mastercycler Gradient PCR machine (Eppendorf, Hamburg, Germany) to identify the optimal annealing temperature. Reactions contained 1 × ThermoPol reaction buffer [New England Biolabs (UK) Ltd., Hitchin, United Kingdom], 200 μM of each dNTP, 500 nM of reverse and forward primers, 0.0125 U μl−1 Taq DNA polymerase [New England Biolabs], 1–2 μl cDNA template, and water to reach a total volume of 20 μl. Amplification was achieved via the following program: initial denaturation at 95 °C for 3 min 30 s followed by 30 cycles of 95 °C for 20 s, 62.5 ± 6.5 °C for 30 s, and 72 °C for 20 s, with a final elongation at 72 °C for 5 min. Amplicon size was verified by 1.5 % (w/v) agarose gel electrophoresis.
Total RNA extraction
The plant material was harvested, snap frozen in liquid nitrogen, and stored at −80 °C. Total RNA was extracted from different plant tissues using the RNeasy® Plus Mini Kit or RNeasy® Midi Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions except for the addition of PVP40,000 to the lysis buffer at a final concentration of 5 mg ml−1. The extracted RNA was treated with RNase-free Ambion® DNaseI (AB Applied Biosystems, Lennik, Belgium), which was subsequently removed during a phenol–chloroform/ethanol purification step. The quantity and quality (A 260/230 and A 260/280) of total RNA were determined using the Nanodrop ND-1000™ spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). Finally, to verify the absence of gDNA in the RNA samples, a qPCR was performed using DNase-treated RNA as template and primers for the EF1 gene. The reaction mixture was identical to that of the RT-qPCR (see below, Two-step real-time RT-PCR section) except that λ-DNA was omitted and instead of 2 μl cDNA template, 1 μl RNA was used. Following the initial polymerase activation at 95 °C for 15 min, 40 cycles of 95 °C for 15 s, 60 °C for 20 s, and 72 °C for 20 s were run. Finally, a melting program as described below (see below, Two-step real-time RT-PCR section) was executed at the end of the real-time PCR run. Only samples for which no amplification could be detected, thereby indicating the absence of DNA contamination, were used.
As the efficiency of enzymes used for PCR is affected by the quality of the RNA samples (Schmittgen and Zakrajsek 2000), only RNA samples with OD260/280 ratios above 1.6 and OD260/230 ratios above 1.8 were used for further analysis. These ratios indicate minimal presence of protein contaminants and organic pollutants, respectively, and were experimentally determined because RNA samples not meeting these criteria yielded irreproducible results with relatively high Ct values (data not shown). Additionally, only RNA samples for which absence of DNA could be ascertained using a RNA qPCR test were further processed.
Two-step real-time RT-PCR
One microgram of each DNA-free RNA sample was reverse-transcribed to cDNA by using an oligo(dT)18 primer and the RevertAid H Minus First Strand cDNA Synthesis Kit (Fermentas, St-Leon Rot, Germany) according to the manufacturer’s instructions. Real-time RT-PCR was performed on the Corbett Rotor-Gene 3000 (Qiagen, Hilden, Germany) using the SYBR Green I technology. In a total volume of 25 μl, the master mix containing 1 × ABsolute™ QPCR SYBR® Green Mix (Thermo Scientific, Epsom, UK), 150 nM of each specific sense and anti-sense primers (Table 2), and 125 ng λ-DNA (Roche Diagnostics, Vilvoorde, Belgium) was mixed with 2 μl of a 50 × diluted template cDNA, control gDNA or water. λ-DNA was added as carrier DNA to minimize absorption and Poisson effects. The following amplification program was used: polymerase activation at 95 °C for 15 min, followed by 45–50 cycles of 95 °C for 15 s, 52–62 °C for 20 s, and 72 °C for 20 s, with a final elongation at 79–81 °C for 15 s. The fluorescence measurement was performed at a temperature of 79–81 °C. To verify the specificity of the amplicon for each primer pair, a melting curve was produced from 55 to 95 °C at the end of each RT-qPCR run. A minimum of five samples from each run were analyzed by agarose gel electrophoresis to verify that the product was a single band of the correct size. A standard curve of six serial four-fold dilutions of pooled cDNA, a no-template control, and the cDNA samples each with two technical replicates were always run concurrently in each assay. The cycle fit-point or threshold Ct was determined for each PCR reaction. When the values of the duplicated samples differed by more than 0.5 cycles, the measurements were repeated or discarded. The incidence of such a difference was rather rare (on average less than one sample per run of 72 samples) irrespective of the experiment or combination of reference genes. Real-time PCR efficiency was determined for each gene by using the slope of a linear regression model of the dilution series [E = 10(−1/slope)] (Pfaffl 2001; Rasmussen 2001) (Table 2). All PCR reactions displayed a correlation coefficient R 2 of above 0.98. The Ct values were imported into Microsoft Excel for further analysis.
Table 2.
Selected candidate reference genes, primers, annealing temperatures, amplicon lengths, and actual amplification efficiencies
| Gene | Primers | Sequence | Annealing temp. (°C)a | Amplicon length (bp) | E (±SD)b |
|---|---|---|---|---|---|
| ACT11 | act11-F3 | CCCAAGGCAAACCGAGAGAAG | 60 | 150 | 1.00 (0.031) |
| act11-R2 | GTGGCTCACACCATCACCAG | ||||
| ACT | act-1 | GAGAAGATACAGTGTCTGGA | 52 | 231 | 0.88 (0.073) |
| act-2 | ATTACCATCGAAATATTAAAAG | ||||
| EF1 | EF1-F2 | CGGAGCGTGAAAGAGGAAT | 62 | 185 | 0.99 (0.069) |
| EF1-R2 | ACCAGCTTCAAAACCACCAG | ||||
| L2 | L2-F2 | AGGGTTCATAGCCACACCAC | 61 | 100 | 1.00 (0.064) |
| L2-R2 | CCGAACTGAGAAGCCCCTAC | ||||
| 25S | 25S-1 | ACATTGTCAGGTGGGGAGTT | 59 | 106 | 0.79 (0.053) |
| 25S-2 | CCTTTTGTTCCACACGAGATT | ||||
| TUB | tub-F1 | TGTTGCATCCTGGTACTGCT | 61 | 112 | 0.98 (0.032) |
| tub-R1 | GGCTTTCTTGCACTGGTACAC |
aAs determined by gradient PCR
bEfficiency of PCR amplification (±standard deviation)
Analysis of the data
The Ct values were converted into relative quantities or expression levels according to the data obtained for the samples of the dilutions series, which are used to create standard curves. Next, the reference gene stability factor (M), defined as the average pair-wise variation between a particular reference gene and all of the other candidate reference genes, was determined using geNorm v3.4 (Vandesompele et al. 2002). Additionally, the same values used as input data for geNorm were analyzed using the NormFinder algorithm (Andersen et al. 2004). Grouping of samples for Normfinder analyses was done according to the treatments described above (with or without acetone, different developmental time points, different varieties, with or without M. fijiensis inoculation, different sucrose treatments, and different varieties for the in vitro, GH development, GH varieties, leaf discs, meristem sucrose and meristem varieties experiment, respectively). ANOVA was used to determine whether differences in the Ct levels between the different experimental treatments within each experiment were significant.
