Graphical abstract
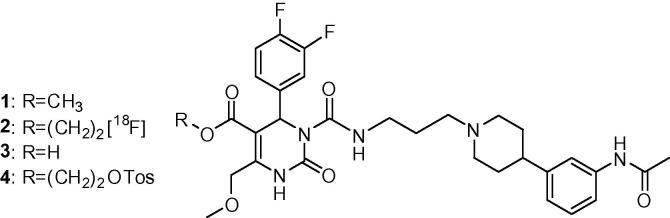
SNAP-7941 derivatives 1–4 (1: SNAP-7941; 2: [18F]FE@SNAP; 3: SNAP-acid; 4: Tos@SNAP).
Keywords: MCHR1, Fluorine-18, PET, Microfluidic, SNAP-7941, Radioligand
Abstract
Changes in the expression of the melanin concentrating hormone receptor 1 (MCHR1) are involved in a variety of pathologies, especially obesity and anxiety disorders. To monitor these pathologies in-vivo positron emission tomography (PET) is a suitable method. After the successful radiosynthesis of [11C]SNAP-7941—the first PET-Tracer for the MCHR1, we aimed to synthesize its [18F]fluoroethylated analogue: [18F]FE@SNAP. Therefore, microfluidic and vessel-based approaches were tested. [18F]fluoroethylation was conducted via various [18F]fluoroalkylated synthons and direct [18F]fluorination. Only the direct [18F]fluorination of a tosylated precursor using a flow-through microreactor was successful, affording [18F]FE@SNAP in 44.3 ± 2.6%.
1. Introduction
Melanin concentrating hormone (MCH) is a cyclic neuropeptide originally isolated from the salmon pituitary gland as a hormone responsible for skin pigmentation in teleost fish.1 In mammals, MCH is predominantly expressed in the lateral hypothalamus and zona incerta,2,3 but is also found in peripheral organs and tissues, such as the pancreas,4 colonic epithelial cells5 or adipocytes.6,7 It plays a key role in energy homeostasis, e.g. the control of food intake, body weight and metabolism.8,9 Furthermore, it is involved in anxiety,10–14 diabetes,4 gut inflammation5 and adiposity.6,7 The biological function of MCH is mediated by two G-protein coupled receptors, MCH receptor 1 and 2 (MCHR115–18 and MCHR2.19–22) The widespread distribution of MCH and its receptors and the involvement in a variety of pathologies makes the MCH system interesting as a target to treat human disorders.
Several MCHR1 antagonists were presented in the last decade; some of them have entered clinical trials for the treatment of obesity,23 some are in discussion of becoming anti-diabetic drugs.24 However, to enable confidence in preclinical to clinical translation of central MCHR1 pharmacology, a suitable positron emission tomography (PET) tracer needs to be developed. Borowsky et al.25 presented the evaluation of the very potent MCHR1 antagonist SNAP-7941 ((+)-methyl (4S)-3-{[(3-{4-[3-(acetylamino)phenyl]-1-piperidinyl}propyl)amino]carbonyl}-4-(3,4-difluorophenyl)-6-(methoxymethyl)-2-oxo-1,2,3,4-tetrahydro-5-pyrimidinecarboxylate hydrochloride, 1, Figure 1) (Kd = 0.18 nM, evaluated on Cos-7 cells expressing the human MCHR1 (hMCHR1)).25 We previously reported the successful radiosynthesis of its radiolabelled analogue, [11C]SNAP-7941—the first PET tracer for the MCHR1.26 Due to the practical advantages of the longer-lived radioisotope 18F (t1/2 = 110 min) instead of 11C (t1/2 = 20 min) and the comparable stability of methyl- and fluoroethylesters,27 we aimed to synthesize a [18F]fluoroethylated analogue: [18F]FE@SNAP (2, Fig. 1). We performed preliminary stability tests by incubation in human plasma at 37 °C which showed almost no degradation (<5%) within 120 min; and incubation with carboxyl esterase in a standard assay revealed only 20% cleavage within 6 h. Additionally, in preliminary binding experiments on CHO-hMCHR1 cell membranes, FE@SNAP evinced similar binding affinity as the parent compound SNAP-7941 (Kd = 0.18 nM).
Figure 1.
SNAP-7941 derivatives 1–4 (1: SNAP-7941; 2: [18F]FE@SNAP; 3: SNAP-acid; 4: Tos@SNAP).
