Abstract
Curative therapies for hepatocellular carcinoma (HCC), such as resection and liver transplantation, can only be applied in selected patients with early tumors. More advanced stages require local or systemic therapies. Resection of HCC offers the only hope for cure. Even in patients undergoing resection, recurrences are common. Chemoembolization, a technique combining intra-arterial chemotherapy with selective tumor ischemia, has been shown by randomized controlled trials to be efficacious in the palliative setting. There is now renewed interest in transarterial embolization/transarterial chemoembolization (TACE) with regards to its use as a palliative tool in a combined modality approach, as a neoadjuvant therapy, in bridging therapy before transplantation, for symptomatic indications, and even as an alternative to resection. There have also been rapid advances in the agents being embolized trans-arterially (genes, biological response modifiers, etc.). The current review provides an evidence-based overview of the past, present and future trends of TACE in patients with HCC.
Keywords: Liver tumors, Embolization, Chemoembolization, Review
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most common cause of cancer-related death in the world[1]. Due to the high prevalence of hepatitis B in Africa and Asia and hepatitic C in America and Europe, the incidence of HCC is increasing[2]. Whilst surgery is curative, only one third of patients with HCC are suitable candidates for hepatic resection[3]. Even in those who undergo resection, the cumulative recurrence of HCC is 70% at 5 years after surgery[4]. Liver transplantation is the treatment of choice in a selected subset of patients, but this modality is plagued by the limited availability of donors[4]. With regards to secondary tumors, only 25% of patients with colorectal metastases are candidates for surgery. Even in those patients who have undergone a successful margin-free resection, 60% develop recurrence[5]. Systemic chemotherapy has been shown to have limited therapeutic effects for primary and secondary hepatic malignancies, with tumor response rates of less than 30%[5,6]. Hence palliative management is the mainstay of therapy for most patients with primary and secondary liver tumors[6]. Most investigative efforts have now converged on local control, with transarterial embolization (TAE) and transarterial chemoembolization (TACE) having an established role in therapy. TAE/TACE is used as an effective means of palliation for unresectable tumors[6-8]. It has been used in the adjuvant setting to manage postoperative recurrent hepatic tumors[8]. Trials with conflicting results have also shown TACE to be useful as an alternative to surgery for resectable tumors[9,10]. This review aims to provide an overview of published studies of TACE in HCC in order to establish an evidence-based practice of this procedure in the management of HCC.
HISTORY
The first attempt at inducing ischemic tumor necrosis and thereby tumor regression was done by operative hepatic artery ligation by Mori et al[11] in 1966. The effect of this procedure was transient due to the rapid development of a collateral circulation. The first successful TACE for liver tumors has been credited to Doyon et al[12], who performed it in 1974. Gelatin sponge as an embolizing agent along with an anticancer agent was first employed by Yamada et al[13] in 1983.
PRINCIPLE
The principle of TACE/TAE revolves around the basic concept of dual blood supply of the normal liver. Liver tumors derive 90% of their blood from the hepatic artery[14]. Park et al[14] conceptualized carcinogenesis of HCC as a multistep process involving parenchymal arterialization, sinusoidal capillarization and development of unpaired arteries (a vital component of neoangiogenesis). All these events lead to a gradual shift in blood supply from portal to arterial circulation. This radical concept has been validated using dynamic imaging modalities by various investigators[15,16]. Sigurdson et al[17] showed that when an agent was infused via the hepatic artery, it attained ten times higher intratumoral concentration as compared to when it was given through the portal vein. Hence, arterial treatment targets the tumor while normal liver is relatively spared. Embolization induces ischemic necrosis of tumor causing a failure of the transmembrane pump, resulting in a greater absorption of agents by the tumor cells[18]. Tissue concentration of agents within the tumor is more than 40 times that of the surrounding normal liver[17,18].
EMBOLIZING AND CHEMOTHERAPEUTIC AGENTS
The use of lipiodol was a major breakthrough in TAE. Lipiodol, a lymphangiographic dye, is iodized poppy seed oil. It is found to remain selectively in the neovasculature and extravascular spaces of liver tumors[18]. It has been shown to persist selectively in the tumor for a few weeks[19]. The reasons for this prolonged intra-hepatic persistence is unclear, but theories include a hemodynamic difference between the hypervascular hepatic tumors and the liver parenchyma with an absence of Kupffer cells in the tumor[18,19]. The maximum safe dose of lipiodol is 15 mL, with the general rule of 1 mL of lipiodol/cm of tumor being used as a pharmacological guide by interventional radiologists[20]. The ideal embolizing agent has to be bigger than the tumor arterio-hepatic-portal shunt and peribiliary plexus, but small enough to enter into the tumor feeding vessels to induce effective tumor embolization[20]. Multiple agents have been used, the most common being gelatin microspheres. Other agents used include autologous blood clots, polyvinyl alcohol particles, cyanoacrylate, absolute alcohol, collagen, starch microspheres, glass microspheres and resin microspheres[21,22]. Doxorubicin is the most commonly used chemotherapeutic agent. It is used either singly or in combination with cisplatin, mitomycin or 5FU. Other agents used include streptozocin, vinblastine and gemcitabine. No clear superiority for any particular cytotoxic agent/regimen has been demonstrated[23,24].
INDICATIONS
Indications for TAE/TACE are hypervascular tumors confined to the liver, or tumors where the intrahepatic component is the main source of morbidity and mortality. Embolization is usually done for HCC, cholangiocarcinoma and hepatic metastases[3,5-7,22]. TAE/TACE has also been used for symptom relief in patients with intractable abdominal pain and hypercalcemia[25,26]. It has also been effectively used for benign liver tumors like giant hemangioma and symptomatic epithelioid hemangioendothelioma[27,28]. An important consideration to be addressed before embolization is the liver function. Different criteria to assess liver function have been used including Child-Pugh classification, Okuda staging and CLIP score, but none of these systems indicate which patients would benefit from TACE[6,24,29]. In the Barcelona Clinic Liver Cancer (BCLC) staging, TACE is recommended for intermediate stage (Okuda 1-2, performance score 0, and large or multinodular HCC), and in selected patients with advanced stage (Okuda 1-2, performance score 1, no extrahepatic HCC)[3,6,7,29].