Results
Selection of candidate reference genes and primer design
Reference genes commonly used for other plant species were investigated to identify genes displaying highly uniform expression patterns in different varieties, tissues, developmental stages, and stress conditions for the non-model crop Musa (banana and plantains). Nine genes from different functional groups were chosen: 18S rRNA, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), elongation factor-1α (EF1), polyubiquitin, actin11 (ACT11), α-tubulin, β-tubulin (TUB), cyclophilin, and ribosomal protein L2 (L2) genes. Banana genes and EST fragments belonging to these gene families were identified by conducting similarity searches (BlastX). The identity of the coding sequence between Arabidopsis or rice and Musa varied between 80 and 97 % (Electronic Supplementary Material 1). At the time this study was performed, no orthologous Musa sequences of sufficient length could be identified for GAPDH, 18S rRNA, and α-tubulin.
Primer pairs were designed for the ACT11, cyclophilin, EF1, L2, TUB, and polyubiquitin genes (Electronic Supplementary Material 1). Using data from other plant species, a primer pair spanning an intron was designed for ACT11; this was not possible for the other genes. To ensure that each primer pair resulted in the production of a single PCR product, gradient PCR was performed on genomic DNA (gDNA) and on cDNA from leaves. For ACT11, EF1, L2, and TUB, a suitable primer pair was identified (Table 2; Electronic Supplementary Material 1). Different primer pairs for the cyclophilin and polyubiquitin genes were designed, butno product and multiple bands, respectively, were observed (Electronic Supplementary Material 1). Additionally, 25S rRNA (25S) and actin (ACT) genes that have been previously used in other banana gene expression studies (Mbeguie-Mbeguie et al. 2007; van den Berg et al. 2007) were included in our analyses. Gradient PCR was performed using previously published primer sequences and the production of one PCR product was confirmed (Electronic Supplementary Material 1). Next, the optimal primer concentration was determined for each primer pair during the first RT-qPCR analysis. Primer concentrations that resulted in the lowest threshold cycles (Ct) along with minimal primer dimers were selected and corresponded to 150 nM for all primers. An overview of the selected primers for RT-qPCR and the expected amplicon sizes is given in Table 2.
Expression analysis
The expression levels of our candidate reference genes were determined in six different experimental set-ups using a total of 78 samples (Table 1). The different types of plant materials analyzed were leaves from in vitro and greenhouse plants, leaf discs from greenhouse plants, and in vitro meristem cultures. Samples were obtained from multiple varieties and exposed to various biotic and abiotic stress conditions (Table 1). The stability of the candidate reference gene expression was examined at the transcript level by RT-qPCR and the results were analyzed using standard statistical analysis and publicly available algorithms NormFinder and geNorm. Within a single experiment, aliquots of the same cDNA synthesis reaction were used for RT-qPCR amplification of all candidate reference genes.
Analysis of candidate reference genes in leaf tissue of in vitro plants
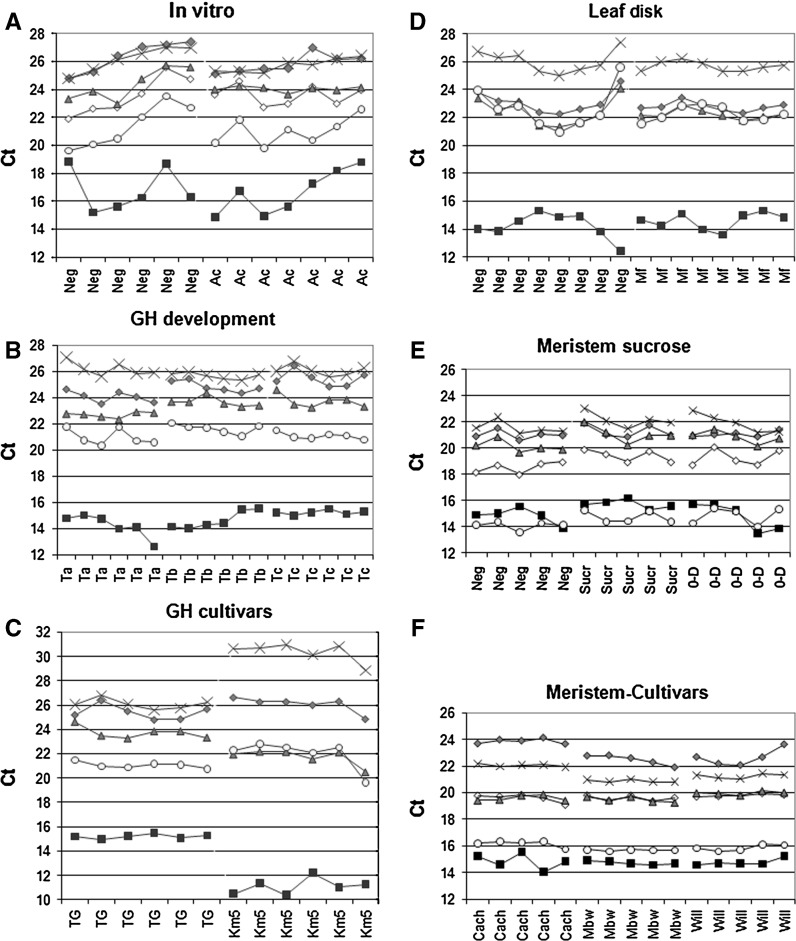
The leaves of each of the six and seven plants grown on the REG medium without and with 0.5 % (v/v) acetone, respectively, were pooled and used for RNA isolation and cDNA synthesis. The Ct values of the six different candidate reference genes exhibited broad variability between the samples, irrespective of the acetone treatment (from 2.3 for L2 up to 4.0 for 25S; Fig. 1a). Firstly, within-group variation was analyzed using ANOVA and showed that the Ct values of the reference genes were not significantly different between the samples obtained from plants grown on the medium with acetone and those grown without acetone (ANOVA, p > 0.05).
Fig. 1.
