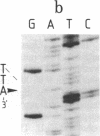
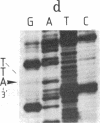
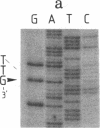
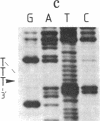
Abstract
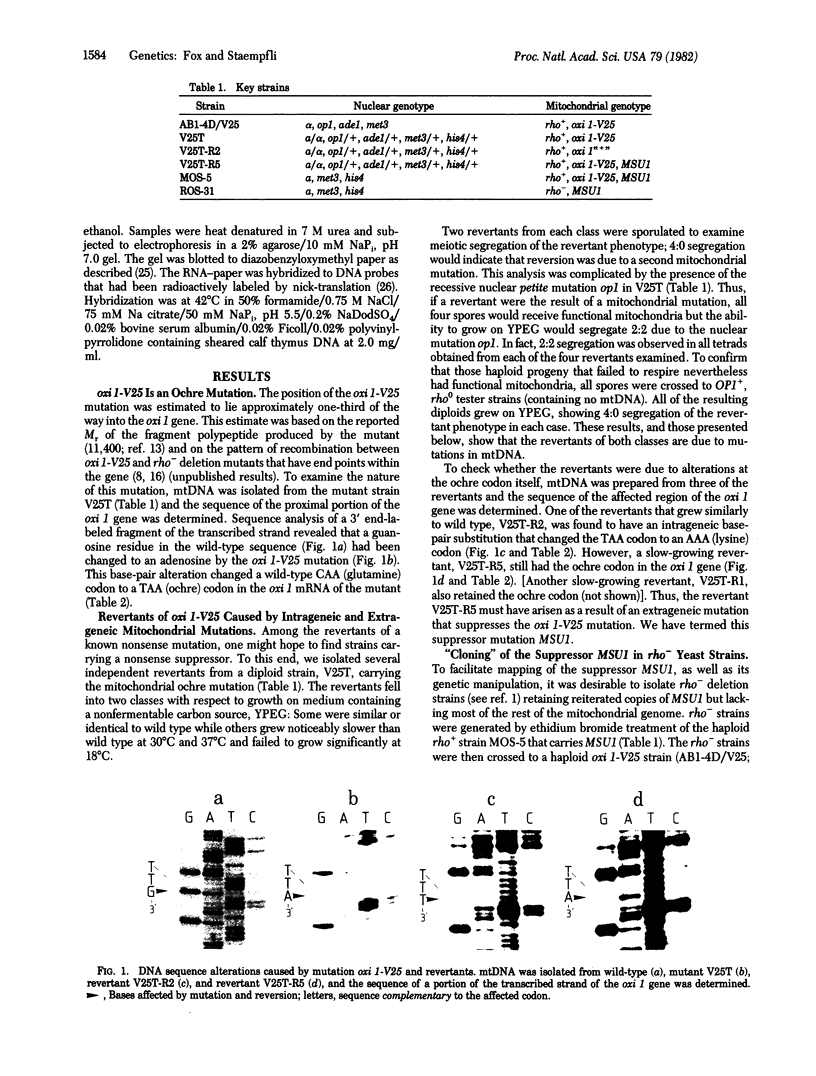
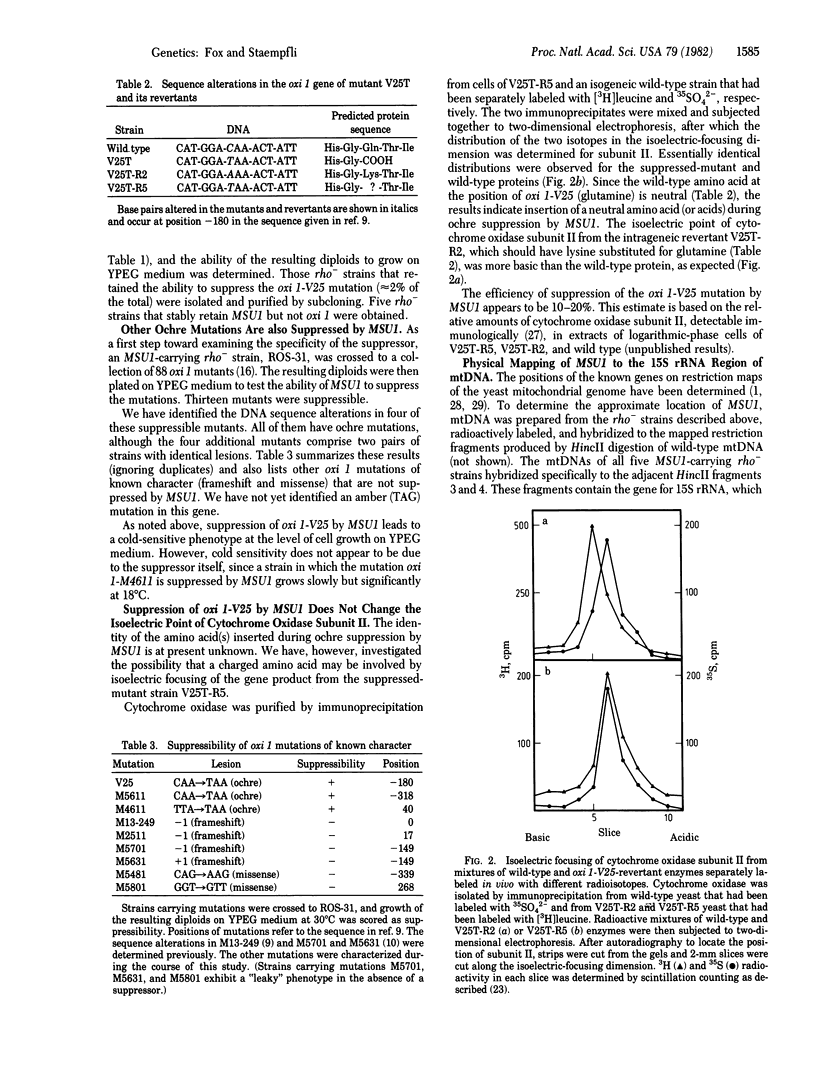
A polypeptide chain-terminating mutation in the yeast mitochondrial oxi 1 gene has been shown to be an ochre (TAA) mutation by DNA sequence analysis. Mitochondrially inherited revertants of this mutation include two types: In the first, the ochre codon has been changed to a sense codon by further mutation in the oxi 1 gene while, in the second, the ochre codon is still present, indicating the occurrence of an extrageneic ochre suppressor mutation. This mitochondrial ochre suppressor, termed MSU1, has been "cloned" in rho- strains of yeast and tested against other oxi 1 mutations. Several additional mutations are also suppressible, and those examined so far are also ochre mutations. MSU1 does not suppress known frameshift or missense mutations at oxi 1. Isoelectric focusing of the gene product (cytochrome oxidase subunit II) from a suppressed-mutant strain indicates that suppression does not involve insertion of charged amino acids. Physical mapping of the mtDNA retained in the MSU1-carrying rho- clones localizes the suppressor mutation to the gene coding the 15S rRNA or a site not more than 300 base pairs from it. No known tRNA genes occur this close to the 15S rRNA gene, and mtDNA from a suppressor-carrying rho- does not hybridize detectably to mitochondrial tRNAs. These results suggest that MSU1 may be an alteration in the 15S rRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Blanc H., Wright C. T., Bibb M. J., Wallace D. C., Clayton D. A. Mitochondrial DNA of chloramphenicol-resistant mouse cells contains a single nucleotide change in the region encoding the 3' end of the large ribosomal RNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3789–3793. doi: 10.1073/pnas.78.6.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezón T., Herzog A., De Wilde M., Villarroel R., Bollen A. Cooperative control of translational fidelity by ribosomal proteins in Escherichia coli. III. A ram mutation in the structural gene for protein S5 (rpx E). Mol Gen Genet. 1976 Feb 27;144(1):59–62. doi: 10.1007/BF00277305. [DOI] [PubMed] [Google Scholar]