CONTRAINDICATIONS
Even though there are no absolute contraindications to TACE, a combination of bad prognostic indicators is considered a contraindication. These include a massive or diffuse tumor involvement of more than 50% of liver, hepatic insufficiency/failure, portal vein invasion, high serum bilirubin (more than 3-5 mg/dL), high α-fetoprotein more than 1000 ng/mL, high levels of serum LDH (more than 425 IU/L) and transaminases (more than 100 IU/L)[6,7,13,23,24,29,30]. Contraindications to the chemotherapeutic agent and anaphylactic reactions to the contrast media also remain a contraindication. Even in patients with poor hepatic functional reserve, superselective TACE has been successfully attempted. Though there is a higher risk of hepatic insufficiency and hepatic infarction, portal vein occlusion is no longer an absolute contraindication[30]. Fan et al[31] showed that TACE can safely be performed in patients with main portal vein occlusion as long as hepatopetal collateral flow is maintained. In these patients a reduced dosage of chemoembolic agents is used and a super-selective administration into tumor-feeding arteries is done[30,31].
PROCEDURE
Pre-procedure workup includes evaluating the hepatic functional reserve and a baseline tumor marker assay. Elevated levels are a good indicator of treatment response, but on the flipside high tumor marker levels also indicate an overall poor prognosis[24]. Cross-sectional imaging is imperative to assess the size and extent of the tumor, for accurate segmental localization and to assess the macroscopic angioinvasion into the hepatic and portal veins. Other imaging studies to assess comorbid and/or metastatic disease are also performed pre-procedure. The procedure is tailored according to the hepatic functional reserve, tumor extent and major portal vein invasion, with every effort being made to preserve nontumorous liver parenchyma from chemoembolization[24,30]. Access is by the Seldinger technique, with initial diagnostic visceral arteriography (Figure 1A) being performed to assess the arterial anatomy and its variations, including the origins of cystic artery, right and left gastric arteries and falciform artery. All tumor feeding arteries need to be identified to avoid non-targeted embolization[32]. If there is a prominent arterio-portal shunting, the collateral/shunt vessels need to be embolized first[32,33]. After appropriate positioning of the catheter, a mixture of iodized oil and chemotherapeutic agent is injected. The endpoint for the mixture administration is stasis in the tumor-feeding arteries and appearance of iodized oil in the peri-tumoral portal vein tributaries[6,7,13,19,20] (Figure 1B).
Figure 1.
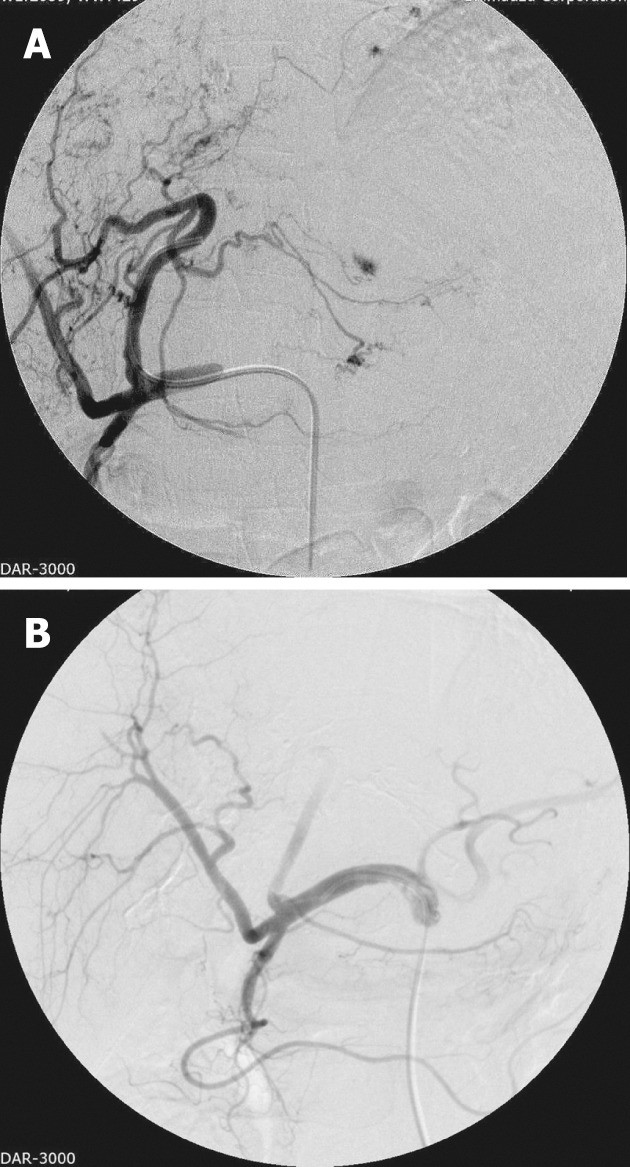
Left lobe hepatocellular carcinoma pre-embolization angiogram (A) and post-embolization angiogram (B).
Post-procedure care includes pain control, which apart from routine narcotic analgesics can be achieved by pre-embolization instillation of intra-arterial lidocaine[6,7,13]. Routine administration of prophylactic antibiotics is not recommended; antibiotics are indicated for special situations such as a stented patient, history of previous invasive interventions in the liver and presence of a biliary-enteric anastomosis[34,35]. Patients are routinely followed up at 2-4 wk with liver function tests and tumor marker assay. A multiphase helical computed tomography is done to determine local or remote tumor recurrence at 4-8 wk (Figure 2). If there is any evidence of viable tumor, a repeat TACE can be performed safely in 4-16 wk[36,37].