Transcriptional profiles of candidate reference genes expressed as absolute Ct values. For each sample group, about 5–8 biological replicates were analyzed. The following reference genes were tested: 25S 25S rDNA (filled square), ACT actin (filled diamond), ACT11 actin11 (open diamond), TUB β-tubulin (filled triangle), L2 ribosomal protein L2 (multiplication sign), and EF1 elongation factor-1α (filled circle). a Leaf tissue from in vitro cultured plants grown in a medium containing 0.5 % (v/v) acetone (Ac) or control medium without acetone (Neg). b Leaf tissue of greenhouse plants sampled at three different time points (Ta samples were harvested 6 months after transfer of the plants to the greenhouse, and Tb and Tc samples were harvested 16 and 26 days later, respectively). c Leaf tissue of greenhouse plants of the varieties Tuu Gia (TG) and Yangambi Km5 (Km5) at Tc. d Leaf discs inoculated with M. fijiensis conidia (Mf) and control leaf discs (Neg). e Meristems cut on day 0 and subsequently either placed on control medium on day 4 and harvested on day 6 (Control samples; Neg) or placed on high sucrose medium on day 4 and harvested on day 6 (Sucrose samples; Sucr) or simply harvested on day 1 (cut samples; 0-D). f Meristems from the varieties Cachaco (Cach), Mbwazirume (Mbw), and Williams (Will) all harvested 6 days after the last cutting and subcultured on the control medium
Secondly, NormFinder was used to determine the stability of the different reference genes. This software ranks candidate reference genes according to their expression stability in an experiment (Andersen et al. 2004). NormFinder can consider the different treatment/sample groups by using a grouping function. The ranking obtained by NormFinder analysis with or without the grouping function as summarized in Table 3 was 25S—EF1—ACT11—ACT—TUB—L2 from least to most stable reference gene, with the best combination being TUB and L2.
Table 3.
Stability of candidate reference genes calculated by NormFinder for the six experiments analyzed
| In vitroa | GH developmentb | GH varietiesc | Leaf discs | Meristem sucrose | Meristem varieties | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grouping | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No |
| 25S | 0.353 | 0.861 | 0.275 | 0.401 | 1.453 | 1.606 | 0.290 | 0.902 | 0.229 | 0.469 | 0.180 | 0.224 |
| EF1 | 0.176 | 0.433 | 0.278 | 0.306 | 0.458 | 0.228 | 0.177 | 0.513 | 0.150 | 0.269 | 0.056 | 0.096 |
| TUB | 0.112 | 0.293 | 0.280 | 0.339 | 0.811 | 0.739 | 0.022 | 0.092 | 0.125 | 0.118 | 0.193 | 0.216 |
| L2 | 0.097 | 0.239 | 0.327 | 0.373 | 1.530 | 1.840 | 0.024 | 0.065 | 0.141 | 0.276 | 0.205 | 0.235 |
| ACT | 0.136 | 0.339 | 0.242 | 0.289 | 0.312 | 0.228 | 0.019 | 0.065 | 0.163 | 0.228 | 0.307 | 0.339 |
| ACT11 | 0.145 | 0.398 | ND | ND | ND | ND | ND | ND | 0.148 | 0.288 | 0.207 | 0.201 |
aLeaf tissue
bGreenhouse development, leaf tissue
cGreenhouse varieties, leaf tissue
Bold the most stable gene; underlined the best reference gene pair. ND not determined
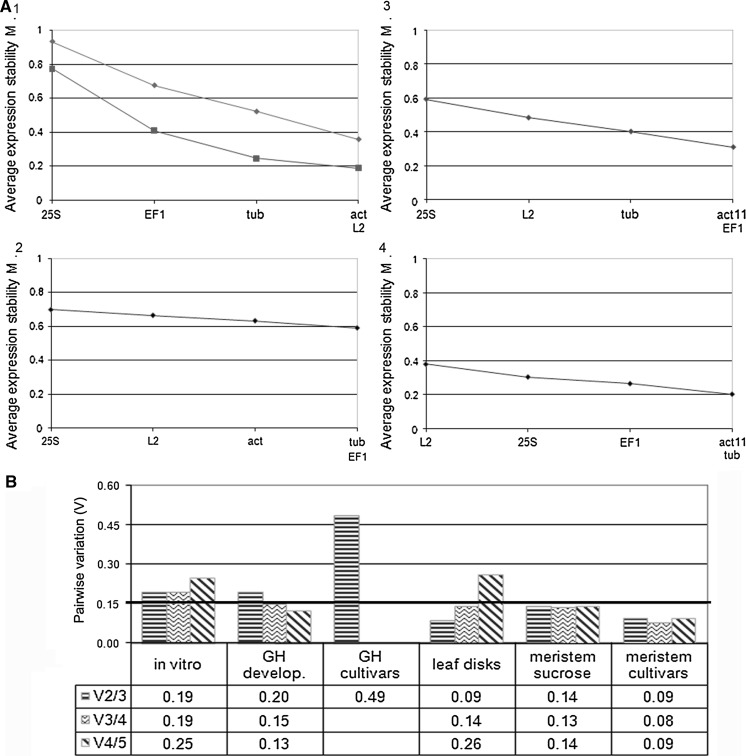
Subsequently, the geNorm software was employed to determine the stability of the different reference genes (Vandesompele et al. 2002). This program calculates the average expression stability value (M) of each reference gene as the average pair-wise variation (V) between a particular reference gene and all other reference genes (Fig. 2). First, geNorm analysis was performed using all reference genes, which clearly revealed that ACT11 was less stable than ACT (data not shown). This result was confirmed by the Ct values obtained, as ACT11 (ΔCt = 3.6) showed larger variability than ACT (ΔCt = 2.6), and by Normfinder analysis (Table 3). Since it is recommended to include only one reference gene per biological pathway in geNorm analysis (Vandesompele et al. 2002), the data from the ACT11 reference gene were discarded for further analysis using the geNorm algorithm. For the in vitro leaf samples, all reference genes exhibited an M-value lower than the default threshold of 1.5, indicating that they were suitable for further geNorm analysis. The most stable reference genes were ACT and L2 and the least stable genes were EF1 and 25S (Fig. 2a-1). The geNorm algorithm also determines the pair-wise variation (Vn/Vn+1), a measure that is used to determine how many additional reference genes should be included in the calculation of the normalization factor for gene expression. A cut-off V-value of 0.15, below which the inclusion of additional reference genes is not required, has been proposed by Vandesompele et al. (2002). For the in vitro plant samples, the best combination is ACT and L2 (Fig. 2a-1) but it is not adequate with a V-value of 0.19 (Fig. 2b, V2/3), and the addition of one (V3/4) or two (V4/5) reference genes resulted in even higher V-values (Fig. 2B). Thus, no suitable combination of reference genes could be identified for these samples.
Fig. 2.