In general, 18F-fluoroalkyl radiolabels can be introduced using either a direct [18F]fluorination of a suitable precursor-compound (already containing a leaving group at the alkyl side chain) or via two step labelling reaction using an 18F-fluoroalkylating synthon. Even for flow-through microreactor procedures both synthesis strategies have already been reported for various radiotracer syntheses: For example, direct radio-fluorinations were presented for 2-[18F]FDG,28–31 [18F]fallypride,32 [18F]annexin,29,31 and N-[18F]fluoroacetyl-N-(2,5-dimethoxybenzyl)-2-phenoxyaniline;33 furthermore, the [18F]fluoroethylation of carboxylic acids,34 [18F]fluoroalkylation of diverse [18F]fluorocholine derivatives using either [18F]fluoroethyl tosylate or [18F]fluoropropyl tosylate35,36 and the syntheses of [18F]fluoroarenes from diaryliodonium salts37 have been presented.
The aim of this work was the preparation of [18F]FE@SNAP in sufficient amounts for future pre-clinical applications. For this purpose, vessel-based as well as microfluidic approaches were conducted, starting from two different precursors: SNAP-acid (3, Fig. 1) (for the two step [18F]fluoroalkylation) and Tos@SNAP (4, Fig. 1) (for direct radiolabelling).
2. Results and discussion
2.1. General
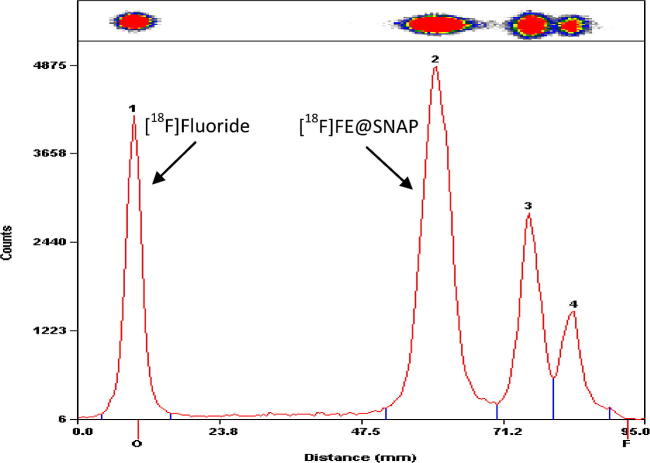
TLC Rf-values were 0.00–0.02 for [18F]fluoride and 0.63–0.69 for [18F]FE@SNAP. A typical radio-TLC sample chromatogram is presented in Figure 2. HPLC retention times were 2.3–2.6 min (k′ = 0.5–0.7) for Tos@SNAP and 5.1–5.9 min (k′ = 2.4–2.9) for [18F]FE@SNAP. All values were verified by inactive reference substances. The TLC Rf-values of the two additional, unknown radio-labelled side products were 0.76–0.82 and 0.87–0.93, ‘respectively’; HPLC retention times were 3.6–4.3 min (k′ = 1.4–1.7) and 6.9–7.7 min (k′ = 3.6–4.1).
Figure 2.
Typical radio-TCL chromatogram of the crude reaction mixture (7.5 mg/mL, 170 °C, 150 μL/min) with results after integration.
2.2. Vessel-based preparation
The production of all [18F]fluoroalkylation agents ([18F]BFE, [18F]FEtTos, [18F]FEtTf) was successful: 2.9 ± 0.9 GBq [18F]BFE (23.1 ± 10.4% EOB), 0.3 ± 0.1 GBq [18F]FEtTos (24.0 ± 0.7% EOB) and 0.3 ± 0.2 GBq [18F]FEtTf (19.2 ± 9.6% EOB) were achieved. However, the [18F]fluoroethylation of SNAP-acid via these synthons did not succeed. No radiochemical incorporation was observed regardless of the conditions applied (reaction temperature, reaction time, solvent, synthon). Furthermore, the direct fluorination of Tos@SNAP in the conventional approach was not successful, either. Again, no radiochemical incorporation was observed.
2.3. Microfluidic preparation
Radiolabelling of the [18F]fluoroalkylation agent [18F]FEtTos was in accordance to the literature.35,36 However, the second step—the [18F]fluoroethylation of SNAP-acid—was not successful using this approach.