Figure 2.
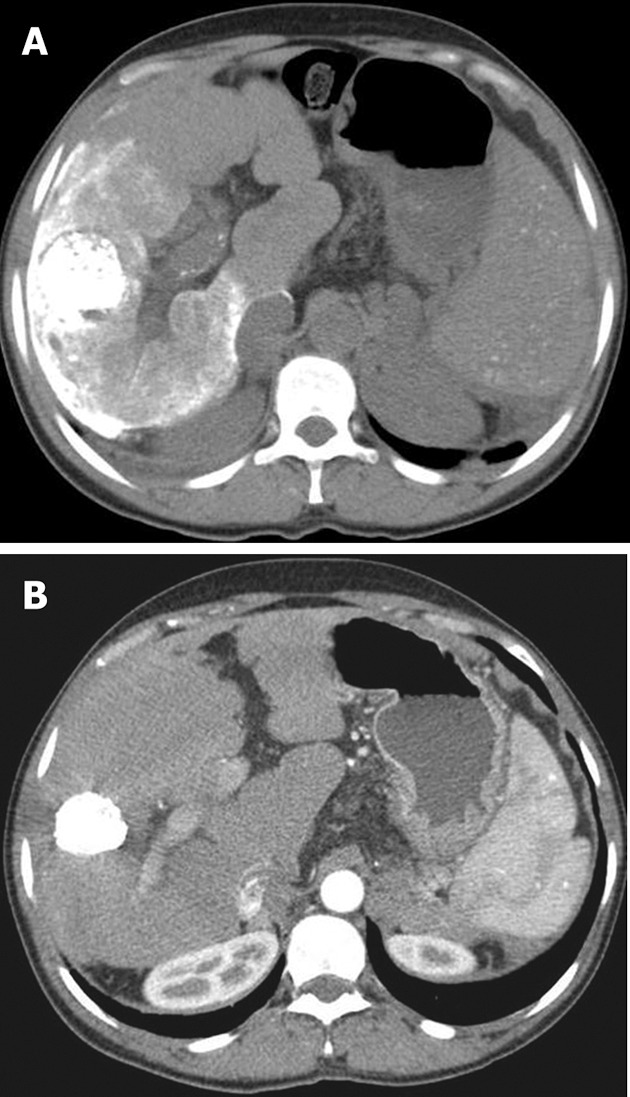
Post transarterial chemoembolization early (A) and late (B) follow-up contrast-enhanced computed tomography.
TACE IN HCC
Initial randomized trials failed to show any significant benefit of TACE in patients with HCC. However, these studies were plagued by heterogeneous techniques and protocols, usage of different chemotherapeutic or embolic agents and varied treatment intervals[38,39]. Pelletier et al[39] compared patients treated by TACE using doxorubicin and gelatin sponge powder with a non-treated group. The survival rate was not significantly different; the main criticism of this study was that gelatin sponge powder was used instead of sponge microspheres, causing a rapid deterioration in liver function[39,40]. The French cooperative group was the other randomized study which failed to show a statistically significant survival rate in patients undergoing TACE[38]. This study was criticized because patients underwent TACE every 2 mo causing deaths unrelated to tumor growth[40]. More recent randomized studies have all shown a survival benefit of TACE in patients with HCC[6,7,41]. Cammà et al[6] in their meta-analysis concluded that TACE significantly reduced the overall 2-year mortality rate (odds ratio 0.54).
TACE plays multiple roles in the management of HCC. It is used in the palliative setting, and it is used in cases with post resection intrahepatic recurrent HCC. TACE is used as an adjuvant therapy for preventing postoperative recurrence, and it is also used as a primary treatment modality in ruptured HCC[4,8,40,42,43]. There is significant interest in the use of TACE as a neoadjuvant therapy in patients with resectable liver tumors. TACE has been performed before hepatic resection, mainly in patients with large tumors to reduce the tumor volume[8,44]. Chua et al[44], in their systematic analysis, concluded that the use of TACE as a neoadjuvant treatment in resectable HCC is safe and efficacious with high rates of pathological responses; however, it did not appear to improve disease-free survival. TACE has been shown to be efficacious as a bridging therapy to inhibit tumor growth for patients awaiting transplantation[8,43,45,46]. Postoperative adjuvant TACE is recommended for patients with high risk of early recurrence[8,42,43]. TACE has also been used as a part of multimodality therapy with an aim to reduce the size of large tumors and obtain a synergistic effect on tumor necrosis[24,40,47]. Different combinations of TACE with percutaneous ethanol injection, radiofrequency ablation and laser ablation have been attempted, with all the combinations showing an improved survival and a reduction in local recurrence rates[24,47-49]. No single multimodality treatment has been shown to be superior to another[49].
TACE IN OTHER LIVER TUMORS
The non-hypervascular nature of colorectal metastases limits the quantum of chemotherapeutic and embolic agent being delivered[50]. TACE is hence used as second-line therapy after systemic chemotherapy has failed, with response rates of approximately 25%[5]. Since neuroendocrine metastases are hypervascular and often confined to the liver, they have a high response rate. These patients demonstrate excellent survival rates after TACE (median survival more than 2 years)[51]. However, it is unclear whether TACE significantly improves survival in this cohort, as prolonged survival is the norm in advanced neuroendocrine disease and, moreover, these tumors are not associated with cirrhosis[51]. TACE produced a major (more than 50% regression) response in 70% of patients with gastrointestinal stromal tumors, lasting 8 to 31 mo. TACE has been recommended for unresectable liver metastasis not responding to imatinib[52].