Expression stability and variation analyses of the candidate reference genes by geNorm. a Average expression stability (M) and ranking of the candidate reference genes. The lower average expression stability M indicates a more stable expression. Experiments: (1) In vitro (filled diamond), leaf discs (filled square), (2) Greenhouse development, (3) meristem sucrose, (4) meristem varieties. b Pair-wise variation (V) analysis of the candidate reference genes. This analysis was conducted to determine the optimal number of reference genes required for normalization. Six experimental set-ups were included in the analysis: in vitro, greenhouse (GH) development, GH varieties, leaf discs, meristem sucrose, and meristem varieties. A cut-off V-value of 0.15, below which the inclusion of additional reference genes is not required, has been proposed by Vandesompele et al. (2002) and is indicated by a bold line. For the greenhouse development experiment, two reference genes had an M value above 1.5 and only three genes were used to calculate the V pair-wise variation and therefore V3/4 and V4/5 could not be calculated. Abbreviations: ACT11: actin11; ACT: actin; EF1: elongation factor-1α; L2: ribosomal protein L2; 25S: 25S rRNA
Analysis of candidate reference genes in greenhouse leaves harvested at different developmental stages and from different varieties
RNA was isolated from leaf samples of six Tuu Gia plants at three different time points (Ta, Tb, and Tc) (Fig. 1b). Additionally, for the last time point (Tc), six samples of variety Yangambi Km5 were also harvested (Fig. 1c). The ACT11 reference gene was not included in these experiments as the experiments described above indicated that ACT11 is less stable than ACT in leaf tissue. Within-group variation was analyzed by ANOVA and showed that the Ct values of all the reference genes except L2 exhibited statistically significant differences (ANOVA, p < 0.05) between leaf samples harvested at different time points, although the differences between the average Ct values of the different groups were smaller than 1.05 Ct except for ACT with a difference of 1.4 between time points Ta and Tc. In contrast, the Ct values for 25S, L2, and TUB showed significant differences between the two varieties and the Ct differences between the average values for L2, 25S, and TUB in the two different varieties is 4.3, 4.1, and 2.0 Ct’s, respectively (Fig. 1c).
For the greenhouse development experiment, NormFinder indicated that ACT is the most stable reference gene, and subsequently 25S, EF1, TUB, L2 and EF1, TUB, L2, 25S with and without grouping function, respectively (Table 3). NormFinder indicated that the most optimal reference gene combination was ACT and EF1. For the greenhouse variety experiment NormFinder indicated that ACT was the most stable reference gene followed by EF1, TUB, 25S, and L2 and the optimal combination is 25S and L2, which are the two reference genes with more than 4 Ct’s difference between the two varieties. GeNorm expression stability analyses of all leaf samples from different time points revealed that all reference genes had an M-value lower than the default threshold of 1.5 (Fig. 2a-2), while for the leaf samples from the two different varieties only ACT, TUB, and EF1 had a M-value below 1.5 (data not shown), thus excluding the other reference genes from further analysis. For the greenhouse development experiment, the most stable reference genes were TUB and EF1 and the least stable genes were 25S and L2 (Fig. 2a-2). The pair-wise variation analysis indicated that the use of at least three genes (EF1, TUB, and ACT) was optimal as the V-value of 0.15 was obtained (Fig. 2b), which is very similar to the ranking obtained by NormFinder without grouping function. For the variety experiment, no combination resulted in a V-value below 0.15 (Fig. 2B).
Analysis of reference genes in samples harvested from leaf discs
The Ct variation for samples obtained from the eight leaf discs inoculated with M. fijiensis was lower than that of the eight control leaf discs (Fig. 1d) and no statistically significant differences between treatment groups were observed.
NormFinder identified ACT as the most stable reference gene and subsequently TUB and L2, although for TUB and L2 the order interchanged depending on whether the grouping factor was used or not. 25S and EF1 were the least stable reference genes (Table 3). The optimal combination according to NormFinder is ACT and TUB. During geNorm analysis, all reference genes showed an M-value lower than the default threshold of 1.5. The most stable reference genes identified by geNorm were ACT and L2 and the least stable gene was 25S (Fig. 2A-1). The pair-wise variation analysis indicated that the use of two reference genes (ACT and L2) was sufficient as the combination yielded a V-value of 0.09 (Fig. 2b). Inclusion of one additional gene (TUB) was possible as this combination yielded a V-value of 0.14 (Fig. 2b). For this experiment, geNorm and Normfinder without grouping function yield an identical ranking of the most stable reference genes.
Analysis of reference genes in shoot meristem cultures
For the sucrose (osmotic stress) experiment, RNA was isolated from five meristems grown either on control medium or on a medium containing a higher sucrose concentration. Additionally, a third group of meristems was harvested 24 h after subculturing to investigate the effect of wounding associated with the cutting and subculturing process. The Ct values of the six different reference genes exhibited the largest variation for 25S (ΔCt = 2.7) and the least variation for ACT (ΔCt = 1.3; Fig. 1e). Differences between the Ct values of the three groups of samples were statistically different for ACT11 and TUB (ANOVA, p < 0.05) although the differences between the average Ct values were less than 1 Ct. For the variety experiment, five meristems of Cachaco, Mbwazirume, and Williams grown under standard conditions on the P4 medium were harvested. The Ct value showed the maximum variation for ACT (ΔCt = 2.2) and the least variation for EF1 (ΔCt = 0.7) over all three varieties (Fig. 1f). Statistical differences between the samples of the different varieties were observed for ACT, L2, TUB, and EF1, with the largest difference in average Ct values observed between the three varieties of 1.4, 1.2, 0.5, and 0.5, respectively.
For the meristem sucrose experiment, NormFinder analysis showed that TUB was the most stable reference gene followed by L2, ACT11, EF1, and ACT, although the order interchanged depending on the grouping factor. 25S was the least stable reference gene (Table 3). For the meristem varieties experiment, EF1 was the most stable reference gene and subsequently 25S, TUB, L2, and ACT11, although the order interchanged depending on the Normfinder grouping factor here as well. ACT was the least stable reference gene (Table 3). NormFinder indicated ACT/TUB and ACT11/L2 as the optimal reference gene combinations for the sucrose and varieties experiments, respectively. From Fig. 1e, f and the Normfinder analyses (Table 3), it is clear that ACT exhibited as much or more variation than ACT11. Therefore, for meristem samples, ACT was not included in the geNorm analysis. Using all samples from the sucrose and varieties experiments, geNorm analysis resulted in M-values below the default threshold 1.5 for all reference genes and the most stable reference genes were ACT11/EF1 and ACT11/TUB, respectively (Fig. 2a-3, -4, respectively). Finally, the pair-wise variation analysis indicated that the use of two genes was sufficient as a V-value of 0.14 (ACT11 and EF1) and 0.09 (ACT11 and TUB) was obtained for the samples of the sucrose and varieties experiments, respectively (Fig. 2b). These genes were also among the three most stable reference genes as identified by the Normfinder analysis without grouping function in each experiment.
Discussion
Quantitative RT-PCR is one of the most commonly applied methods for the analysis of mRNA expression levels, because of its accuracy and sensitivity. Recent studies have clearly advocated the use of multiple suitable reference genes for normalization of sample gene expression (Bustin 2002; Gutierrez et al. 2008b; Huggett et al. 2005; Vandesompele et al. 2002) and have recommended a thorough assessment of these reference genes for expression stability. Screening multiple reference genes allows distinction between variations in the amount of cDNA input and variations in gene expression. Suitable reference genes for normalization are often selected using software programs such as geNorm (Vandesompele et al. 2002) and NormFinder (Andersen et al. 2004). Recent studies have indicated that the traditional reference genes are not always stably expressed in different species, tissues, and experimental treatments (Artico et al. 2010). For example, in Arabidopsis the reference genes coding for actin, tubulin, ubiquitin, and elongation factor showed high variability (Gutierrez et al. 2008a), confirming the need for the assessment of even traditional reference genes in a specific species and under relevant environmental treatments. A recent study in banana by Chen et al. (2011) also strongly suggests that a thorough validation of the stability of candidate reference genes under specific experimental conditions is required.