- Cabral F., Solioz M., Rudin Y., Schatz G., Clavilier L., Slonimski P. P. Identification of the structural gene for yeast cytochrome c oxidase subunit II on mitochondrial DNA. J Biol Chem. 1978 Jan 10;253(1):297–304. [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The sequence of Escherichia coli ribosomal 16 S RNA determined by new rapid gel methods. FEBS Lett. 1978 Oct 1;94(1):152–156. doi: 10.1016/0014-5793(78)80926-0. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence of subunit 2 of yeast cytochrome oxidase. J Biol Chem. 1979 Sep 25;254(18):9324–9330. [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin G., Pajot P., Groudinsky O., Slonimski P. P. Long range control circuits within mitochondria and between nucleus and mitochondria. I. Methodology and phenomenology of suppressors. Mol Gen Genet. 1980;179(3):469–482. doi: 10.1007/BF00271736. [DOI] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Five TGA "stop" codons occur within the translated sequence of the yeast mitochondrial gene for cytochrome c oxidase subunit II. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6534–6538. doi: 10.1073/pnas.76.12.6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D. Genetic and physical analysis of the mitochondrial gene for subunit II of yeast cytochrome c oxidase. J Mol Biol. 1979 May 5;130(1):63–82. doi: 10.1016/0022-2836(79)90552-7. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Identification of phage SP01 proteins coded by regulatory genes 33 and 34. Nature. 1976 Aug 26;262(5571):748–753. doi: 10.1038/262748a0. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Weiss-Brummer B. Leaky +1 and -1 frameshift mutations at the same site in a yeast mitochondrial gene. Nature. 1980 Nov 6;288(5786):60–63. doi: 10.1038/288060a0. [DOI] [PubMed] [Google Scholar]
- Goldring E. S., Grossman L. I., Krupnick D., Cryer D. R., Marmur J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol. 1970 Sep 14;52(2):323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Hawthorne D. C., Leupold U. Suppressors in yeast. Curr Top Microbiol Immunol. 1974;64(0):1–47. doi: 10.1007/978-3-642-65848-8_1. [DOI] [PubMed] [Google Scholar]
- Ishiguro J., Ono B. I., Masurekar M., McLaughlin C. S., Sherman F. Altered ribosomal protein S11 from the SUP46 suppressor of yeast. J Mol Biol. 1981 Apr 15;147(3):391–397. doi: 10.1016/0022-2836(81)90491-5. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R., Rabinowitz M. Physical mapping of the yeast mitochondrial genome: derivation of the fine structure and gene map of strain D273-10B and comparison with a strain (MH41-7B) differing in genome size. Mol Gen Genet. 1979 Feb 16;170(1):25–48. [PubMed] [Google Scholar]
