Abstract
To identify the major serum biomarkers predicting the response to methotrexate (MTX) treatment in patients with early rheumatoid arthritis (RA), we evaluated the relationships between the individual response to MTX and various associated factors utilizing the 1H nuclear magnetic resonance (1H NMR)-based metabolomic method. Thirty-eight early RA patients were enrolled in this cohort study, and they received MTX (10 mg/week) orally as monotherapy for 24 weeks. According to the American College of Rheumatology criteria for improvement, clinical evaluation following MTX treatment was carried out at baseline and at the end of 24 weeks. Furthermore, collected serum samples were analyzed using 600 M 1H NMR for spectral binning. The obtained data were processed by both the unsupervised principal component analysis (PCA) and the supervised partial least squares discriminant analysis (PLS-DA). Lastly, multivariate analyses were performed to recognize the spectral pattern of endogenous metabolites related to MTX treatment. Differential clustering of 1H NMR spectra identified by PCA was found between the effective (n=25) and non-effective (n=13) group of RA patients receiving MTX treatment. Multivariate statistical analysis showed a difference in metabolic profiles between the two groups using PLS-DA (R2=0.802, Q2=0.643). In targeted profiling, 11 endogenous metabolites of the effective group showed a significant difference when compared with those of the non-effective group (p<0.05). Serum metabolites correlated with MTX treatment in patients with early RA were identified, which may be the major predictive factors for evaluating the response to MTX treatment in patients with early RA. Furthermore, our results highlight the usefulness of 1H NMR-based metabolomics as a feasible and efficient prognostic tool for predicting therapeutic efficacy to MTX treatment.
Keywords: metabolomics, nuclear magnetic resonance, methotrexate, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic chronic inflammatory joint disease, which is characterized by persistent synovitis, systemic inflammation and autoantibodies (1). Methotrexate (MTX) is the most widely used and is regarded as the anchor drug in the treatment of RA. Despite the advent of newer biologic therapies, MTX retains its central role since it is relatively inexpensive, broad experience with its use exists, and it is widely used in combination regimens with other disease-modifying antirheumatic drugs (DMARDs) (2). In the US and European countries, the recommended general dose of MTX is 15–20 mg/week, but individual optimal dose is in the range of 5–25 mg/week. In China, the conventional dose of MTX is 10 mg/week, but in practice 5–20 mg/week is prescribed based on individual sensitivity to and tolerance of MTX (3,4). Although well proven, it is recognized that there are large individual differences in the optimal dose of MTX for RA patients (5). The reasons for those individual differences are thought to be different concentrations of intracellular MTX-polyglutamates (MTX-PGs) and different enzyme activities at MTX-active sites (6). Levels of various cytokines and other mediators of inflammation, such as tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10), may also correlate with the efficacy of MTX (7,8). However, rapid clinical assays to measure these such as MTX-PGs, enzyme and cytokines are technically difficult and not available in most clinical facilities (9,10). To date, there are no useful and reliable clinical or molecular markers to predict the efficacy of MTX treatment.
Recent advances in metabolomics offer us a chance to identify changes in the concentration of small endogenous metabolites in biological samples caused by drug therapy. Metabolomics refers to the total metabolites present in biofluids (e.g., blood and urine) and chemometric techniques combining 1H nuclear magnetic resonance (1H NMR) has been applied for metabolomic profiling and characterization of individually tailored therapeutic approaches (11,12). Various studies have been performed to identify the major factors predicting the response to MTX therapy in RA based on the traditional method through evaluating the concentration of MTX-PGs (13). In addition, 1H NMR metabolomic study of rat urine was used to predict the risk of adverse effects caused by nonsteroid anti-inflammatory drugs (NSAIDs). A few endogenous metabolites of allantoin, taurine and dimethylamine were selected as putative biomarkers for gastric damage (14). Very recently, an alternative approach to understanding such inter-subject variability has been developed using metabolomics, and has been used to predict the metabolism and toxicity of a dosed substance, based solely on the analysis and modeling of a predose metabolic profile (15).