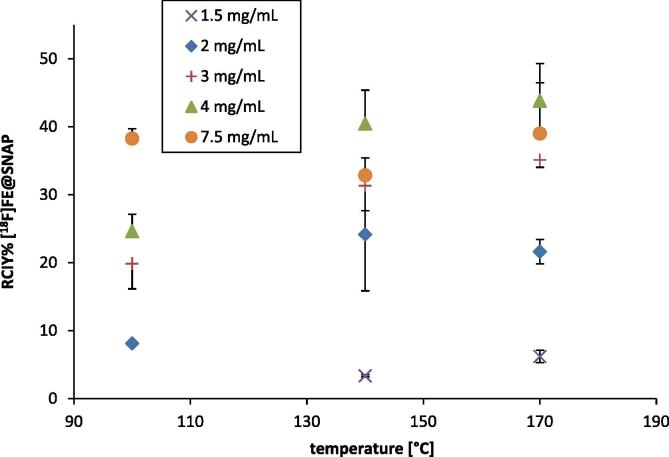
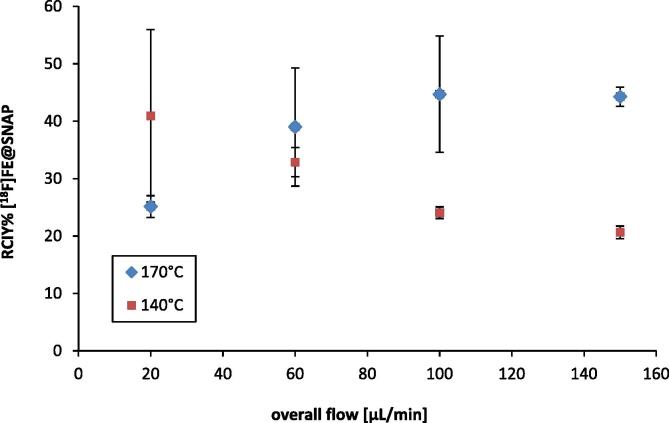
The direct [18F]fluorination via the microfluidic system finally resulted in the production of [18F]FE@SNAP. The RCIY was increasing with higher reaction temperatures and increasing amounts of precursor. Highest RCIYs were obtained at 170 °C using 4 mg/mL of precursor in the reaction mixture (43.8 ± 2.2%). Further raise of precursor amount did not improve synthetic outcome (Fig. 3). Reasonable radiolabelling yields and no formation of any side products could be observed at 100 °C. At 140 °C radiochemical incorporation was increased but two unknown radiolabelled side products were formed additionally. When increasing the reaction temperature up to 170 °C side product formation was even more pronounced. At the same time degradation of precursor with increasing temperature could be observed in the UV channel of the HPLC chromatogram. This degradation also increased with slow flow rates. Therefore, the dependency upon the flow rate was determined both at 140 °C and 170 °C (7.5 mg/mL Tos@SNAP, Fig. 4). At 170 °C the RCIY increased with increasing flow rates. The inverse trend was observed at 140 °C. RCIYs decreased with increasing flow rates. However, best RCIYs were observed using fast flow rates up to 150 μL/min at 170 °C. In this set-up the residence time within the heated microreactor (15.6 μL) was only 6.24 s. This time was sufficient to radiolabel the tosylated precursor and short enough to avoid extensive precursor degradation. RCIY of [18F]FE@SNAP was increased from 25.1 ± 1.8% (20 μL/min) to 44.3 ± 2.6% (150 μL/min) applying 7.5 mg/mL precursor at 170 °C.
Figure 3.
Dependency of the RCIY upon precursor concentration. Reactions were performed with a flow rate of 60 μL/min at 100 °C, 140 °C and 170 °C. Values are decay-corrected and given as arithmetic means ± SD (n ⩾ 3). Error bars are depicted unidirectional for better visualization but represent deviations in both directions.
Figure 4.
Influence of the overall flow rate on the RCIY. Reactions were conducted using 7.5 mg/mL Tos@SNAP in reaction mixture. Values are decay-corrected and given as arithmetic means ± SD (n ⩾ 3).
Due to previous findings, 38 the reaction volume was examined as additional parameter. No “bolus volume effect” could be observed for this set-up. For reactions using 3 mg/mL Tos@SNAP at 170 °C and a flow rate of 150 μL/min, RCIY was similar for small-scale reactions (20 μL) and for enlarged reaction boluses (300 μL): 30.5 ± 8.9%.
All these results led to the optimum reaction conditions for the radiochemical preparation of [18F]FE@SNAP summarized in Table 1.