Musa is a non-model plant with limited sequence information available, and thus a limited number of candidate reference genes. Therefore, in this study, we selected candidate reference genes for which such sequence information was publicly available. RT-qPCR protocols were developed for four different reference genes (ACT11, EF1, L2, and TUB) as well as two previously reported reference genes in Musa (ACT and 25S RNA). Recently, Chen et al. (2011) selected 18 candidate reference genes from a proprietary banana transcriptome sequence database. The stability of expression of these genes and that of two additional genes from publicly available sequences was analyzed in six sample sets, all originating from the Cavendish dessert banana. None of these genes was researched in the present investigation although members of the same gene families were analyzed in both studies (actin, elongation factor 1α, ribosomal protein L, and tubulin). Due to a non-specific amplification we did not process the ubiquitin gene, while Chen et al. (2011) validated the usefulness of the UBQ2 gene despite a similar problem of non-specific amplification.
Factors known to affect the reliability of gene expression data such as RNA quality, DNase I treatment, two-step RT-qPCR, PCR efficiency, and non-specific amplification were controlled (Derveaux et al. 2010; Maroufi et al. 2010). Of the two reference genes that were annotated as actin genes, only the gene exhibiting the lowest level of Ct variation in the samples was retained for further analysis with the geNorm algorithm. Hence, for in vitro leaf samples, ACT11 was excluded from the geNorm analyses, whereas for meristem samples these analyses were executed without ACT. Similarly, in fruit tissues of dessert banana, differences were seen in the expression stability of the different actin genes analyzed (Chen et al. 2011).
Our study showed that the expression levels of all reference genes investigated exhibited high Ct variability in the leaf samples of the Musa plants grown in vitro. No significant Ct differences between the control group and the acetone-treated group were identified. NormFinder identified L2 as the most stable reference gene and subsequently TUB. Furthermore, the software analysis revealed that the combination of these two reference genes would give the most reliable gene expression outcome. The geNorm algorithm indicated that the combination of the ACT and L2 reference genes is preferred although it is not sufficient to normalize gene expression levels in these in vitro leaf samples. Moreover, the use of additional reference gene(s) resulted in even more unacceptable reference gene combinations for normalization, indicating that suitable reference genes for in vitro gene expression studies are scarce. These results also suggest that plants grown in vitro might be stressed and show variable expression levels of genes involved in basic biological processes. Analysis of the expression of genes of interest in such samples is thus difficult and requires careful examination of candidate reference genes prior to any analysis.
For greenhouse leaf samples harvested at different developmental stages and for leaf discs, the geNorm analysis demonstrated that the combinations EF1/TUB/ACT and L2/ACT, respectively, allow reliable normalization despite the occurrence of significant Ct differences between different sample groups in the former for all but one (L2) reference gene. NormFinder analyses resulted in similar results and indicated that the combinations ACT/EF1 and ACT/TUB are optimal for normalization of leaf samples at different developmental stages and leaf discs, respectively. For leaf samples from different varieties the ANOVA indicated significant differences for L2, 25S, and TUB with large differences (ΔCt > 4.0) in average Ct’s for L2 and 25S, which were both excluded from the geNorm analysis, resulting in the inability to identify a suitable combination of reference genes. NormFinder identified ACT as most stable and L2 and 25S as least stable reference genes, but surprisingly indicated L2 and 25S as the most suitable reference gene pair. A glance at the raw Ct’s shows that these genes are relatively stable within each variety, but the level of L2 and 25S is ±4 Ct’s higher and ±4 Ct’s lower, respectively, in Km5 than in TG (Fig. 1). The recent reference gene validation study in Cavendish banana by Chen et al. (2011) mostly dealt with fruit tissues (141 out of 144 samples). The only tissue common to this study and our study is leaf tissue, but it was isolated from mature plants in the field whereas we sampled in vitro and greenhouse plants. For the sample set examining different tissues including three leaf samples, ACT2 was the third most stable gene and the preferential pair included a GTP-binding nuclear protein encoding gene and a ribosomal protein 2 gene (Chen et al. 2011).
In meristem cultures a different set of reference genes seemed more stable than in leaf samples, although some statistically significant Ct differences between the sample groups were observed in these tissue samples as well. The geNorm algorithm yielded multiple reference gene combinations useful for both the sucrose and varieties experiments. The minimum suitable combinations for gene expression normalization are ACT11/EF1 and ACT11/TUB, respectively. Alternatively, NormFinder proposed TUB/ACT and L2/ACT11 as the most suitable reference gene combinations for the sucrose and varieties experiments, respectively.
Numerous reference gene expression studies have used both geNorm and NormFinder (Barsalobres-Cavallari et al. 2009; Chen et al. 2011; Cruz et al. 2009; Exposito-Rodriguez et al. 2008; Hong et al. 2008; Hu et al. 2009; Lee et al. 2010; Maroufi et al. 2010; Paolacci et al. 2009; Remans et al. 2008) and reported limited variation in stability ranking by these software tools, whereas other studies have reported significantly different results depending on the sofware (Lin and Lai 2010; Paolacci et al. 2009; Schmidt and Delaney 2010). These variations stem from differences between geNorm and NormFinder in the mathematical approaches used to calculate expression stability. We have noted that without grouping function, the rankings by NormFinder and geNorm were more consistent than when using the grouping function of Normfinder. GeNorm determines the stability of the candidate reference gene against that of all other candidate reference genes under investigation by pair-wise comparison of variation of expression ratios. One of the drawbacks of geNorm is that it is sensitive to co-regulation (Vandesompele et al. 2002), which is why it is important to use reference genes involved in different biological processes. Further, geNorm identifies the appropriate number of reference genes for accurate normalization, whereas NormFinder selects two genes with minimal combined inter- and intra-group expression variation to take into account systematic differences between sample subgroups. Our results confirm the observation of Rytkönen et al. (2010) that ANOVA tests in some cases indicate statistically significant differences between sample groups for reference genes that were ranked by NormFinder and/or geNorm as the most stable candidate genes. From the greenhouse leaf variety experiment and its Normfinder analysis it becomes clear that the use of reference genes showing significant different expression levels in different varieties might still be considered suitable when used in combination. However, it should be noted that geNorm analysis of these samples failed to identify a suitable reference gene combination.
The expression levels of some of the reference genes investigated clearly differed between banana varieties tested. Nevertheless, for most of these genes the level of stability seemed similar across different varieties. This suggests that reference genes validated in one banana variety might be suitable candidates in other banana varieties, but this should always be confirmed prior to expression studies of genes of interest. Based on our results, we propose the use of ACT, TUB, and EF1 for reliable normalization of gene expression in banana leaf samples and multiple combinations of TUB, ACT, ACT11, EF1, and L2 for gene expression studies in banana meristem cultures. Similarly, Chen et al. (2011) concluded that each experimental condition tested demands a specific set of reference genes, since for the six banana sample sets analyzed six different pairs of optimal reference genes were identified. Graeber et al. (2011) concluded that for different Brassicaceae species the reference gene expression stability is higher for a given developmental process between distinct species than for distinct developmental processes within a given single species.