In this study, we categorized the subsets of early RA patients receiving MTX treatment for 24 weeks into two groups according to the clinic assessment criterion, i.e., effective group and non-effective group. Furthermore, 11 endogenous metabolites of the effective group were significant different than the non-effective group based on 1H NMR metabolomic analysis and multivariate statistical analysis (p<0.05). As a potential strategy and a more convenient technique, the 1H NMR-based metabolomic approach deserves further evaluation for assessing the efficacy of MTX treatment in patients with early RA.
Patients and methods
Patient background and medical records
This cohort study was conducted from January 2009 to September 2010 at a single investigational site, the Department of Integrated Chinese Traditional and Western Medicine, Tongji Hospital (Wuhan, China). Patient demographic and clinical details were collected on standardized data collection forms. All patients underwent physical examination, electrocardiography and routine blood testing. The duration of RA, age at which MTX administration was initiated, gender, body weight, laboratory data; clinical data including anti-cyclic citrullinated peptide (anti-CCP), serum C-reactive protein (CRP) concentration, rheumatoid factor (RF), erythrocyte sedimentation rate (ESR), serum creatinine clearance (SCR) and alanine transaminase (ALT) were obtained from the medical records.
Patients and study protocol
Patients 18 years of age and older with early (<0.5 year) active RA, as defined by the American College of Rheumatology (ACR; formerly, the American Rheumatism Association) (16), were included in the study and were scheduled to receive MTX monotherapy for 24 weeks. In all cases, the starting dose of oral MTX was 10 mg/week. Active disease was defined as following: ≥8 swollen joints, ≥10 tender joints and an ESR of ≥28 mm/h or a CRP concentration of ≥7.5 mg/l. Exclusion criteria were the following: previous use of glucocorticoids and/or DMARDs within a period of three months before inclusion in the study, alcohol abuse, serious comorbidity or recent major surgery. A total of 20 healthy age-matched volunteers, who had no rheumatic disease, were used as normal controls. Blood samples were collected at baseline (before starting MTX treatment) and 24 weeks after MTX monotherapy.
Ethical approval was obtained from the Ethics Committee of Tongji Medical College, China. Written informed consent was obtained from each participant in accordance with the principles outlined in the Declaration of Helsinki.
Clinical assessment
Treatment efficacy was evaluated in early RA patients receiving MTX monotherapy for 24 weeks, according to the European League Against Rheumatism (EULAR) response criteria (17). To obtain a better analysis, the patients were categorized into the ‘effective’ or ‘non-effective’ group based on the amount of change in the DAS28 and the level of DAS28 reached. The effective group was defined as patients who had a decrease in DAS28 from baseline (ΔDAS28) of >1.2 and a DAS28 at the sixth month of <3.2; and had either ΔDAS28 of >1.2 and a DAS28 at the sixth month of ≥3.2 or ΔDAS28 of 0.6 to 1.2 and a DAS28 at the sixth month of <5.1. The non-effective group consisted of those who had either ΔDAS28 of <0.6 or a DAS28 at the sixth month of ≥5.1.
Sample preparation
Venous blood samples were collected in the morning preprandial, after overnight fasting, using vacuum tubes (BD, 8.5 ml, USA) and left at room temperature to coagulate for 45 min. After this, samples were centrifuged at 1000 x g for 10 min, and serum was collected and immediately stored at −80°C until being used for metabolomic analysis (18).
Preparation of serum samples for metabolomic analysis by 1H NMR was performed manually according to Beckonert et al (19). After defrosting at room temperature, 200 μl of serum was mixed with 400 μl of 0.9% saline solution (wt/vol, NaCl in 90% H2O/10%/D2O) and then centrifuged at 12000 x g for 5 min at 4°C. After that, 550 μl of the supernatant was added to a 5-mm NMR tube and NMR acquisition was performed immediately.
NMR acquisition
The NMR spectra were acquired using a Bruker Avance III 600 spectrometer (Bruker BioSpin, Rheinstetten, Germany), operating at a 1H frequency of 600.13 MHz, and equipped with a 5-mm 1H TXI probe. Samples were afforded 5 min within the spectrometer to equilibrate before acquisition. Standard one-dimensional (1D) 1H NMR spectra were acquired using a single 90º pulse experiment with water presaturation using a recycle delay of 3 sec. Each data set was averaged over 64 transients using 32 K time domain points. The data were Fourier transformed, and spectra were referenced to the TSP signal at 0 ppm.