Table 1.
Optimum reaction conditions and outcome for the preparation of [18F]FE@SNAP
| Amount of precursor (mg/mL) | 4 |
| Reaction temperature (°C) | 170 |
| Flow rate (μL/min) | 150 |
| RCIY (%) | 43.8 ± 2.2 |
3. Conclusion
[18F]FE@SNAP could not be synthesized in any vessel-based approach neither using direct radio-fluorination of the tosylated precursor nor through the coupling of a [18F]fluoroalkylation agent to SNAP-acid. Once again, flow-through microreactor technology was demonstrated to be a valuable addition in the portfolio of radiolabelling techniques for the preparation of new PET tracers. Solely, a microfluidic procedure enabled the radiosynthesis of [18F]FE@SNAP. Further experiments comprising an up-scaling, purification and formulation of [18F]FE@SNAP will enable its use in preclinical investigations.
4. Experimental section
4.1. General
Chemicals and solvents were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (Vienna, Austria). All reagents were at least of analytical grade and used without purification. [18F]fluoride was produced via the 18O(p,n)18F reaction in a GE PETtrace cyclotron (16.5-MeV protons; GE Medical Systems, Uppsala, Sweden). H218O (HYOX18; >98%) was purchased from Rotem Europe (Leipzig, Germany). Anion-exchange cartridges (PS-HCO3) for [18F]fluoride trapping were obtained from Macherey-Nagel (Dueren, Germany). 2-bromoethyl triflate39 (BETfO) and ethylene glycol bis triflate40 were synthesized in cooperation with the Department of Drug and Natural Product Synthesis of the University of Vienna (Austria). Alumina cartridges (Sep-Pak® Light Alum N) were purchased from Waters (Waters® Associates Milford, MA, USA). For conventional small-scale preparations, the reactions were carried out in Wheaton 1.0 mL v-vials (clear glass) assembled with TFE/silicone septa. Microfluidic reactions were carried out within an Advion NanoTek® unit (Ithaca, NY, USA) comprising a concentrator unit (CE) and a liquid flow reaction unit (LF) with dedicated control software (Advion, version 1.4). The set-up is illustrated in Figure 5. Microreactors were made of fused silica tubing (inner diameter 0.1 μm; length 2.0 m), wound-up and held in a brass ring filled with a thermoresistant polymer to hold the tubing in its place. Drying and collecting v-vials (3.0 and 5.0 mL, clear glass) were obtained from Alltech (Deerfield, IL, USA). Radiochemical incorporation yields (RCIYs) were determined by analysing the reaction mixtures by radio-analytical thin-layer chromatography (radio-TLC) and analytical high performance liquid chromatography (HPLC). Radio-TLC was performed using silica gel 60 RP-18 F254S plates from Merck (Darmstadt, Germany) (mobile phase: acetonitrile/water 95/5 v/v). Analyses of radio-TLC plates were done using a Canberra-Packard Instant Imager (Perkin Elmer, Watford, UK). Analytical HPLC was performed using an Agilent system (Boeblingen, Germany) consisting of an autosampler 1100, a quaternary pump 1200 (flow: 1 mL/min), a diode array detector 1200 (operated at 255 nm) and a lead-shielded NaI-radiodedector (Raytest Isotopenmessgeräte GmbH, Straubenhardt, Germany). HPLC column (Chromolith® Performance RP-18e; 100–4.6 mm) was purchased from Merck (Darmstadt, Germany). HPLC mobile phase consisted of (water/acetic acid 97.5/2.5 v/v; 2.5 g/L ammonium acetate; pH 3.5)/acetonitrile 70/30 v/v.
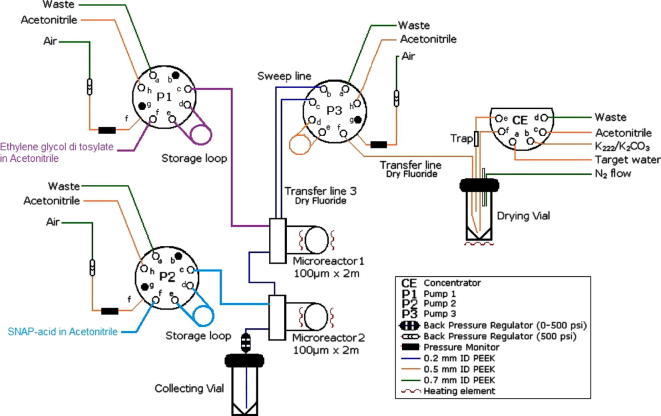
Figure 5.