COMPLICATIONS
Major complications following TACE are rare (less than 5%) with procedure-related mortality occurring in 0.5% of the patients[53]. The major predisposing factors for complications include main portal vein obstruction, compromised hepatic functional reserve, biliary obstruction, previous biliary surgery, excessive lipiodol injection and nonselective embolization[6,22,23,30-34]. A unique complication associated with TACE is the post-embolization syndrome (PES). Even though this is a self-limited condition, it prolongs hospitalization and postpones additional treatment. PES commonly presents with fever, nausea, vomiting and right upper quadrant pain associated with elevated transaminases. The exact etiology is not known but proposed theories include acute ischemia of liver parenchyma, distension of liver capsule and gallbladder ischemia following cystic artery embolization. Treatment is mainly supportive with antiemetics, analgesics and antipyretics, with steroids being reserved for the more severe cases. Pre-TACE intra-arterial administration of lidocaine has also been shown to reduce the incidence of PES[6,8,22,23,30,36,54]. Liver failure is the most serious complication associated with TACE. It is seen in 20% of patients with 3% of the patients going on to irreversible failure. Predisposing factors for liver failure include hyperbilirubinemia, higher dose of anticancer drug and advanced cirrhosis[6,8,24,30,31,36,55].
Liver infarction following TACE occurs due to a combined arterial and portal blockage. Patient with portal vein obstruction, hepatic insufficiency and biliary obstruction are at a higher risk of liver infarction[24,31]. Reducing the dosage of the agent, super-selective administration into the tumor feeding artery, a lower dose of iodized oil in Child-Pugh class B/C patients and pre-procedure decompression biliary system are some of the measures used to decrease the risk of post-embolization liver infarction[6,30,31,36,55].
Symptomatic bacterial infections occur in 4% of cases with septicemia occurring in about 1%[34]. The major predisposing factors for these complications include portal vein obstruction, biliary obstruction, presence of ascites and biliary-enteric anastomosis. Prophylactic antibiotics have been recommended for patients with the above-mentioned risk factors[24,30,34,35]. Biliary injury is observed in 8% of the patients, with most cases being asymptomatic. Intrahepatic bile ducts are most often affected and they present as intrahepatic bilomas, focal strictures of the duct or as diffuse dilations of intrahepatic bile duct[30,56]. TACE-induced biliary injury occurs mainly due to blockage of the peribiliary capillary plexus which is the primary blood supply for intrahepatic bile ducts[56].
Nontargeted embolization can lead to gallbladder ischemia/infarctions when the cystic artery is occluded. Most of the cases have a self-limited clinical course[30,53]. Acute pancreatitis can occur when there is regurgitation of embolic material into the pancreaticoduodenal artery leading to ischemia of the pancreas[24]. Splenic infarction has also been known to occur when there is a reflux of embolic material into the splenic artery during embolization[30]. This commonly occurs in patients with celiac artery stenosis with reversal of flow direction in the common hepatic artery. Skin complications such as painful induration and discoloration and transmural necrosis occur due to accidental embolization of the internal mammary artery, intercostal artery, and the falciform artery[30]. Pulmonary embolism occurs when the embolic agent leaves the liver through the normal hepatic vasculature or an arteriovenous hepatic shunt. The amount of the iodized oil injected is the most important factor predicting pulmonary embolism[32,33]. Non-targeted embolization can be prevented by administering the agents superselective via a microcatheter and by being wary of any large arteriovenous hepatic shunts[22,24,30,32,33]. Other procedure-related complications include iatrogenic arterial injuries such as dissection and pseudoaneurysm, which most commonly occur in the celiac and proper hepatic arteries. Most often this dissection heals spontaneously, and subsequent TACE is possible[57].
FUTURE TRENDS
Sorafenib, a multitargeted VEGF and Raf kinase inhibitor, has been shown to improve survival in the SHARP trial by Llovet et al[58]. Recent studies using sorafenib in combination with TACE have shown encouraging results. Sansonno et al[59] used a conventional TACE procedure followed by sorafenib treatment and showed that it resulted in a significantly delayed time to progression in patients with intermediate-stage HCV-related HCC, with no unexpected side effects[60]. Biological response modifying agents (BRMs), including GM-CSF, interleukin-2 (IL-2) and tumor necrosis factor-α (TNF-α), have a direct cytotoxic and cytostatic activity against tumor cells, thereby augmenting the anti-tumor effect of anticancer agents[61]. They also inhibit DNA and RNA synthesis in tumor cells[61]. BRMs act by polarizing the T-cell response to a helper T-cell 1 (Th1)-dominant state, activating cytotoxic T lymphocytes and tumor infiltrating lymphocytes. These agents are selectively embolized into the tumor, a process labeled transarterial immunoembolization (TIE). Preoperative TIE has been shown to suppress recurrences of HCC after surgery[61,62]. The suppressive effect might be caused by increased levels of helper T-cell 1 cytokines, accumulation and maturation of dendritic cells, and by the suppression of T-regulatory lymphocytes[61,62].
Gene embolization is the novel technique where cytokines (TNF-α, IL-2, IFN-γ, etc.) and/or p53 genes are embolized using an adenovirus, cytomegalovirus, or Epstein-Barr virus, as vector with lipiodol, with an aim to selectively transfer them into the tumor[60,63-65]. Prolonged residence of the vector within the tumor vessels with the embolic agent increases contact between the vector and tumor cells, resulting in enhanced gene expression within the tumor and limited gene transfer to the surrounding normal tissues[63,64]. Intra-arterial injection of adenovirus vector-mediated immunogene therapy has been shown to be an effective therapeutic method for liver cancer in rats[63-65].
Anti-angiotherapy embolization is a technique where tumor angiogenesis-targeting agents like bevacizumab are selectively embolized into the tumor; the main advantage being low toxicity and a selective effect on tumor vasculature[60,66,67]. There have been reports from China regarding the use of a traditional Chinese medicinal herb Bletilla striata as an embolic material in TACE for HCC. It contains mucilage, starch and a little volatile oil. The embolized herb disintegrates platelets, agglutinates erythrocytes and forms a thrombus, leading to a prolonged anticancer effect and inhibition of collateralization and metastasis[68,69].