Of the candidate reference genes evaluated in this study, ACT and 25S have been used previously as single “controls” in banana (Mbeguie-Mbeguie et al. 2007; van den Berg et al. 2007). More specifically, the reference gene ACT was used as the control gene in an experiment investigating changes in gene expression during fruit development (Mbeguie-Mbeguie et al. 2007). In another study involving Fusarium wilt-infected roots, the 25S gene was used for normalization of gene expression (van den Berg et al. 2007). Neither of these studies reported on the stability of the reference gene under the experimental conditions investigated. This information is also lacking in the expression studies of the newly discovered banana dehydrin gene (Shekhawat et al. 2011) and MADS-box genes (Elitzur et al. 2010), although two reference genes were included in these reports (ACT/EF1α and a ribosomal RNA gene/GAPDH, respectively). In our study, ACT was found to be one of the most stable reference genes whereas Chen et al. (2011) revealed that the selected banana actin genes were not within the preferential pair for five of the six experimental conditions. The results presented here also showed that the 25S gene was relatively unstable in leaf tissues. This study confirms that multiple reference genes should be screened for each tissue type and stress condition. The identification of reliable reference genes is time-consuming and expensive but at the same time necessary for accurate gene expression analyses. The present study provides a strong set of candidate reference genes for researchers working on Musa gene expression in leaf and meristem tissues from different banana varieties and complements the study of Chen et al. (2011) that mainly deals with fruit tissues.
In summary, this is a detailed study aimed at validating candidate reference genes for the quantification of transcript levels in various banana varieties under different experimental conditions and in different non-fruit tissues. Identification of suitable reference genes for normalization is indeed challenging in the case of some tissues and conditions. In our study, this was the case for in vitro leaf samples. We recommend classical reference genes, namely EF1, ACT, and TUB, and appropriate primer sequences as references for normalization in expression studies in leaves of greenhouse plants, ACT and L2 for leaf discs, and we advocate the use of combinations of TUB, ACT/ACT11, and EF1 for expression studies in meristems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
IMH and SR were supported by the Bijzonder Onderzoeksfonds and the Industrieel Onderzoeksfonds of K.U.Leuven, respectively. The authors thank Jean-Pierre Busogoro and Gabriella Kovács for practical advice on the leaf disc assay. Technical assistance by Hien Do and Els Thiry is much appreciated. Access to the Syngenta Musa 3′ EST database, donated by Syngenta to Bioversity International for use within the framework of the Global Musa Genomics Consortium, is acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
Nancy Podevin and An Krauss have contributed equally to this work.
Nancy Podevin: The views and positions expressed in this work are solely those of the author, who acts in his personal capacity, and do not necessarily represent the views of the EFSA or of the European Union.
Contributor Information
Nancy Podevin, Email: nancy.podevin@gmail.com.
An Krauss, Email: ann_krauss@yahoo.com.
Isabelle Henry, Email: imhenry@ucdavis.edu.
Rony Swennen, Email: Rony.Swennen@biw.kuleuven.be.
Serge Remy, Email: Serge.Remy@biw.kuleuven.be.
References
- Abadie C, Zapater MF, Pignolet L, Carlier J, Mourichon X. Artificial inoculation on plants and banana leaf pieces with Mycosphaerella spp., responsible for Sigatoka leaf spot diseases. Fruits. 2008;63(5):319–323. doi: 10.1051/fruits:2008030. [DOI] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64(15):5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Artico S, Nardeli SM, Brilhante O, Grossi-de-Sa MF, Alves-Ferreira M. Identification and evaluation of new reference genes in Gossypium hirsutum for accurate normalization of real-time quantitative RT-PCR data. BMC Plant Biol. 2010;10:49. doi: 10.1186/1471-2229-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalobres-Cavallari CF, Severino FE, Maluf MP, Maia IG. Identification of suitable internal control genes for expression studies in Coffea arabica under different experimental conditions. BMC Mol Biol. 2009;10:1. doi: 10.1186/1471-2199-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner AM, Yakovlev IA, Strauss SH. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004;4:14. doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29(1):23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Carpentier SC, Coemans B, Podevin N, Laukens K, Witters E, Matsumura H, Terauchi R, Swennen R, Panis B. Functional genomics in a non-model crop: transcriptomics or proteomics? Physiol Plant. 2008;133(2):117–130. doi: 10.1111/j.1399-3054.2008.01069.x. [DOI] [PubMed] [Google Scholar]
- Castro P, Román B, Rubio J, Die J (2011) Selection of reference genes for expression studies in Cicer arietinum L.: analysis of cyp81E3 gene expression against Ascochyta rabiei. Mol Breed 27. doi:10.1007/s11032-11010-19544-11038
- Chen L, H-y Zhong, J-f Kuang, Li J-g, Lu W-j, Chen J-y. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta. 2011;234(2):377–390. doi: 10.1007/s00425-011-1410-3. [DOI] [PubMed] [Google Scholar]
- Coemans B, Matsumura H, Terauchi R, Remy S, Swennen R, Sagi L. SuperSAGE combined with PCR walking allows global gene expression profiling of banana (Musa acuminata), a non-model organism. Theor Appl Genet. 2005;111(6):1118–1126. doi: 10.1007/s00122-005-0039-7. [DOI] [PubMed] [Google Scholar]
- Cordoba EM, Die JV, Gonzalez-Verdejo CI, Nadal S, Roman B. Selection of reference genes in Hedysarum coronarium under various stresses and stages of development. Anal Biochem. 2011;409(2):236–243. doi: 10.1016/j.ab.2010.10.031. [DOI] [PubMed] [Google Scholar]
- Cruz F, Kalaoun S, Nobile P, Colombo C, Almeida J, Barros LMG, Romano E, Grossi-de-Sa MF, Vaslin M, Alves-Ferreira M. Evaluation of coffee reference genes for relative expression studies by quantitative real-time RT-PCR. Mol Breed. 2009;23(4):607–616. doi: 10.1007/s11032-009-9259-x. [DOI] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139(1):5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepak SA, Kottapalli KR, Rakwal R, Oros G, Rangappa KS, Iwahashi H, Masuo Y, Agrawal GK. Real-time PCR: revolutionizing detection and expression analysis of genes. Curr Genomics. 2007;8(4):234–251. doi: 10.2174/138920207781386960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derveaux S, Vandesompele J, Hellemans J. How to do successful gene expression analysis using real-time PCR. Methods. 2010;50(4):227–230. doi: 10.1016/j.ymeth.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Dheda K, Huggett JF, Chang JS, Kim LU, Bustin SA, Johnson MA, Rook GA, Zumla A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal Biochem. 2005;344(1):141–143. doi: 10.1016/j.ab.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Die JV, Roman B, Nadal S, Gonzalez-Verdejo CI. Evaluation of candidate reference genes for expression studies in Pisum sativum under different experimental conditions. Planta. 2010;232(1):145–153. doi: 10.1007/s00425-010-1158-1. [DOI] [PubMed] [Google Scholar]