Schematic illustration of the set-up of the Advion NanoTek® system for two-step reactions.
4.2. Preparation of precursor compounds and reference standard
Syntheses of precursor compounds (SNAP-acid26 and Tos@SNAP41,42) and reference standard (FE@SNAP43) were performed in cooperation with the Department of Drug and Natural Product Synthesis of the University of Vienna (Austria). 1H- and 13C-NMR data and spectra as well as 19F-NMR, MS, HRMS and IR data, are available as Supplementary data.
4.3. Azeotropic drying
Azeotropic drying of cyclotron produced [18F]fluoride was always performed within the concentrator unit of the Advion NanoTek® synthesizer using a programmed procedure, in order to avoid differences between manual and automatic azeotropic drying. N.C.A [18F]fluoride (1.5–31.7 GBq) was trapped on to an anion exchange cartridge (PS-HCO3) and released with a solution containing Kryptofix 2.2.2 (4,7,13,16,21,24-hexaoxa-1,10-diaza-bi-cyclo[8.8.8]hexacosane; 10 mg, 26.6 μmol) and potassium carbonate (2.25 mg, 16.6 μmol) in acetontrile/water (70/30 v/v; V = 0.5 mL). Iterative azeotropic drying was performed at 110 °C by addition of 3 times 300 μL of dry acetonitrile.
4.4. Vessel-based preparation
4.4.1. Fluoroethylation via 1-bromo-2 [18F]fluoroethane ([18F]BFE)
[18F]BFE was produced according to Zuhayra et al.44 Briefly, to the dried [18F]fluoride-aminopolyether a mixture of 30 μL BETfO and 500 μL 1,2-dichlorobenzene (o-DCB) was added. After heating at 100 °C for 10 min, [18F]BFE was distilled at 130 °C under helium flow into the respective solvent (DMF, DMSO and 2-butanone). The precursor SNAP-acid (4 mg/mL) was activated by adding an equimolar amount of tetrabutylammonium hydroxide (TBAH) and dissolved in the respective solvent. The [18F]BFE solution was added to the precursor solution (1:1) and the reaction mixture was heated at 75–140 °C for 10–20 min.
4.4.2. Fluoroethylation via [18F]fluoroethyl tosylate ([18F]FEtTos)
Ethylene glycol di-p-tosylate (9.25 mg/mL in acetonitrile) was added to the dried [18F]fluoride-aminopolyether and heated at 85 °C for 15 min. The resulted [18F]FEtTos was added to SNAP-acid (activated with TBAH, 4 mg/mL in acetonitrile) (1:1) and the reaction mixture was heated at 75 °C for 10–30 min.
4.4.3. Fluoroethylation via [18F]fluoroethyl triflate ([18F]FEtTf)
[18F]FEtTf was produced according to Kiesewetter et al.40 Briefly, a solution of ethylene glycol bis triflate (1.0 mg in 100 μL acetonitrile) was added to the dried [18F]fluoride-aminopolyether. After heating at 125 °C for 1 min, the solution was cooled in an ice bath and triflic anhydride (5 μL) was added. The solution was loaded onto an alumina cartridge and [18F]FEtTf was eluted with acetonitrile (500 μL). The solution of [18F]FEtTf was added to SNAP-acid (activated with TBAH, 4 mg/mL in acetonitrile) (1:1) and heated at 75 °C for 10–30 min.
4.4.4. Direct [18F]fluorination
The tosylated precursor Tos@SNAP (2 mg/mL) was dissolved in acetonitrile and added to the dried [18F]fluoride-aminopolyether. The reaction mixture was heated at 75 °C for 10–90 min.