- Needleman R. B., Tzagoloff A. Breakage of yeast: a method for processing multiple samples. Anal Biochem. 1975 Apr;64(2):545–549. doi: 10.1016/0003-2697(75)90466-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ono B. I., Stewart J. W., Sherman F. Serine insertion caused by the ribosomal suppressor SUP46 in yeast. J Mol Biol. 1981 Apr 15;147(3):373–379. doi: 10.1016/0022-2836(81)90489-7. [DOI] [PubMed] [Google Scholar]
- Osinga K. A., Evers R. F., Van der Laan J. C., Tabak H. F. A putative precursor for the small ribosomal RNA from mitochondria of Saccharomyces cerevisiae. Nucleic Acids Res. 1981 Mar 25;9(6):1351–1364. doi: 10.1093/nar/9.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosset R., Gorini L. A ribosomal ambiguity mutation. J Mol Biol. 1969 Jan 14;39(1):95–112. doi: 10.1016/0022-2836(69)90336-2. [DOI] [PubMed] [Google Scholar]
- Saltzgaber-Müller J., Schatz G. Heme is necessary for the accumulation and assembly of cytochrome c oxidase subunits in Saccharomyces cerevisiae. J Biol Chem. 1978 Jan 10;253(1):305–310. [PubMed] [Google Scholar]
- Smirnov V. N., Surguchov A. P., Smirnov V. V., Berestetskaya Y. V. Recessive nonsense-suppression in yeast: involvement of 60S ribosomal subunit. Mol Gen Genet. 1978 Jul 6;163(1):87–90. doi: 10.1007/BF00268967. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Séquence nucléotidique du gène de l'ARN ribosomique 15S mitochondrial de la levure. C R Seances Acad Sci D. 1980 Dec 8;291(12):933–936. [PubMed] [Google Scholar]
- Thalenfeld B. E., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequence of the oxi 2 gene of yeast mitochondrial DNA. J Biol Chem. 1980 Jul 10;255(13):6173–6180. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembath M. K., Monk B. C., Kellerman G. M., Linnane A. W. Biogenesis of mitochondria 40. Phenotypic suppression of a mitochondrial mutation by a nuclear gene in Saccharomyces cerevisiae. Mol Gen Genet. 1975 Oct 22;140(4):333–337. [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Gupta R., Siegel R. B., Stahl D. A., Kop J., Crawford N., Brosius J., Gutell R., Hogan J. J. Secondary structure model for bacterial 16S ribosomal RNA: phylogenetic, enzymatic and chemical evidence. Nucleic Acids Res. 1980 May 24;8(10):2275–2293. doi: 10.1093/nar/8.10.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Garvin R. T., Gorini L. Alteration of a 30S ribosomal protein accompanying the ram mutation in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2263–2267. doi: 10.1073/pnas.68.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]