- Dombrowski JE, Martin RC. Evaluation of reference genes for quantitative RT-PCR in Lolium temulentum under abiotic stress. Plant Sci. 2009;176(3):390–396. doi: 10.1016/j.plantsci.2008.12.005. [DOI] [Google Scholar]
- Dong L, Sui C, Liu Y, Yang Y, Wei J, Yang Y. Validation and application of reference genes for quantitative gene expression analyses in various tissues of Bupleurum chinense. Mol Biol Rep. 2011;38(8):5017–5023. doi: 10.1007/s11033-010-0648-3. [DOI] [PubMed] [Google Scholar]
- Elitzur T, Vrebalov J, Giovannoni JJ, Goldschmidt EE, Friedman H. The regulation of MADS-box gene expression during ripening of banana and their regulatory interaction with ethylene. J Exp Bot. 2010;61(5):1523–1535. doi: 10.1093/jxb/erq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito-Rodriguez M, Borges AA, Borges-Perez A, Perez JA. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008;8:131. doi: 10.1186/1471-2229-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2009) FAOSTAT http://faostat.fao.org. Accessed 27 Nov 2011
- Gachon C, Mingam A, Charrier B. Real-time PCR: what relevance to plant studies? J Exp Bot. 2004;55(402):1445–1454. doi: 10.1093/jxb/erh181. [DOI] [PubMed] [Google Scholar]
- Garg R, Sahoo A, Tyagi AK, Jain M. Validation of internal control genes for quantitative gene expression studies in chickpea (Cicer arietinum L.) Biochem Biophys Res Commun. 2010;396(2):283–288. doi: 10.1016/j.bbrc.2010.04.079. [DOI] [PubMed] [Google Scholar]
- Global Musa Genomics Consortium (2011) www.musagenomics.org. Accessed 13 Feb 2011
- Goncalves S, Cairney J, Maroco J, Oliveira MM, Miguel C. Evaluation of control transcripts in real-time RT-PCR expression analysis during maritime pine embryogenesis. Planta. 2005;222(3):556–563. doi: 10.1007/s00425-005-1562-0. [DOI] [PubMed] [Google Scholar]
- Graeber K, Linkies A, Wood ATA, Leubner-Metzger G. A guideline to family-wide comparative state-of-the-art quantitative RT-PCR analysis exemplified with a Brassicaceae cross-species seed germination case study. Plant Cell. 2011;23(6):2045–2063. doi: 10.1105/tpc.111.084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenin S, Mauriat M, Pelloux J, Van Wuytswinkel O, Bellini C, Gutierrez L. Normalization of qRT-PCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. J Exp Bot. 2009;60(2):487–493. doi: 10.1093/jxb/ern305. [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Mauriat M, Guenin S, Pelloux J, Lefebvre JF, Louvet R, Rusterucci C, Moritz T, Guerineau F, Bellini C, Van Wuytswinkel O. The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol J. 2008;6(6):609–618. doi: 10.1111/j.1467-7652.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Mauriat M, Pelloux J, Bellini C, Van Wuytswinkel O. Towards a systematic validation of references in real-time RT-PCR. Plant Cell. 2008;20(7):1734–1735. doi: 10.1105/tpc.108.059774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez N, Gimenez MJ, Palomino C, Avila CM (2011) Assessment of candidate reference genes for expression studies in Vicia faba L. by real-time quantitative PCR. Mol Breed 28(1):13–24
- Hong SY, Seo PJ, Yang MS, Xiang F, Park CM. Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant Biol. 2008;8:112. doi: 10.1186/1471-2229-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SM, Bahn SC, Lyu A, Jung HS, Ahn JH. Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant Cell Physiol. 2010;51(10):1694–1706. doi: 10.1093/pcp/pcq128. [DOI] [PubMed] [Google Scholar]
- Hu R, Fan C, Li H, Zhang Q, Fu YF. Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol Biol. 2009;10:93. doi: 10.1186/1471-2199-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6(4):279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Iskandar HM, Simpson RS, Casu RE, Bonnett GD, Maclean DJ, Manners JM. Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression in sugarcane. Plant Mol Biol Rep. 2004;22(4):325–337. doi: 10.1007/BF02772676. [DOI] [Google Scholar]
- Israeli Y, Lahav E. Injuries to banana caused by adverse climate and weather. In: Jones DR, editor. Diseases of Banana, Abacá and Enset. Wallingford, UK: CAB International; 2000. pp. 351–374. [Google Scholar]
- Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun. 2006;345(2):646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- Jarosova J, Kundu JK. Validation of reference genes as internal control for studying viral infections in cereals by quantitative real-time RT-PCR. BMC Plant Biol. 2010;10:146. doi: 10.1186/1471-2229-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DR (2009) Disease and pest constraints to banana production. In: Proceedings of the international symposium on recent advances in banana crop protection for sustainable production and improved livelihoods, White River, South Africa, 10–14 September 2007. Acta Hort 828:21–36
- Kim BR, Nam HY, Kim SU, Kim SI, Chang YJ. Normalization of reverse transcription quantitative-PCR with housekeeping genes in rice. Biotechnol Lett. 2003;25(21):1869–1872. doi: 10.1023/A:1026298032009. [DOI] [PubMed] [Google Scholar]
- Lee JM, Roche JR, Donaghy DJ, Thrush A, Sathish P. Validation of reference genes for quantitative RT-PCR studies of gene expression in perennial ryegrass (Lolium perenne L.) BMC Mol Biol. 2010;11:8. doi: 10.1186/1471-2199-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Qin Y, Xiao X, Tang C. Screening of valid reference genes for real-time RT-PCR data normalization in Hevea brasiliensis and expression validation of a sucrose transporter gene HbSUT3. Plant Sci. 2011;181(2):132–139. doi: 10.1016/j.plantsci.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Libault M, Thibivilliers S, Bilgin D, Radwan O, Benitez M, Clough S, Stacey G. Identification of four soybean reference genes for gene expression normalization. Plant Genome. 2008;1:44–54. doi: 10.3835/plantgenome2008.02.0091. [DOI] [Google Scholar]
- Lilly ST, Drummond RSM, Pearson MN, MacDiarmid RM. Identification and validation of reference genes for normalization of transcripts from virus-infected Arabidopsis thaliana. Mol Plant Microbe Interact. 2011;24(3):294–304. doi: 10.1094/MPMI-10-10-0236. [DOI] [PubMed] [Google Scholar]