4.5. Microfluidic preparation
4.5.1. Fluoroethylation via [18F]fluoroethyl tosylate ([18F]FEtTos)
Reaction educts were dissolved in acetonitrile and drawn into the respective loops (loop 1: ethylene glycol di-p-tosylate (10 mg/mL); loop 2: SNAP-acid (activated with TBAH, 20 mg/mL); loop 3: dried [18F]fluoride-aminopolyether in 500 μL acetonitrile). Before starting the experiments, transfer-lines were filled with the solutions from the storage loops and the reactors were finally swept with the reaction solvent via pump 3 (Fig. 2). The synthesis was carried out within the Advion NanoTek® using the ‘discovery mode’ for two step reactions. The conversion to [18F]FEtTos (first reaction) was performed by pushing the previously filled solutions simultaneously from loop 1 and 3 through the first temperature controlled (180 °C) flow-through microreactor (flow rate: 30–50 μL/min). In a second phase the [18F]FEtTos-mixture was swept out of the reactor through the storage line towards the other reactor. The second reaction starts when the [18F]FEtTos-mixture and the precursor solution (loop 2) are pushed through the second microreactor (100–180 °C) with defined velocity (20–150 μL/min) followed by a final sweeping where the reaction mixture is finally flushed out of the system into a collecting vial filled with 200 μL of solvent. Total reaction bolus volumes between 28 μL and 90 μL (7 + 7 + 14 and 15 + 15 + 60 μL) were tested.
4.5.2. Direct [18F]fluorination
Dried [18F]fluoride-aminopolyether dissolved in 500 μL acetonitrile and Tos@SNAP (3–10 mg/mL in acetonitrile) precursor solution were drawn and stored in the respective loops of pumps 1 and 3 of the LF unit. Before starting the experiments, transfer lines 1 and 3 were filled with the solutions from the storage loops and the reactor was swept with the reaction solvent via pump 3. Optimal reaction conditions were determined using the pre-installed ‘discovery’ mode for one step reactions: small volumes (5–200 μL) of the previously filled solutions were simultaneously pushed through the temperature-controlled reactor (100–200 °C) with defined overall flow rates (60–150 μL/min). Subsequently, the crude product solution was swept out of the micro reactor with a defined volume of 200 μL of acetonitrile into collection vials.
4.6. Statistical analyses
Quantitative data (both in text and figures) are expressed as arithmetic mean ± standard deviation. If not stated otherwise all experiments were performed in triplicates.
Acknowledgement
This research was part of an ongoing study, funded by the Austrian Science Fund (FWF P20977-B09; P.I.: M. Mitterhauser).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2012.07.051.
Supplementary data
References and notes
- 1.Kawauchi H., Kawazoe I., Tsubokawa M., Kishida M., Baker B.I. Nature. 1983;305:321. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- 2.Bittencourt J.C., Presse F., Arias C., Peto C., Vaughan J., Nahon J.L., Vale W., Sawchenko P.E. J. Comp. Neurol. 1992;319:218. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 3.Casatti C.A., Elias C.F., Sita L.V., Frigo L., Furlani V.C.G., Bauer J.A., Bittencourt J.C. Neuroscience. 2002;115:899. doi: 10.1016/s0306-4522(02)00508-0. [DOI] [PubMed] [Google Scholar]
- 4.Tadayyon M., Welters H.J., Haynes A.C., Cluderay J.E., Hervieu G. Biochem. Biophys. Res. Commun. 2000;275:709. doi: 10.1006/bbrc.2000.3357. [DOI] [PubMed] [Google Scholar]
- 5.Kokkotou E., Moss A.C., Torres D., Karagiannides I., Cheifetz A., Liu S., O’Brian M., Maratos-Flier E., Pothoulakis C. Proc. Natl. Acad. Sci. U.S.A. 2008;105:10613. doi: 10.1073/pnas.0804536105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley R.L., Kokkotou E.G., Maratos-Flier E., Cheatham B. Diabetes. 2000;1073:49. doi: 10.2337/diabetes.49.7.1073. [DOI] [PubMed] [Google Scholar]