Each NMR spectrum was reduced to 0.04 ppm wide segments between 0.00 and 10.0 ppm using Chenomx NMR Suite Professional software version 4.6 (Chenomx Inc., Edmonton, Canada), giving a total of 230 integrated regions per NMR spectrum. The spectrum regions of water (δ=2.6–3.0 ppm) and urea (δ=4.3–4.7 ppm) were removed from the analysis for all groups in order to prevent variation in each sample. Each NMR variable was normalized to the total area in order to allow a spectrum-to-spectrum comparison. Metabolites were assigned based on comparisons with the chemical shifts of standard compounds in the Chenomx software. Custom library entries were created for unidentified resonances in order to carry them through the analysis for relative concentration comparison. Typically, the concentrations of serum metabolites were reported as ratios with creatinine.
Multivariate data analysis
The spectral data were converted to Microsoft Excel format and imported into SIMCA-P software (version 11.5, Umetrics, Umeå, Sweden) for multivariate analysis in which all spectral data were mean-centered with no scaling. Initially, principal component analysis (PCA) was applied to the clustering of all identified outliers. Partial least squares-discrimination analysis (PLS-DA) was then advanced for pattern recognition analysis from the converted spectral data (20). All the statistical analyses were carried out using binned bucket data and the identified metabolites for spectral binning and targeted profiling. PLS-DA made it possible to rotate the projection to latent variables that focus on class separation. Thus, PLS-DA aimed to find a model that separated classes of observations based on their x variables. The PLS-DA model was validated by permutation method by describing R2Y and Q2Y values. In addition, the choice of input variables for ANOVA analysis was based on magnitude of the variable influence on projection (VIP) from SIMCA-P where a VIP >1 indicated contribution to the model.
Statistical analysis
Clinic data are expressed as the means ± standard deviation (SD), and the data were analyzed for statistical significance by ANOVA test using SPSS version 17.0. Post-hoc tests were applied to perform all pairwise comparisons between group means by least significant difference (LSD) t-test. A value of p<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of the patients and metabolite identification
Demographics and characteristics of the patients at baseline and at the end of 24 weeks of MTX treatment are provided in Table I. Overall, 38 patients with early RA were enrolled in this study, and 20 healthy age-matched volunteers (15 females and 5 males, 55.3±3.6 years of age) were used as healthy controls. According to the EULAR DAS28 response criteria, the individuals treated with MTX montherapy were divided into two subsets, i.e., effective and non-effective group. Furthermore, the patients in the effective group showed a reduction in RA signs and symptoms, inhibited progression of structural damages, and improved physical function as compared with that of non-effective group (Table I).
Table I.
Demographics and clinical characteristics of the RA patients at baseline and after MTX treatment (10 mg/week) for 24 weeks.
| Group | Healthy controls (n=20) | RA patients (n=38) | Early RA patients receiving MTX treatment
|
|
|---|---|---|---|---|
| Non-effective (n=13) | Effective (n=25) | |||
| Female/male | 15/5 | 26/5 | 10/1 | 16/4 |
| Age (years) | 55.3±3.6 | 56.4±2.8 | 54.7±3.4 | 54.6±2.5 |
| Disease duration (years) | - | 4.5±1.9 | 4.4±1.2 | 4.2±0.8 |
| DAS28 | - | 5.9±1.4 | 4.6±1.2 | 2.3±0.8a,b |
| Anti-CCP (RU/ml) | 6.3±1.9 | 38.3±5.1 | 36.3±7.8 | 15.3±3.4a,b |
| CRP (mg/l) | 1.3±0.6 | 30.2±2.7 | 28.2±3.6 | 3.5±1.2a,b |
| RF (IU/ml) | 40.7±5.2 | 378.8±80.6 | 336.8±97.6 | 166.7±62.1a,b |
| ESR (mm/h) | 8.3±4.2 | 57.3±9.6 | 52.8±5.3 | 18.2±2.1a,b |
| SCR (mg/dl) | 53.6±9.5 | 58.6±7.4 | 68.2±9.8 | 60.7±8.2 |
| ALT (U/l) | 15.8±5.1 | 16.2±5.4 | 21.3±8.2 | 23.9±6.3 |
MTX, methotrexate; DAS, disease activity score; anti-CCP, anti-cyclic citrullinated peptide; CRP, c-reactive protein; RF, rheumatoid factor; ESR, erythrocyte sedimentation rate; SCR, serum creatinine clearance; ALT, alanine transaminase.