- Lin YL, Lai ZX. Reference gene selection for qPCR analysis during somatic embryogenesis in longan tree. Plant Sci. 2010;178(4):359–365. doi: 10.1016/j.plantsci.2010.02.005. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DeltaDeltaCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mallona I, Lischewski S, Weiss J, Hause B, Egea-Cortines M. Validation of reference genes for quantitative real-time PCR during leaf and flower development in Petunia hybrida. BMC Plant Biol. 2010;10(1):4. doi: 10.1186/1471-2229-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroufi A, Van Bockstaele E, De Loose M. Validation of reference genes for gene expression analysis in chicory (Cichorium intybus) using quantitative real-time PCR. BMC Mol Biol. 2010;11:15. doi: 10.1186/1471-2199-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia T, Santovito E, Gallitelli D, Cillo F. Evaluation of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in infected tomato plants. Mol Plant Pathol. 2010;11(6):805–816. doi: 10.1111/j.1364-3703.2010.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbeguie-Mbeguie D, Hubert O, Sabau X, Chillet M, Fils-Lycaon B, Baurens FC. Use of suppression subtractive hybridization approach to identify genes differentially expressed during early banana fruit development undergoing changes in ethylene responsiveness. Plant Sci. 2007;172(5):1025–1036. doi: 10.1016/j.plantsci.2007.02.007. [DOI] [Google Scholar]
- Migocka M, Papierniak A. Identification of suitable reference genes for studying gene expression in cucumber plants subjected to abiotic stress and growth regulators. Mol Breed. 2011;28(3):343–357. doi: 10.1007/s11032-010-9487-0. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nicot N, Hausman JF, Hoffmann L, Evers D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot. 2005;56(421):2907–2914. doi: 10.1093/jxb/eri285. [DOI] [PubMed] [Google Scholar]
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1(3):1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- Obrero A, Die JV, Roman B, Gomez P, Nadal S, Gonzalez-Verdejo CI. Selection of reference genes for gene expression studies in zucchini (Cucurbita pepo) using qPCR. J Agric Food Chem. 2011;59(10):5402–5411. doi: 10.1021/jf200689r. [DOI] [PubMed] [Google Scholar]
- OligoAnalyzer (2011) www.idtdna.eu/analyzer/Applications/OligoAnalyzer. Accessed 13 Feb 2011
- Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol. 2009;10(1):11. doi: 10.1186/1471-2199-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: bestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26(6):509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Primer3 (2011) http://frodo.wi.mit.edu/primer3/. Accessed 13 Feb 2011
- Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313(4):856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Rasmussen R. Quantification on the lightcycler. In: Meuer S, Wittwer C, Nakagawara K, editors. Rapid cycle real-time PCR, methods and applications. Heidelberg, Germany: Springer; 2001. pp. 21–34. [Google Scholar]
- Reid KE, Olsson N, Schlosser J, Peng F, Lund ST. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006;6:27. doi: 10.1186/1471-2229-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T, Smeets K, Opdenakker K, Mathijsen D, Vangronsveld J, Cuypers A. Normalisation of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta. 2008;227(6):1343–1349. doi: 10.1007/s00425-008-0706-4. [DOI] [PubMed] [Google Scholar]
- Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG, Phillips AL, Hedden P. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 2008;53(3):488–504. doi: 10.1111/j.1365-313X.2007.03356.x. [DOI] [PubMed] [Google Scholar]
- Rytkonen KT, Renshaw GMC, Ashton KJ, Williams-Pritchard G, Leder EH, Nikinmaa M. Elasmobranch qPCR reference genes: a case study of hypoxia preconditioned epaulette sharks. BMC Mol Biol. 2010;11:27. doi: 10.1186/1471-2199-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt GW, Delaney SK. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genomics. 2010;283(3):233–241. doi: 10.1007/s00438-010-0511-1. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46(1–2):69–81. doi: 10.1016/S0165-022X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285(2):194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- Shekhawat UKS, Ganapathi TR, Srinivas L. Cloning and characterization of a novel stress-responsive WRKY transcription factor gene (MusaWRKY71) from Musa spp. cv. Karibale Monthan (ABB group) using transformed banana cells. Mol Biol Rep. 2011;38(6):4023–4035. doi: 10.1007/s11033-010-0521-4. [DOI] [PubMed] [Google Scholar]
- Silveira ED, Alves-Ferreira M, Guimaraes LA, da Silva FR, Carneiro VTD. Selection of reference genes for quantitative real-time PCR expression studies in the apomictic and sexual grass Brachiaria brizantha. BMC Plant Biol. 2009;9:84. doi: 10.1186/1471-2229-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosse H, Schoofs H, Panis B, Andre E, Reyniers K, Swennen R. Development of embryogenic cell suspensions from shoot meristematic tissue in bananas and plantains (Musa spp.) Plant Sci. 2006;170(1):104–112. doi: 10.1016/j.plantsci.2005.08.007. [DOI] [Google Scholar]
- Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999;75(2–3):291–295. doi: 10.1016/S0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Thomas-Hall S, Campbell PR, Carlens K, Kawanishi E, Swennen R, Sagi L, Schenk PM. Phylogenetic and molecular analysis of the ribulose-1,5-bisphosphate carboxylase small subunit gene family in banana. J Exp Bot. 2007;58(10):2685–2697. doi: 10.1093/jxb/erm129. [DOI] [PubMed] [Google Scholar]
- Tong ZG, Gao ZH, Wang F, Zhou J, Zhang Z. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol Biol. 2009;10:71. doi: 10.1186/1471-2199-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, Bustin SA, Orlando C. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem. 2002;309(2):293–300. doi: 10.1016/S0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Tu LL, Zhang XL, Liu DQ, Jin SX, Cao JL, Zhu LF, Deng FL, Tan JF, Zhang CB. Suitable internal control genes for qRT-PCR normalization in cotton fiber development and somatic embryogenesis. Chin Sci Bull. 2007;52(22):3110–3117. doi: 10.1007/s11434-007-0461-0. [DOI] [Google Scholar]
- van Asten PJA, Fermont AM, Taulya G. Drought is a major yield loss factor for rainfed East African highland banana. Agric Water Manage. 2011;98:541–552. doi: 10.1016/j.agwat.2010.10.005. [DOI] [Google Scholar]
- van den Berg N, Berger DK, Hein I, Birch PRJ, Wingfield MJ, Viljoen A. Tolerance in banana to Fusarium wilt is associated with early up-regulation of cell wall-strengthening genes in the roots. Mol Plant Pathol. 2007;8(3):333–341. doi: 10.1111/j.1364-3703.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):RESEARCH0034 .1–research0034.11 [DOI] [PMC free article] [PubMed]
- Wan HJ, Zhao ZG, Qian CT, Sui YH, Malik AA, Chen JF. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal Biochem. 2010;399(2):257–261. doi: 10.1016/j.ab.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Wang YA, Wu J, Xu BY, Liu JH, Zhang JB, Jia CH, Jin ZQ. Cloning of an ADP-ribosylation factor gene from banana (Musa acuminata) and its expression patterns in postharvest ripening fruit. J Plant Physiol. 2010;167(12):989–995. doi: 10.1016/j.jplph.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hou S, Cui G, Chen S, Wei J, Huang L. Characterization of reference genes for quantitative real-time PCR analysis in various tissues of Salvia miltiorrhiza. Mol Biol Rep. 2010;37(1):507–513. doi: 10.1007/s11033-009-9703-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.