- 7.Bradley R.L., Mansfield J.P., Maratos-Flier E., Cheatham B. Am. J. Physiol. Endocrinol. Metab. 2002;283:E584. doi: 10.1152/ajpendo.00161.2002. [DOI] [PubMed] [Google Scholar]
- 8.Marsh D.J., Weingarth D.T., Novi D.E., Chen H.Y., Turmbauer M.E., Chen A.S., Guan X.M., Jiang M.M., Feng Y., Camacho R.E., Shen Z., Frazier E.G., Yu H., Metzger J.M., Kuca S.J., Shearman L.P., Gopal-Truter S., MacNeil D.J., Strack A.M., MacIntyre D.E., Van der Ploeg L.H.T., Qian S. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3240. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito M., Gomori A., Ishihara A., Oda Z., Mashiko S., Matsushita H., Yumoto M., Ito M., Sano H., Tokita S., Moriya M., Iwaasa H., Kanatani A. Am. J. Physiol. Endocrinol. Metab. 2003;284:E940. doi: 10.1152/ajpendo.00529.2002. [DOI] [PubMed] [Google Scholar]
- 10.Monzón M.E., De Barioglio S.R. Physiol. Behav. 1999;67:813. doi: 10.1016/s0031-9384(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 11.Kela J., Salmi P., Rimondini-Giorgini R., Heilig M., Wahlestedt C. Regul Pept. 2003;114:109. doi: 10.1016/s0167-0115(03)00114-9. [DOI] [PubMed] [Google Scholar]
- 12.Smith D.G., Davis R.J., Rorick-Kehn L., Morin M., Witkin J.M., McKenzie D.L., Nomikos G.G., Gehlert D.R. Neuropsychopharmacology. 2006;31:1135. doi: 10.1038/sj.npp.1300913. [DOI] [PubMed] [Google Scholar]
- 13.Roy M., David N.K., Danao J.V., Baribault H., Tian H., Giorgetti M. Neuropsychopharmacology. 2006;31:112. doi: 10.1038/sj.npp.1300805. [DOI] [PubMed] [Google Scholar]
- 14.Roy M., David N., Cueva M., Giorgetti M. Biol. Psychiatry. 2007;61:174. doi: 10.1016/j.biopsych.2006.03.076. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y., Nothacker H.P., Wang Z., Lin S.H., Leslie F., Civelli O. Nature. 1999;400:265. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- 16.Shimomura Y., Mori M., Sugo T., Ishibashi Y., Abe M., Kurokawa T., Onda H., Nishimura O., Sumino Y., Fujino M. Biochem. Biophys. Res. Commun. 1999;261:622. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- 17.Chambers J., Ames R.S., Bergsma D., Muir A., Fitzgerald L.R., Hervieu G., Dytko G.M., Foley J.J., Martin J., Liu W.S., Park J., Ellis C., Ganguly S., Konchar S., Cluderay J., Leslie R., Wilson S., Sarau H.M. Nature. 1999;400:261. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- 18.Lembo P.M.C., Grazzini E., Cao J., Hubatsch D.A., Pelletier M., Hoffert C., St-Onge S., Pou C., Labreque J., Groblewski T., O’Donnell D., Payza K., Ahmad S., Walker P. Nat. Cell. Biol. 1999;1:267. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- 19.Sailer A.W., Sano H., Zeng Z., McDonald T.P., Pan J., Pong S.S., Feighner S.D., Tan C.P., Fukami T., Iwaasa H., Hreniuk D.L., Morin N.R., Sadowski S.J., Ito M., Ito M., Bansal A., Ky B., Figueroa D.J., Jiang Q., Austin C.P., MacNeil D.J., Ishihara A., Ihara M., Kanatani A., Van der Ploeg L.H.T., Howard A.D., Liu Q. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7564. doi: 10.1073/pnas.121170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill J., Duckworth M., Murdock P., Rennie G., Sabido-David C., Ames R.S., Szekeres P., Wilson S., Bergsma D.J., Gloger I.S., Levy D.S., Chambers J.K., Muir A.I. J. Biol. Chem. 2001;276:20125. doi: 10.1074/jbc.M102068200. [DOI] [PubMed] [Google Scholar]
- 21.Wang S., Behan J., O’Neill K., Weig B., Fried S., Laz T., Bayne M., Gustafson E., Hawes B.E. J. Biol. Chem. 2001;276:34664. doi: 10.1074/jbc.M102601200. [DOI] [PubMed] [Google Scholar]