Precision TACE is a technique where drug eluting beads (DEB) are used. Five hundred to 700 μm embolic beads are loaded with a drug which elutes into the liver parenchyma over a period of 7-14 d[60,70-73]. The main purported advantages are controlled sustained release of the agent allowing for higher intra-tumor levels and lower levels in the systemic circulation[73-77]. A randomized phase II study comparing TACE and TACE-DEB reported a significant reduction in liver toxicity and drug-related adverse events for the latter arm, associated with a non-significant trend of better antitumoral effect[70]. Other studies with the use of this method have also not shown a significant difference in survival or tumor recurrence rates as compared to conventional TACE[60,70-73,76,77]. Yttrium-90 (90Y) is a source of β energy and is used for radioembolization. The agent is impregnated into glass microspheres and embolized into the tumor, causing tumor necrosis through radiation exposure. Studies comparing radioembolization vs chemoembolization have failed to show any significant difference in tumor-free survival and recurrence rates[78-81]. Cohort studies reporting long-term outcomes showed a median survival time of 17.2 mo for patients with intermediate stage HCC and 12 mo for patients at advanced stage HCC with portal vein invasion. Objective response rates range from 35% to 50%[82-85].
Stem cell therapy is an exciting new avenue which has been combined with TACE. Infusion of bone marrow stem cells (BMC) before trans-arterial chemoembolization may help increase liver volume and consequently increase hepatic reserve in patients with HCC, and this may improve the outcome of this procedure[86,87]. In a recent study from Egypt, BMC infusion into the hepatic artery synchronized with TACE for patients with chronic liver disease complicated with HCC has been shown to be safe, feasible, and it also demonstrated an improvement in both biological and radiological volumetric parameters[88].
CONCLUSION
TACE has been shown to be an effective means of palliation for unresectable liver tumors. There is active research with encouraging results into expanding its application for resectable as well recurrent tumors. Even though there are no absolute contraindications, a combination of bad prognostic indicators is considered a contraindication for TACE. The BCLC staging best indicates ideal candidates for TACE. The treatment is tailored according to the hepatic functional reserve, tumor extent and major portal vein invasion, with the aim to preserve nontumorous liver parenchyma from chemoembolization at all times. TACE is a safe procedure with a morbidity of less than 5% and a mortality of 0.6%. With avid research happening in this field, there is a need for an evidence-based incorporation of these newer modalities into the standardized treatment protocol of HCC.
Footnotes
Peer reviewer: Hadi Rokni Yazdi, MD, Associate Professor, Department of Radiology, Central Radiology, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran 1419733141, Iran
S- Editor Cheng JX L- Editor Logan S E- Editor Xiong L
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, Alter HJ. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA. 2002;99:15584–15589. doi: 10.1073/pnas.242608099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Bismuth H, Majno PE. Hepatobiliary surgery. J Hepatol. 2000;32 Suppl 1:208–224. doi: 10.1016/s0168-8278(00)80427-4. [DOI] [PubMed] [Google Scholar]
- 5.Vassiliou I, Arkadopoulos N, Theodosopoulos T, Fragulidis G, Marinis A, Kondi-Paphiti A, Samanides L, Polydorou A, Gennatas C, Voros D, et al. Surgical approaches of resectable synchronous colorectal liver metastases: timing considerations. World J Gastroenterol. 2007;13:1431–1434. doi: 10.3748/wjg.v13.i9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 8.Jelic S, Sotiropoulos GC. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v59–v64. doi: 10.1093/annonc/mdq166. [DOI] [PubMed] [Google Scholar]
- 9.Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P’eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122–126. doi: 10.1002/bjs.1800820141. [DOI] [PubMed] [Google Scholar]
- 10.Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701; discussion 701-703. doi: 10.1097/00000658-199712000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori W, Masuda M, Miyanaga T. Hepatic artery ligation and tumor necrosis in the liver. Surgery. 1966;59:359–363. [PubMed] [Google Scholar]
- 12.Doyon D, Mouzon A, Jourde AM, Regensberg C, Frileux C. [Hepatic, arterial embolization in patients with malignant liver tumours (author’s transl)] Ann Radiol (Paris) 1974;17:593–603. [PubMed] [Google Scholar]
- 13.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397–401. doi: 10.1148/radiology.148.2.6306721. [DOI] [PubMed] [Google Scholar]
- 14.Park YN, Yang CP, Fernandez GJ, Cubukcu O, Thung SN, Theise ND. Neoangiogenesis and sinusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol. 1998;22:656–662. doi: 10.1097/00000478-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Fujimoto Y, Hosoki Y, Suzuki M, Sakurai S, Ohhira M, Saito H, Kohgo Y. Clinical utility of sequential imaging of hepatocellular carcinoma by contrast-enhanced power Doppler ultrasonograpy. Eur J Radiol. 2003;48:214–219. doi: 10.1016/S0720-048X(03)00272-9. [DOI] [PubMed] [Google Scholar]
- 16.Tajima T, Honda H, Taguchi K, Asayama Y, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Shimada M, Masuda K. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178:885–897. doi: 10.2214/ajr.178.4.1780885. [DOI] [PubMed] [Google Scholar]