p<0.05 compared with healthy controls;
p<0.05 compared with non-effective group.
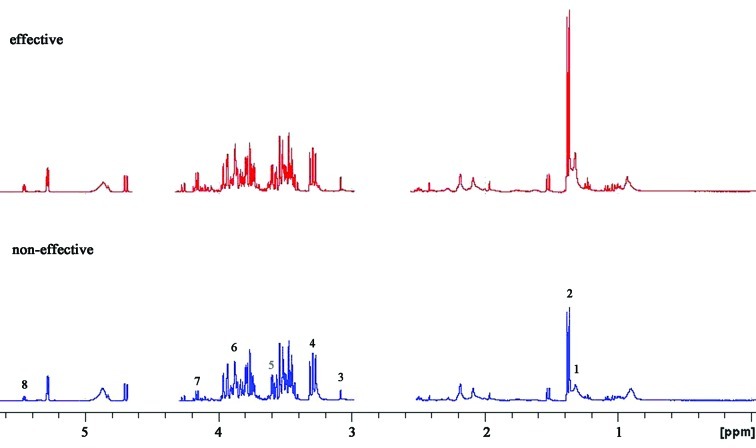
A 600 MHz 1D 1H NMR spectrum of a typical human serum sample is shown in Fig. 1. This revealed extensive complexity and significant information of biofluid. Regions of most significant metabolite signals usually fell between δ1–5.6 ppm, including alanine (δ1.3–1.35 ppm), acetate (δ1.35–1.4 ppm), citrate (δ3.0–3.2 ppm), cysteine (δ3.2–3.4 ppm), taurine (δ3.5–3.6 ppm), methionine (δ3.8–4.0 ppm), hypoxanthine (δ4.0–4.2 ppm) and uracil (δ5.4–5.6 ppm). Research in relation to the locations of the different chemical groups and various molecular-weight metabolites has already been reported (21,22). To accurately detect the difference between the two groups, specific software was used in the chemometric analysis.
Figure 1.
Representative 600-MHz 1H NMR spectrum of serum samples from the ‘effective’ group (red, top) and ‘non-effective’ group (blue, bottom). Typical serum samples are shown and peak assignments of major metabolites on 1H NMR spectra. The spectrum regions of water (δ=2.6–3.0 ppm) and urea (δ=4.3–4.7 ppm) were removed from the analysis for all groups in order to prevent variation in each sample. 1, alanine; 2, acetate; 3, citrate; 4, cysteine; 5, taurine; 6, methionine; 7, hypoxanthine; 8, uracil.
