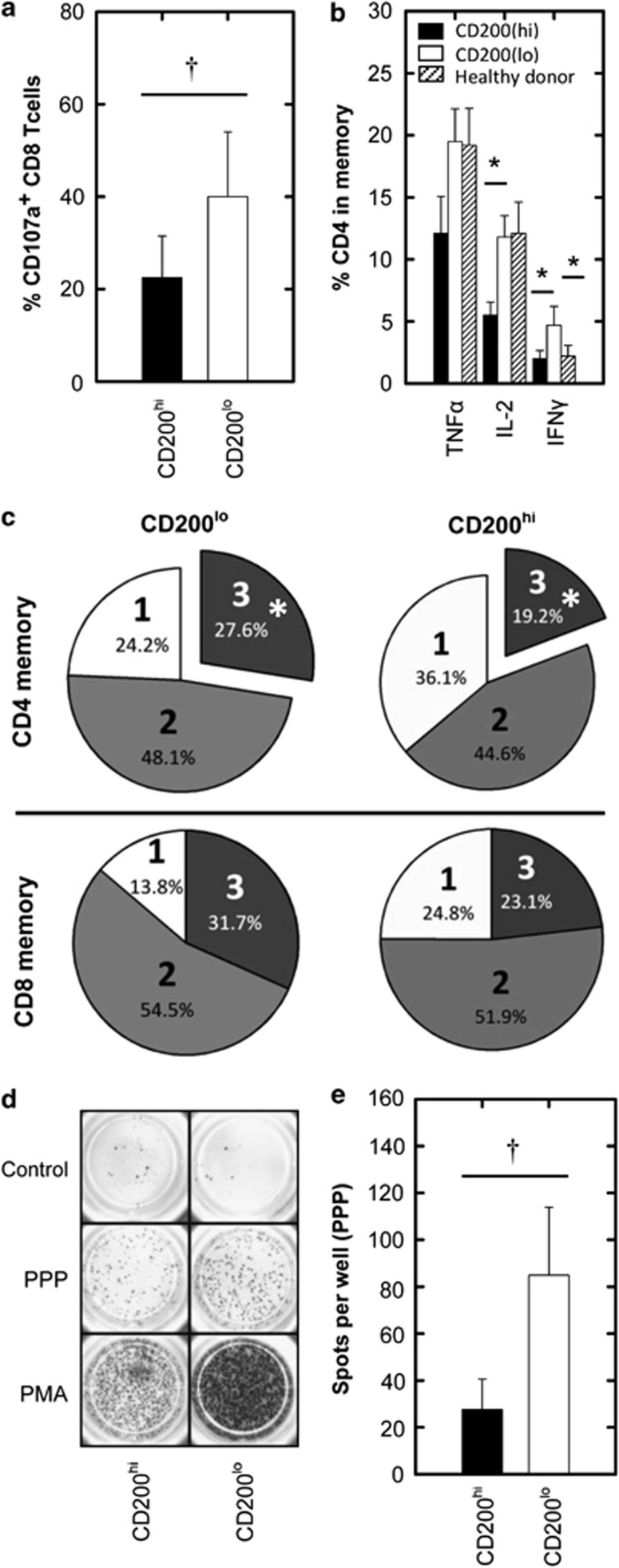
Previous studies have shown that immunosuppression in acute myeloid leukemia (AML) is associated with changes in the adaptive immune compartment. Such changes include the suppression of memory T-cell function1 and the suppression of Th1 cytokine (TNFα, IL-2 and IFNγ)-producing cells.2 A suppressed immune response in AML is associated with a worse patient outcome and increased risk of relapse,3 as well as increased risk of infection impairing patient recovery.4 The over-expression of the immunosuppressive ligand CD200 is also associated with an increased risk of relapse in AML (hazard ratio 1.7); an observation consistent with a hypothesis in which CD200 inhibits clearance of residual disease.5, 6 As memory T-cell responses are central for tumor immunosurveillance and contribute to prolonged molecular remission,7 we carried out this study to establish how these responses were affected in AML patients over-expressing CD200 (Supplemental Table S1). We initially investigated whether CD200 expression on AML blasts influenced CD8+ T-cell cytotoxic potential and the frequency of TNFα-, IL-2- and IFNγ-producing CD4+/CD8+ memory T-cells (Supplementary Materials and Methods and Supplemental Figure S1 for gating strategy). Using CD107a as a marker of cytotoxic function, AML cells were activated with PMA/ionomycin. We show that the frequency of CD107a+CD8+ memory T-cells was significantly reduced by ∼50% for CD200hi patients when compared with CD200lo AML, demonstrating that cytotoxic memory T-cell activity was compromised in CD200hi patients (Figure 1a). Furthermore, the frequencies of TNFα-, IL-2- and IFNγ-producing CD4+ memory cells were also reduced by ∼50% for CD200hi patients when compared with CD200lo AML (Figure 1b), significantly so in the case of IL-2 and IFNγ. Interestingly, CD200lo patients displayed a higher IFNγ response, not only with respect to