- 17.Sigurdson ER, Ridge JA, Kemeny N, Daly JM. Tumor and liver drug uptake following hepatic artery and portal vein infusion. J Clin Oncol. 1987;5:1836–1840. doi: 10.1200/JCO.1987.5.11.1836. [DOI] [PubMed] [Google Scholar]
- 18.Konno T. Targeting cancer chemotherapeutic agents by use of lipiodol contrast medium. Cancer. 1990;66:1897–1903. doi: 10.1002/1097-0142(19901101)66:9<1897::aid-cncr2820660907>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Kan Z, Sato M, Ivancev K, Uchida B, Hedgpeth P, Lunderquist A, Rosch J, Yamada R. Distribution and effect of iodized poppyseed oil in the liver after hepatic artery embolization: experimental study in several animal species. Radiology. 1993;186:861–866. doi: 10.1148/radiology.186.3.8381552. [DOI] [PubMed] [Google Scholar]
- 20.Nakao N, Uchida H, Kamino K, Nishimura Y, Ohishi H, Takayasu Y, Miura K. Determination of the optimum dose level of lipiodol in transcatheter arterial embolization of primary hepatocellular carcinoma based on retrospective multivariate analysis. Cardiovasc Intervent Radiol. 1994;17:76–80. doi: 10.1007/BF00193921. [DOI] [PubMed] [Google Scholar]
- 21.Gunji T, Kawauchi N, Ohnishi S, Ishikawa T, Nakagama H, Kaneko T, Moriyama T, Matsuhashi N, Yazaki Y, Imawari M. Treatment of hepatocellular carcinoma associated with advanced cirrhosis by transcatheter arterial chemoembolization using autologous blood clot: a preliminary report. Hepatology. 1992;15:252–257. doi: 10.1002/hep.1840150213. [DOI] [PubMed] [Google Scholar]
- 22.Brown DB, Pilgram TK, Darcy MD, Fundakowski CE, Lisker-Melman M, Chapman WC, Crippin JS. Hepatic arterial chemoembolization for hepatocellular carcinoma: comparison of survival rates with different embolic agents. J Vasc Interv Radiol. 2005;16:1661–1666. doi: 10.1097/01.RVI.0000182160.26798.A2. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H, Mitani T, Murakami T, Hashimoto T, Tsuda K, Nakanishi K, Ishida T, Tomoda K, Hori S, Kozuka T. Five-year survival after transcatheter chemoembolization for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33 Suppl:S89–S92. doi: 10.1007/BF00686675. [DOI] [PubMed] [Google Scholar]
- 24.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Young N, Hollands M, Wong KP. Symptom relief and survival after chemo-embolization with adriamycin, lipiodol and gelfoam for hepatocellular carcinoma. Australas Radiol. 1993;37:173–176. doi: 10.1111/j.1440-1673.1993.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Kono N, Ono A, Osuga Y, Kiyokawa H, Mineo I, Matsuda Y, Miyoshi S, Kawata S, Minami Y. Transcatheter arterial chemo-embolization for humoral hypercalcemia of hepatocellular carcinoma. Gastroenterol Jpn. 1988;23:29–36. doi: 10.1007/BF02918853. [DOI] [PubMed] [Google Scholar]
- 27.Duxbury MS, Garden OJ. Giant haemangioma of the liver: observation or resection? Dig Surg. 2010;27:7–11. doi: 10.1159/000268108. [DOI] [PubMed] [Google Scholar]
- 28.Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, Schirmacher P, Weitz J, Friess H, Buchler MW, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108–2121. doi: 10.1002/cncr.22225. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 30.Chung JW, Park JH, Han JK, Choi BI, Han MC, Lee HS, Kim CY. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198:33–40. doi: 10.1148/radiology.198.1.8539401. [DOI] [PubMed] [Google Scholar]
- 31.Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, Zhou XD, Ye SL. Multimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal vein. World J Gastroenterol. 2001;7:28–32. doi: 10.3748/wjg.v7.i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung JW, Park JH, Im JG, Han JK, Han MC. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993;187:689–693. doi: 10.1148/radiology.187.3.8388567. [DOI] [PubMed] [Google Scholar]
- 33.Lin MT, Kuo PH. Pulmonary lipiodol embolism after transcatheter arterial chemoembolization for hepatocellular carcinoma. JRSM Short Rep. 2010;1:6. doi: 10.1258/shorts.2009.090352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geschwind JF, Kaushik S, Ramsey DE, Choti MA, Fishman EK, Kobeiter H. Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. J Vasc Interv Radiol. 2002;13:1163–1166. doi: 10.1016/s1051-0443(07)61959-9. [DOI] [PubMed] [Google Scholar]
- 35.Khan W, Sullivan KL, McCann JW, Gonsalves CF, Sato T, Eschelman DJ, Brown DB. Moxifloxacin prophylaxis for chemoembolization or embolization in patients with previous biliary interventions: a pilot study. AJR Am J Roentgenol. 2011;197:W343–W345. doi: 10.2214/AJR.10.6019. [DOI] [PubMed] [Google Scholar]
- 36.Okusaka T, Okada S, Ueno H, Ikeda M, Yoshimori M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Iwata R, et al. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. Oncology. 2000;58:293–299. doi: 10.1159/000012115. [DOI] [PubMed] [Google Scholar]
- 37.Kubota K, Hisa N, Nishikawa T, Fujiwara Y, Murata Y, Itoh S, Yoshida D, Yoshida S. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom Imaging. 2001;26:184–190. doi: 10.1007/s002610000139. [DOI] [PubMed] [Google Scholar]