Principal component analysis of the serum samples in the patients receiving MTX treatment
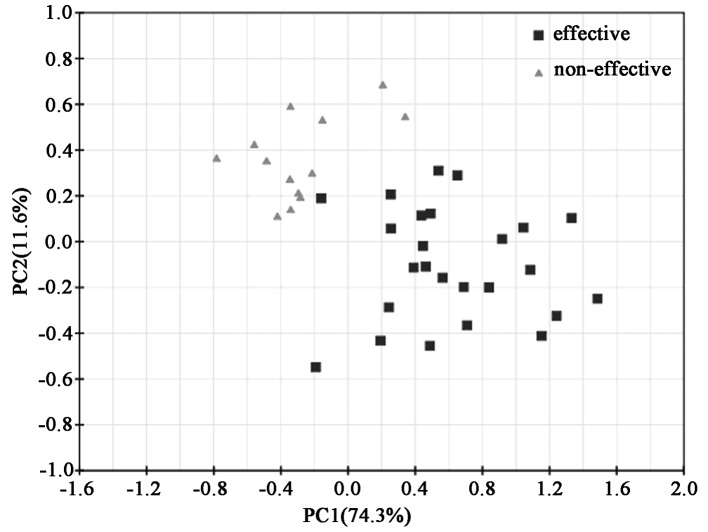
The mean-centered data from 1D 1H NMR spectra were input into the SIMCA-P software and processed by the unsupervised method of the principal component analysis (PCA), and the resulting score loading plots for both the effective and the non-effective group are shown (Fig. 2). PCA score plots showed clustering of serum samples in the first and second principal components. The diverged score plots signified a separation between the effective and the non-effective group along the axes corresponding to PC 1 and 2 with these two principal components accounting for 74.3 and 11.6% of the variation in the data, respectively. A tendency for grouping according to the effects of MTX treatment was noted along the first principal components. For further elucidation of the effective group following MTX treatment and providing validation, supervised analysis of the pattern recognition such as PLS and PLS-DA was performed.
Figure 2.
The score plot of PCA components in the effective and non-effective groups. Each point represented a single serum spectrum, with the position determined by the contribution of the tracked features. Black squares indicate the PCA plot of the ‘effective’ group in early RA patients receiving MTX treatment and grey triangles indicate the ‘non-effective’ group.
Partial least squares (PLS) and PLS-discriminant analysis (PLS-DA)
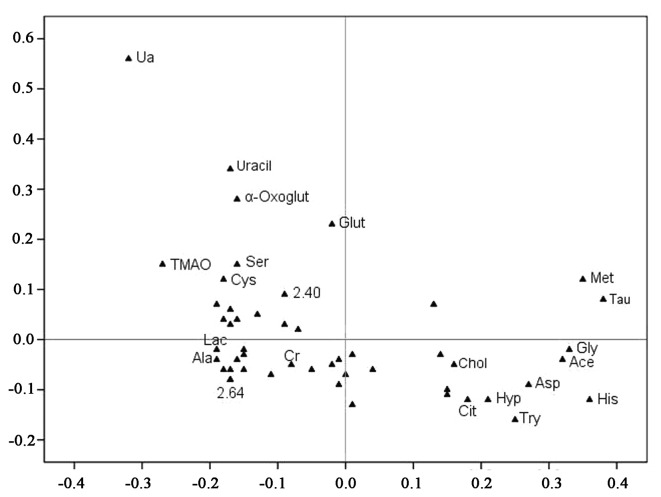
The spectral data of the serum samples were collected from the effective (n=25) and non-effective group (n=13), respectively, and the metabolic profiles of the serum samples are depicted in Fig. 3. The loading plots of the PCA demonstrated that the outer subset of compounds was important in the discrimination of the two groups. Furthermore, several features in the NMR metabolite profile were identified by the Chenomx NMR Suite (Table II).
Figure 3.
Loading plot from the PLS-DA analysis. Unknown resonances are indicated by the numerical ppm value or unmarked if not significant between the two groups. Each point represents the number of a metabolite included in the PLS-DA analysis (total, 20 metabolic data points are shown), and the axes are associated with the score plot of Fig. 2. Met, methionine; His, histidine; Gly, glycine; Ala, alanine; Cys, cysteine; Ser, serine; Tau, taurine; Glut, glutamine; Chol, cholesterol; Try, tryptophan; Hyp, hypoxanthine; Asp, aspartate; α-Oxoglut, α-oxoglutarate; Ace, acetate; TMAO, trimethylamine-N-oxide.
Table II.