CD200hi patients but also in comparison to healthy donors, suggesting a role for this cytokine in AML, which is attenuated by CD200. No difference was observed for TNFα-, IL-2- and IFNγ-producing CD8+ memory cells between CD200hi, CD200lo and healthy donors (data not shown). CD200 has also been reported to mediate suppression of the Th1 response in chronic lymphocytic leukemia as well as solid tumors,8, 9 suggesting that CD200-mediated Th1 suppression is a central mechanism in cancer immunomodulation.
Figure 1.
Cytotoxic T-cell response and Th1 memory/recall response in CD200hi and CD200lo AML patients. AML patient cytotoxic and intracellular Th1 cytokine memory T-cell responses were measured by flow cytometry following PMA/ionomycin stimulation (for full methods and flow cytometric gating strategies see Supplementary Materials and Methods and Supplemental Figure S1). (a) Summary data illustrating a significant difference in CD107a+CD8+ memory T-cells between CD200hi and CD200lo AML patients. (b) The production of TNFα, IL-2 or IFNγ for CD200hi, CD200lo and healthy donors for CD4+ memory T-cells. (c) Pie charts summarizing the proportion of CD4+ and CD8+ memory cells capable of producing one (1=TNFα, IL-2 or IFNγ), two (2=TNFα/Il-2, TNFα/IFNγ or IFNγ/IL-2) or three (3=TNFα/IL-2/IFNγ) cytokines simultaneously for CD200hi or CD200lo (refer to Supplemental Figure S1 for Boolean analysis). The ability of T-cells from CD200hi and CD200lo AML patients to mount recall responses to common microbial antigens (PPP, Supplementary Materials and Methods) was assessed by IFNγ ELISPOT. (d) Representative ELISPOT wells for CD200hi and CD200lo patients. (e) Average spots per well for CD200hi and CD200lo patients. AML patient data represents mean±1 s.d., n=9 for CD200hi and n=12 for CD200lo. †P<0.05, analyzed by one-tailed unpaired t-test; *P<0.05, analyzed by one-way ANOVA with Tukey's multiple comparison test.