- 38.A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332:1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier G, Roche A, Ink O, Anciaux ML, Derhy S, Rougier P, Lenoir C, Attali P, Etienne JP. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990;11:181–184. doi: 10.1016/0168-8278(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 40.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 41.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 42.Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2010;40:943–953. doi: 10.1111/j.1872-034X.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- 43.Vogl TJ, Naguib NN, Nour-Eldin NE, Rao P, Emami AH, Zangos S, Nabil M, Abdelkader A. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol. 2009;72:505–516. doi: 10.1016/j.ejrad.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Chua TC, Liauw W, Saxena A, Chu F, Glenn D, Chai A, Morris DL. Systematic review of neoadjuvant transarterial chemoembolization for resectable hepatocellular carcinoma. Liver Int. 2010;30:166–174. doi: 10.1111/j.1478-3231.2009.02166.x. [DOI] [PubMed] [Google Scholar]
- 45.Bouchard-Fortier A, Lapointe R, Perreault P, Bouchard L, Pomier-Layrargues G. Transcatheter arterial chemoembolization of hepatocellular carcinoma as a bridge to liver transplantation: a retrospective study. Int J Hepatol. 2011;2011:974514. doi: 10.4061/2011/974514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lesurtel M, Müllhaupt B, Pestalozzi BC, Pfammatter T, Clavien PA. Transarterial chemoembolization as a bridge to liver transplantation for hepatocellular carcinoma: an evidence-based analysis. Am J Transplant. 2006;6:2644–2650. doi: 10.1111/j.1600-6143.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhu AX, Abou-Alfa GK. Expanding the treatment options for hepatocellular carcinoma: combining transarterial chemoembolization with radiofrequency ablation. JAMA. 2008;299:1716–1718. doi: 10.1001/jama.299.14.1716. [DOI] [PubMed] [Google Scholar]
- 48.Wang N, Guan Q, Wang K, Zhu B, Yuan W, Zhao P, Wang X, Zhao Y. TACE combined with PEI versus TACE alone in the treatment of HCC: a meta-analysis. Med Oncol. 2011;28:1038–1043. doi: 10.1007/s12032-010-9620-2. [DOI] [PubMed] [Google Scholar]
- 49.Cabibbo G, Latteri F, Antonucci M, Craxì A. Multimodal approaches to the treatment of hepatocellular carcinoma. Nat Clin Pract Gastroenterol Hepatol. 2009;6:159–169. doi: 10.1038/ncpgasthep1357. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi A, Taniguchi H, Kunishima S, Koh T, Yamagishi H. Correlation between angiographically assessed vascularity and blood flow in hepatic metastases in patients with colorectal carcinoma. Cancer. 2000;89:1236–1244. doi: 10.1002/1097-0142(20000915)89:6<1236::aid-cncr7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 51.Lee E, Leon Pachter H, Sarpel U. Hepatic arterial embolization for the treatment of metastatic neuroendocrine tumors. Int J Hepatol. 2012;2012:471203. doi: 10.1155/2012/471203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mavligit GM, Zukwiski AA, Ellis LM, Chuang VP, Wallace S. Gastrointestinal leiomyosarcoma metastatic to the liver. Durable tumor regression by hepatic chemoembolization infusion with cisplatin and vinblastine. Cancer. 1995;75:2083–2088. doi: 10.1002/1097-0142(19950415)75:8<2083::aid-cncr2820750809>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 53.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321–326. doi: 10.1016/s1051-0443(07)61911-3. [DOI] [PubMed] [Google Scholar]
- 55.Caturelli E, Siena DA, Fusilli S, Villani MR, Schiavone G, Nardella M, Balzano S, Florio F. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue-long-term prospective study. Radiology. 2000;215:123–128. doi: 10.1148/radiology.215.1.r00ap21123. [DOI] [PubMed] [Google Scholar]
- 56.Kim HK, Chung YH, Song BC, Yang SH, Yoon HK, Yu E, Sung KB, Lee YS, Lee SG, Suh DJ. Ischemic bile duct injury as a serious complication after transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol. 2001;32:423–427. doi: 10.1097/00004836-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Sueyoshi E, Hayashida T, Sakamoto I, Uetani M. Vascular complications of hepatic artery after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2010;195:245–251. doi: 10.2214/AJR.08.2301. [DOI] [PubMed] [Google Scholar]
- 58.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 59.Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17:359–366. doi: 10.1634/theoncologist.2011-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 61.Saito T, Tsuchiya T, Sato Y, kenjo A, Kimura T, Anazawa T, Terashima T, Takahashi M, Ohira A, Mitsukazu G. Effect of Transarterial Immunoembolization as Preoperative Treatment for Hepatocellular Carcinoma. Ann Cancer Res Therap. 2011;19:26–33. [Google Scholar]
- 62.Sato T, Sullivan KL, Eschelman DJ, Gonsalves CF, Terai M, Sakashita H, McCue PA, Berd D, Mastrangelo MJ. Immunoembolization of malignant liver tumor with granulocyte/macrophage colony stimulating factor (GM-CSF) and ethiodized oil followed by gelatin sponge pledgets. J Clin Oncol. 2005;23:2514. [Google Scholar]
- 63.Hanyu K, Iida T, Shiba H, Ohashi T, Eto Y, Yanaga K. Immunogene therapy by adenovirus vector expressing CD40 ligand for metastatic liver cancer in rats. Anticancer Res. 2008;28:2785–2789. [PubMed] [Google Scholar]
- 64.Shiba H, Okamoto T, Futagawa Y, Ohashi T, Eto Y. Efficient and cancer-selective gene transfer to hepatocellular carcinoma in a rat using adenovirus vector with iodized oil esters. Cancer Gene Ther. 2001;8:713–718. doi: 10.1038/sj.cgt.7700368. [DOI] [PubMed] [Google Scholar]
- 65.Shiba H, Okamoto T, Futagawa Y, Misawa T, Yanaga K, Ohashi T, Eto Y. Adenovirus vector-mediated gene transfer using degradable starch microspheres for hepatocellular carcinoma in rats. J Surg Res. 2006;133:193–196. doi: 10.1016/j.jss.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 66.O'Reilly MS. The combination of antiangiogenic therapy with other modalities. Cancer J. 2002;8 Suppl 1:S89–S99. [PubMed] [Google Scholar]