- 22.An S., Cutler G., Zhao J.J., Huang S.G., Tian H., Li W., Liang L., Rich M., Bakleh A., Du J., Chen J.L., Dai K. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7576. doi: 10.1073/pnas.131200698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luthin D.R. Life Sci. 2007;81:423. doi: 10.1016/j.lfs.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Gattrell W.T., SambrookSmith C.P., Smith A.J. Bioorg. Med. Chem. Lett. 2012;22:2464. doi: 10.1016/j.bmcl.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Borowsky B., Durkin M.M., Ogozalek K., Marzabadi M.R., DeLeon J., Lagu B., Heurich R., Lichtblau H., Shaposhnik Z., Daniewska I., Blackburn T.P., Branchek T.A., Gerald C., Vaysse P.J., Forray C. Nat. Med. 2002;8:825. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- 26.Philippe C., Schirmer E., Mitterhauser M., Shanab K., Lanzenberger R., Karanikas G., Spreitzer H., Viernstein H., Wadsak W. Appl. Radiat. Isot. 2012 doi: 10.1016/j.apradiso.2012.07.010. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nics L., Haeusler D., Wadsak W., Wagner K.H., Dudczak R., Kletter K., Mitterhauser M. Nucl. Med. Biol. 2011;38:13. doi: 10.1016/j.nucmedbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Lee C.C., Sui G., Elizarov A., Shu C.J., Shin Y.S., Dooley A.N., Huang J., Daridon A., Wyatt P., Stout D., Kolb H.C., Witte O.N., Satyamurthy N., Heath J.R., Phelps M.E., Quake S.R., Tseng H.R. Science. 2005;310:1793. doi: 10.1126/science.1118919. [DOI] [PubMed] [Google Scholar]
- 29.Gillies J.M., Prenant C., Chimon G.N., Smethurst G.J., Dekker B.A., Zweit J. Appl. Radiat. Isot. 2006;64:333. doi: 10.1016/j.apradiso.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Steel C.J., O’Brien A.T., Luthra S.K., Brady F.J. Label. Compd. Radiopharm. 2007;50:308. [Google Scholar]
- 31.Gillies J.M., Prenant C., Chimon G.N., Smethurst G.J., Perrie W., Hamblett I., Dekker B.A., Zweit J. Appl. Radiat. Isot. 2006;64:325. doi: 10.1016/j.apradiso.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Lu S., Giamis A.M., Pike V.W. Curr. Radiopharm. 2009;2:49. doi: 10.2174/1874471010902010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briard E., Zoghbi S.S., Simeon F.G., Imaizumi M., Gourley J.P., Shetty H.U., Lu S., Fujita M., Innis R.B., Pike V.W. J. Med. Chem. 2009;52:688. doi: 10.1021/jm8011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu S.Y., Watts P., Chin F.T., Hong J., Musachio J.L., Briard E., Pike V.W. Lab chip. 2004;4:523. doi: 10.1039/b407938h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascali G., Mazzone G., Saccomanni G., Manera C., Salvadori P.A. Nucl. Med. Biol. 2010;37:547. doi: 10.1016/j.nucmedbio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Pascali G., Nannavecchia G., Pitzianti S., Salvadori P.A. Nucl. Med. Biol. 2011;38:637. doi: 10.1016/j.nucmedbio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Chun J.H., Lu S., Lee Y.S., Pike V.W. J. Org. Chem. 2010;75:3332. doi: 10.1021/jo100361d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ungersboeck, J.; Richter, S.; Collier, L.; Mitterhauser, M.; Karanikas, G.; Lanzenberger, R.; Dudczak, R.; Wadsak, W. Nucl. Med. Biol., 2012, May 23. [Epub ahead of print] (http://dx.doi.org/10.1016/j.nucmedbio.2012.04.004). [DOI] [PubMed]
- 39.Chi D.Y., Kilbourn M.R., Katzenellenbogen J.A., Welch M.J. J. Org. Chem. 1987;52:658. [Google Scholar]
- 40.Kiesewetter D.O., Brücke T., Finn R.D. Appl. Radiat. Isot. 1989;40:455. doi: 10.1016/0883-2889(89)90126-3. [DOI] [PubMed] [Google Scholar]
- 41.Schirmer, E. EHEC 2010—XXIVth European Colloquium on Heterocyclic Chemistry, August 23–27 2010, Vienna, Austria, book of abstracts, publ.: book-of-abstracts.com, H. Krebs, Gumpoldskirchen, Austria. ISBN: 978-3-9502992-0-5, PO-57.
- 42.Schirmer, E. PACCON 2010—Pure and Applied Chemistry International Conference, January 21 23 2010, Ubon Ratchathani, Thailand, book of abstracts, ISBN: 978-974-523-221-1, ORC-PO-47.
- 43.Schirmer, E. JMMC 2011—VII Joint Meeting on Medicinal Chemistry, Catania, Italy, June 30th-July 2nd 2011, book of abstracts, 127, www.jmmc2011.it.
- 44.Zuhayra M., Alfteimi A., Papp L., Lützen U., Lützen A., Von Forstner C., Meller B., Henze E. Bioorg. Med. Chem. 2008;16:9121. doi: 10.1016/j.bmc.2008.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.