The ability to simultaneously produce TNFα, IL-2 and IFNγ is an important indicator of ‘T-cell quality' in anti-tumor/viral responses.10 We therefore simultaneously measured the production of all these cytokines in CD200hi and CD200lo patients after PMA/ionomycin stimulation (Supplemental Figure S1). A significant reduction (30%) in CD4+ memory T-cells capable of simultaneously producing TNFα, IL-2 and IFNγ was observed in CD200hi compared with CD200lo AML patients (Figure 1c). Although a similar reduction was observed within the CD8+ memory cells, the changes in this subpopulation were less consistent and were not statistically significant (Supplemental Figure S2). To assess if CD200 expression on AML blasts influences the memory Th1 response through an antigen-specific mechanism, we compared T-cell responses with common microbial recall antigens (PPP) by ELISPOT (Supplementary Materials and Methods).11 We observed a significant 75% reduction in the frequency of IFNγ-secreting T-cells towards PPP in CD200hi vs CD200lo AML (Figures 1d and e). CD200 expression level did not influence the overall frequency of CD3+ lymphocytes (Supplemental Figure S3), demonstrating that the difference observed in these cohorts was due to T-cell inhibition in CD200hi patients and not due to a decrease in overall T-cell frequency or increase in AML blasts. Thus, we show for the first time (in any context) that CD200 expression in AML is associated with the suppression of Th1 memory T-cell quality and function. Not only does this finding demonstrate that the memory T-cell response in CD200hi patients is suppressed through an antigen-specific mechanism, but suggests that CD200 expression may exacerbate the susceptibility of leukemia patients to common microbial infections, which may impair patient recovery.4 This notion is supported by a study from Snelgrove et al., who demonstrated in a murine model that CD200 expression suppresses T-cell responses towards influenza.12
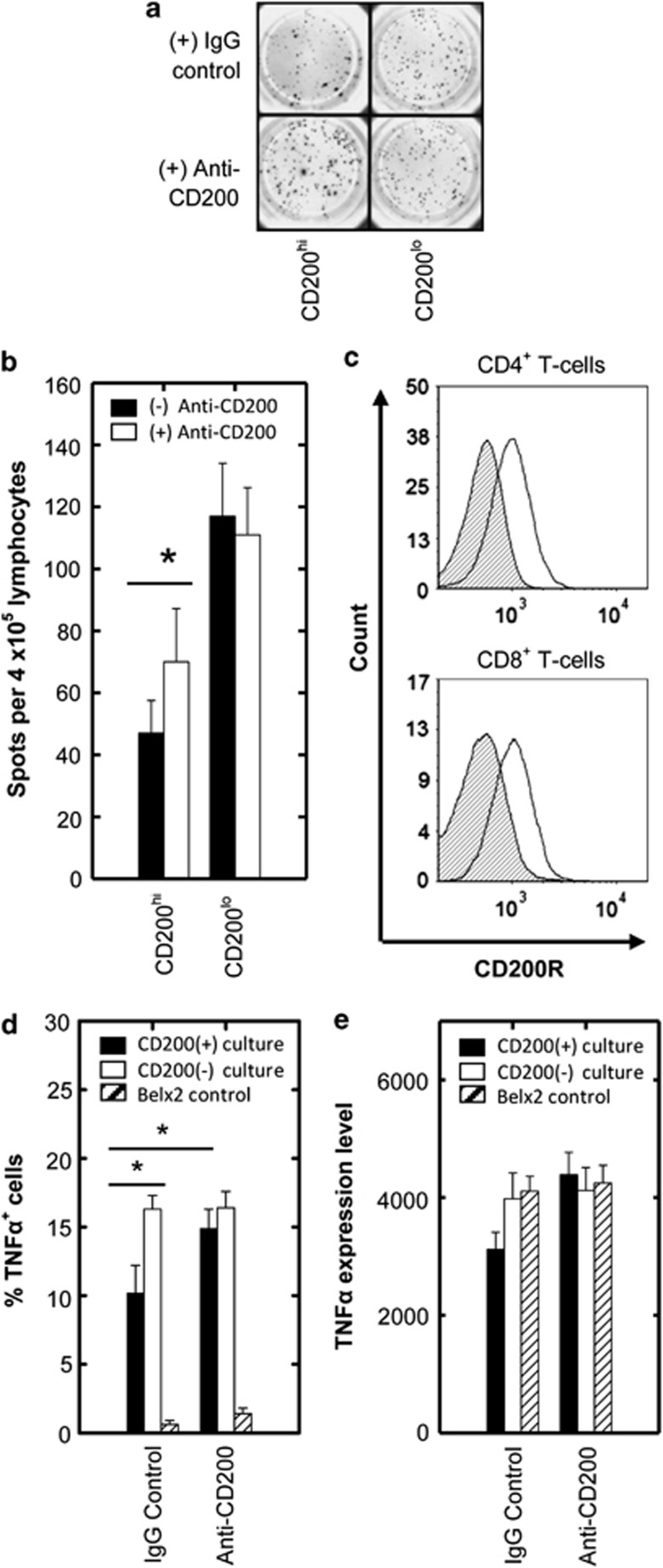
To demonstrate that this immunosuppression was functionally driven by CD200, we asked whether blocking CD200 could also recover the AML Th1 memory T-cell response. Figures 2a and b show a significant recovery of memory T-cells secreting IFNγ for CD200hi patients in an ELISPOT assay, raising the possibility that AML blast CD200 was directly interacting with memory T-cells via CD200R. Flow cytometric data confirmed CD200R expression on memory T-cells from AML patients (Figure 2c), supporting previous literature using healthy subjects.13 To rule out the possibility of indirect suppression through antigen-presenting cells, we next carried out a refined assay in which a CD4+ T-cell clone (Belx2)14 was co-cultured with K562 cells, which differed solely in their expression of CD200 (Supplemental Figure S4).15 We showed a significantly impaired TNFα response in CD200hi K562 cells compared with control co-cultures (Figure 2d). We also observed a significant suppression in IL-2 and IFNγ production with a loss of multi-functionality in terms of TNFα and IFNγ production in the presence of CD200+ cells (Supplemental Figure S4), though the frequency of Belx2 cells producing IFNγ and IL-2 was minimal compared with TNFα under these assay conditions. Adding anti-CD200 to the CD3/CD28-stimulated assay could significantly recover TNFα