- 67.Jiang H, Meng Q, Tan H, Pan S, Sun B, Xu R, Sun X. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int J Cancer. 2007;121:416–424. doi: 10.1002/ijc.22655. [DOI] [PubMed] [Google Scholar]
- 68.Zheng C, Feng G, Zhou R. [New use of Bletilla striata as embolizing agent in the intervention treatment of hepatic carcinoma] Zhonghua Zhongliu Zazhi. 1996;18:305–307. [PubMed] [Google Scholar]
- 69.Feng G, Kramann B, Zheng C, Zhou R. [Comparative study on the long-term effect of permanent embolization of hepatic artery with Bletilla striata in patients with primary liver cancer] J Tongji Med Univ. 1996;16:111–116. doi: 10.1007/BF02887970. [DOI] [PubMed] [Google Scholar]
- 70.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM, Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 72.Syha R, Ketelsen D, Heller S, Schmehl J, Mangold S, Heuschmid M, Springer F, Claussen CD, Brechtel K. Hepatocellular carcinoma: initial tumour response after short-term and long-interval chemoembolization with drug-eluting beads using modified RECIST. Eur J Gastroenterol Hepatol. 2012:Epub ahead of print. doi: 10.1097/MEG.0b013e32835724bc. [DOI] [PubMed] [Google Scholar]
- 73.Martin R, Geller D, Espat J, Kooby D, Sellars M, Goldstein R, Imagawa D, Scoggins C. Safety and efficacy of trans arterial chemoembolization with drug-eluting beads in hepatocellular cancer: a systematic review. Hepatogastroenterology. 2012;59:255–260. doi: 10.5754/hge10240. [DOI] [PubMed] [Google Scholar]
- 74.van Malenstein H, Maleux G, Vandecaveye V, Heye S, Laleman W, van Pelt J, Vaninbroukx J, Nevens F, Verslype C. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011;34:368–376. doi: 10.1159/000329602. [DOI] [PubMed] [Google Scholar]
- 75.Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012:Epub ahead of print. doi: 10.1016/j.jhep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 76.Gaur SK, Friese JL, Sadow CA, Ayyagari R, Binkert CA, Schenker MP, Kulke M, Baum R. Hepatic arterial chemoembolization using drug-eluting beads in gastrointestinal neuroendocrine tumor metastatic to the liver. Cardiovasc Intervent Radiol. 2011;34:566–572. doi: 10.1007/s00270-011-0122-1. [DOI] [PubMed] [Google Scholar]
- 77.Raoul JL, Guyader D, Bretagne JF, Heautot JF, Duvauferrier R, Bourguet P, Bekhechi D, Deugnier YM, Gosselin M. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26:1156–1161. doi: 10.1002/hep.510260511. [DOI] [PubMed] [Google Scholar]
- 78.Kan RW, Tsang SH, Poon RT, Cheung TT. Update on yttrium-90-based radio-embolization for treatment of hepatocellular carcinoma. ANZ J Surg. 2012;82:505–509. doi: 10.1111/j.1445-2197.2012.06121.x. [DOI] [PubMed] [Google Scholar]
- 79.López-Benítez R, Hallscheidt P, Kratochwil C, Ernst C, Kara L, Rusch O, Vock P, Kettenbach J. Protective Embolization of the Gastroduodenal Artery with a One-HydroCoil Technique in Radioembolization Procedures. Cardiovasc Intervent Radiol. 2012:Epub ahead of print. doi: 10.1007/s00270-012-0361-9. [DOI] [PubMed] [Google Scholar]
- 80.Shaheen M, Hassanain M, Aljiffry M, Cabrera T, Chaudhury P, Simoneau E, Kongkaewpaisarn N, Salman A, Rivera J, Jamal M, et al. Predictors of response to radio-embolization (TheraSphere®) treatment of neuroendocrine liver metastasis. HPB (Oxford) 2012;14:60–66. doi: 10.1111/j.1477-2574.2011.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burgmans MC, Irani FG, Chan WY, Teo TK, Kao YH, Goh AS, Chow PK, Lo RH. Radioembolization After Portal Vein Embolization in a Patient with Multifocal Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2012:Epub ahead of print. doi: 10.1007/s00270-012-0376-2. [DOI] [PubMed] [Google Scholar]
- 82.Xie F, Zang J, Guo X, Xu F, Shen R, Yan L, Yang J, He J. Comparison of transcatheter arterial chemoembolization and microsphere embolization for treatment of unresectable hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2012;138:455–462. doi: 10.1007/s00432-011-1117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, Van Buskirk M, Roberts CA, Goin JE. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194–S205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 84.Townsend A, Price T, Karapetis C. Selective internal radiation therapy for liver metastases from colorectal cancer. Cochrane Database Syst Rev. 2009;(4):CD007045. doi: 10.1002/14651858.CD007045.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 86.Nakamoto Y, Mizukoshi E, Tsuji H, Sakai Y, Kitahara M, Arai K, Yamashita T, Yokoyama K, Mukaida N, Matsushima K, et al. Combined therapy of transcatheter hepatic arterial embolization with intratumoral dendritic cell infusion for hepatocellular carcinoma: clinical safety. Clin Exp Immunol. 2007;147:296–305. doi: 10.1111/j.1365-2249.2006.03290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mohamadnejad M, Ashrafi M, Alimoghaddam K, Vosough M, Mardpour S, Azimian V, Aghdami N, Bagheri M, Abdollahzadeh L, Bashtar M, et al. Surveillance for Hepatocellular Carcinoma after Autologous Stem Cell Transplantation in Cirrhosis. Middle East J Dig Dis. 2012;4:145–149. [PMC free article] [PubMed] [Google Scholar]
- 88.Ismail A, AlDorry A, Shaker M, Elwekeel R, Mokbel K, Zakaria D, Meshaal A, Eldeen FZ, Selim A. Simultaneous injection of autologous mononuclear cells with TACE in HCC patients; preliminary study. J Gastrointest Cancer. 2011;42:11–19. doi: 10.1007/s12029-010-9218-0. [DOI] [PubMed] [Google Scholar]