List of the metabolites identified by the Chenomx NMR Suite in the serum samples.
| Metabolites | Chemical shift (ppm) and multiplicity |
|---|---|
| α-oxoglutarate | δ2.44 (t), 3.07 (t) |
| Glycine | δ3.55 (s) |
| Citrate | δ2.55 (d), 2.65 (d) |
| Aspartate | δ3.56 (s), 6.74 (m), 7.08 (m) |
| Acetate | δ1.92 (s) |
| Alanine | δ1.32 (d), 3.72 (q) |
| Cholesterol | δ3.60 (s) |
| Creatinine | δ3.00 (s), 3.96 (s) |
| Cysteine | δ2.72 (s) |
| Histidine | δ3.96 (d), 6.49 (t), 7.73–7.76 (m) |
| Hypoxanthine | δ3.14 (d), 4.05 (t), 5.31–5.37 (m), 6.77 (d) |
| Lactate | δ1.32 (s), 4.23 (s) |
| Glutamine | δ2.04 (d) |
| Methionine | δ1.36 (s), 2.42 (t), 3.40–3.42 (q), 3.56 (d), 3.98 (s) |
| Serine | δ2.40 (s) |
| Taurine | δ3.36 (t), 3.68 (t) |
| Tryptophan | δ4.43 (s), 7.47 (t) |
| Trimethylamine-N-oxide | δ3.18 (s) |
| Uracil | δ5.81 (d), 7.53 (d) |
| Uric acid | δ3.13 (t), 5.46 (m) |
s, singlet; d, doublet; t, triplet; q, quarter; m, multiplet.
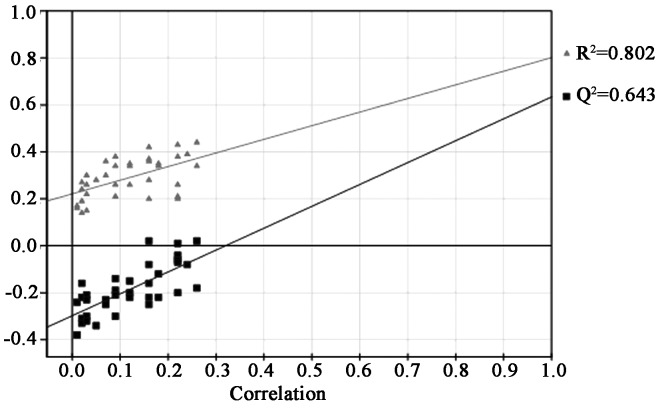
The quality of the principal component models was evaluated with the parameters R2 and Q2, and the goodness of fit was quantified by R2 while the predictive ability was indicated by Q2. Generally, R2Y and Q2Y explained variation in the data, where 0 was no variation explained, and 1, for which 100% variation was accounted for. The results indicated that this PLS-DA model had a high R2Y value of 0.802 and Q2Y value of 0.643, which suggested that although each patient in the two groups was treated with the same concentration of MTX, and it was possible to metabolically discriminate with different physical constitutions (Fig. 3).
The permutation testing was performed to validate PLS-DA models. It provided the statistical significance of the estimated predicted power of the models by comparing R2Y and Q2Y values of the original model with those of the re-ordered model, which was created newly whenever y data was permutated at random. It is known that models with the R2Y intercept <0.4 and a Q2Y intercept <0.05 indicate valid models. As shown in Fig. 4, the PLS-DA model had a proper R2Y intercept value of 0.208 and a Q2Y intercept value of −0.285.
Figure 4.
Quality of the principal component models was evaluated with the parameters R2 and Q2. R2 is the explained variance, Q2 is the predictive ability of the model validation, and correlation is the degree of overlap between the permuted and original models. The validation model exhibited good predictive ability, as indicated by the high Q2 value. Grey triangles, the goodness of fit was quantified by R2; black squares, the predictive ability was indicated by Q2 (R2Y=0.802, Q2Y=0.643).