production in CD200+ co-cultures to the same level as CD200− co-cultures (Figure 2d), thus demonstrating that blockade of CD200 alone is sufficient to recover memory T-cell activity. The data also show that the intensity of TNFα was decreased (though not significantly) in CD200+ cultures, which was fully recovered by the addition of anti-CD200 (Figure 2e). This finding indicates that CD200 can suppress both the magnitude and intensity of the memory Th1 response in AML and that blocking CD200 in this disease may be therapeutically advantageous.
Figure 2.
Assessment of monoclonal anti-CD200 in relieving the inhibition of Th1 responses in CD200hi AML patients with direct assessment of CD200R engagement on CD4+ memory T-cells. (a) Representative ELISPOT wells show the effect of anti-CD200 (+) or isotype control antibody (−) on IFNγ release following PPP stimulation for CD200hi and CD200lo AML patients. (b) Summary data illustrating IFNγ release in response to PPP stimulation as measured by ELISPOT for CD200hi/lo AML patients +/− anti-CD200. (c) Representative flow cytometric plots illustrate CD200R expression on both CD4+ and CD8+ memory T-cells for AML patients (filled histograms represent immunoglobulin isotype-matched control and open histograms represent CD200R expression). Data represents mean±1 s.d., n=7. *P<0.05, analyzed by one-tailed paired t-test. (d) To directly assess CD200R engagement on CD4+ memory T-cells, the CD4+ memory T-cell clone; Belx2 were co-cultured with control or CD200+ K562 cells. The percentage of TNFα-producing Belx2 T-cell clones in co-culture assays in response to CD3/CD28 costimulation was measured by flow cytometry following intracellular staining. Summary data illustrates the frequency of TNFα-producing T-cells in CD200+ and CD200- K562 co-cultures +/− anti-CD200. (e) Summary data illustrating MFI of the TNFα+ T-cell population in CD200+ and CD200- K562 co-cultures +/− anti-CD200. Data represents mean±1 s.d., n=6. *P<0.05, analyzed by one-tailed paired t-test.
Previously, we have shown that CD200 on AML cells directly impairs NK cell function.15 However, CD200 expression may not always promote immunosuppression in every context. One study using a CD200+ mouse plasmacytomal model showed that CD200 had the capacity to decrease production of the suppressive cytokine IL-10 from tumor-associated myeloid cells resulting in an improved anti-tumor response.16 Whether this mechanism exists in human AML remains to be elucidated. Taken together, the data presented here this suggests that at diagnosis, when the disease burden is high, the main mechanism of CD200 is to drive immunosuppression through direct interaction of CD200 on leukemia cells with CD200R on cells of the adaptive immune system. The situation may be different following reduction of tumor burden post-chemotherapy, where the influence of Treg cells may become a dominant factor in immunosuppression.17
In conclusion, we show for the first time that CD4+ Th1 memory and memory cytotoxic responses are significantly compromised in CD200hi AML patients, which may contribute to the increased risk of relapse and worse overall survival observed in these patients. Most importantly we demonstrate that CD200 on leukemia cells directly suppresses T-cell responses, supporting the use of CD200-blocking therapy for the treatment of AML.
Acknowledgments
This work was funded by Leukaemia and Lymphoma Research UK. Dr Steve Coles is currently funded by NISCHR, UK. We are grateful to the patients for access to material enrolled in the NCRI clinical trials.
SJC designed and performed the experiments, analyzed all data and co-wrote the manuscript. RKH provided statistical guidance. ECYW provided biological insight. AKB provided resources and clinical insight. SM, RLD and AT contributed to experimental design and co-wrote the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Liepert A, Grabrucker C, Kremser A, Dreyssig J, Ansprenger C, Freudenreich M, et al. Quality of T-cells after stimulation with leukemia-derived dendritic cells (DC) from patients with acute myeloid leukemia (AML) or myeloid dysplastic syndrome (MDS) is predictive for their leukemia cytotoxic potential. Cell Immunol. 2010;265:23–30. doi: 10.1016/j.cellimm.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Ersvaer E, Liseth K, Skavland J, Gjertsen BT, Bruserud O. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol. 2010;11:38. doi: 10.1186/1471-2172-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenghui Z, Yixiang H, Jianbo W, Kang Y, Laixi B, Yan Z, et al. Elevated frequencies of CD4(+)CD25(+)CD127(lo) regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer. 2010;129:1373–1381. doi: 10.1002/ijc.25791. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Akiyama N, Fujita H, Miura K, Miyatake J, Handa H, et al. Analysis of bacteremia/fungemia and pneumonia accompanying acute myelogenous leukemia from 1987 to 2001 in the Japan Adult Leukemia Study Group. Int J Hematol. 2011;93:66–73. doi: 10.1007/s12185-010-0746-y. [DOI] [PubMed] [Google Scholar]
- Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK, et al. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21:566–568. doi: 10.1038/sj.leu.2404559. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M, et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117:6267–6276. doi: 10.1182/blood-2010-12-324004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Qiao Z, Zhu L, Wang H, Su L, Lu Y, et al. Th1/Th2 cytokine profiles and their relationship to clinical features in patients following nonmyeloablative allogeneic stem cell transplantation. Am J Hematol. 2004;75:78–83. doi: 10.1002/ajh.10443. [DOI] [PubMed] [Google Scholar]
- McWhirter JR, Kretz-Rommel A, Saven A, Maruyama T, Potter KN, Mockridge CI, et al. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc Natl Acad Sci USA. 2006;103:1041–1046. doi: 10.1073/pnas.0510081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2008;57:987–996. doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Smith KL, Tristram A, Gallagher KM, Fiander AN, Man S. Epitope specificity and longevity of a vaccine-induced human T cell response against HPV18. Int Immunol. 2005;17:167–176. doi: 10.1093/intimm/dxh197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102:173–179. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KM, Man S. Identification of HLA-DR1- and HLA-DR15-restricted human papillomavirus type 16 (HPV16) and HPV18 E6 epitopes recognized by CD4+ T cells from healthy young women. J Gen Virol. 2007;88:1470–1478. doi: 10.1099/vir.0.82558-0. [DOI] [PubMed] [Google Scholar]
- Coles SJ, Wang EC, Man S, Hills RK, Burnett AK, Tonks A, et al. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25:792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu JQ, Talebian F, El-Omrani HY, Khattabi M, Yu L, et al. Tumor expression of CD200 inhibits IL-10 production by tumor-associated myeloid cells and prevents tumor immune evasion of CTL therapy. Eur J Immunol. 2010;40:2569–2579. doi: 10.1002/eji.201040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SJ, Hills RK, Wang ECY, Burnett AK, Man S, Darley RL, et al. Increased CD200 expression in acute myeloid leukemia is linked with an increased frequency of FoxP3+ regulatory T–cells. Leukemia. 2012;26:2148–2151. doi: 10.1038/leu.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.