VIP value of the major contributing metabolites
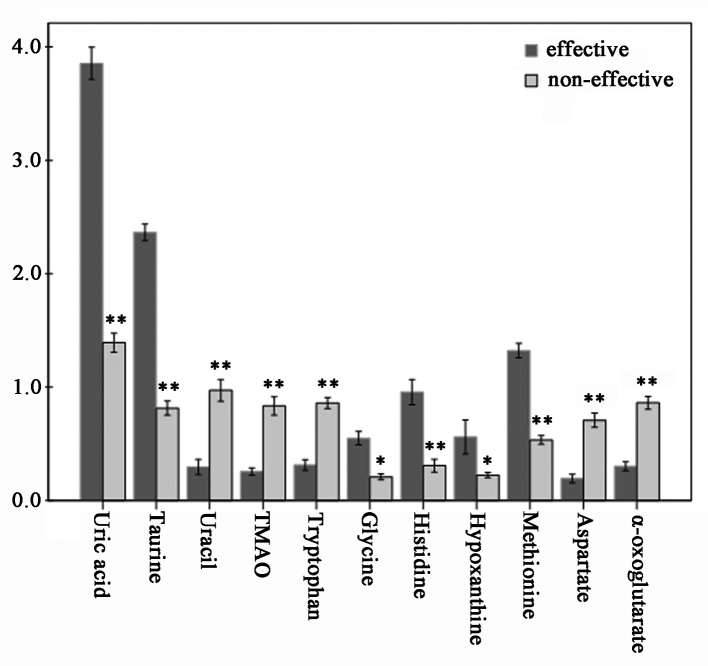
VIP values of several metabolites assigned by using SIMCA-P are listed in Table III. Usually, the metabolites with VIP >1 were considered to be influential for separating each sample in these PLS-DA models. Consequently, we performed an ANOVA test to determine the relative intensities of the important metabolites contributing to the separation of the two groups. Eleven metabolites were selected as key metabolites for the classification of each group. VIP analysis showed the order of metabolites according to their critical influence on clustering, with metabolites on the x-axis arranged according to their VIP values (VIP >1), and the y-axis illustrating the relative intensities of the metabolites (Table III). The mean uric acid, taurine, histidine, methionine, glycine and hypoxanthine concentrations in the effective group were elevated (p<0.05), while uracil, TMAO, α-oxoglutarate, aspartate and tryptophan were statistically significant decreased (p<0.05) (Fig. 5).
Table III.
VIP values of the major contributing metabolites for the separation in the score plots derived from PLS-DA.
| Metabolites | VIP value |
|---|---|
| α-oxoglutarate | 1.328 |
| Glycine | 1.942 |
| Citrate | 0.704 |
| Aspartate | 1.213 |
| Acetate | 2.348 |
| Alanine | 0.841 |
| Cholesterol | 0.536 |
| Cysteine | 0.964 |
| Histidine | 2.815 |
| Hypoxanthine | 1.224 |
| Lactate | 0.872 |
| Glutamine | 1.116 |
| Methionine | 2.478 |
| Serine | 0.836 |
| Taurine | 3.624 |
| Tryptophan | 1.462 |
| Trimethylamine-N-oxide | 2.367 |
| Uracil | 2.437 |
| Uric acid | 3.837 |
VIP, variable importance in the projection.
Figure 5.
Absolute concentrations of 11 discriminate metabolites from the PLS-LDA plot are shown. Based on creatinine, the relative concentrations of 11 endogenous metabolites were determined, where the effective group showed a significant difference when compared to those of the non-effective group. Dark and light grey squares represent the concentrations of metabolites between the ‘effective’ and ‘non-effective’ group, respectively. A significant difference is indicated by *p<0.05; and **p<0.01.
Discussion
Metabolomics is a novel scientific discipline focused on the association between disease and metabolic profile in tissue and biofluids, as determined by techniques including 1H NMR spectroscopy, mass spectroscopy and liquid chromatography mass spectroscopy. Moreover, metabolomic analysis can also be utilized to identify biomarkers of drug treatment (23). From a therapeutic point of view, it is well accepted that MTX is effective in only 60–75% of RA patients (24). In this study, we performed an 1H NMR-based metabolomic approach in early RA patients with MTX treatment to identify potential biomarkers for therapeutic evaluation. Using PCA and PLS-DA analysis, we detected the relationships between the individual response of 38 patients with early RA receiving MTX mono-therapy and various associated factors in the effective group. Furthermore, 11 endogenous metabolites as the major predictive factors for the response to MTX were identified in the metabolic profile. Significantly, the current study demonstrates the feasibility of using serum for diagnostic or prognostic testing in patients with early RA receiving MTX treatment.
Biological fluids, such as blood and urine, contain a large number of metabolites that may provide valuable information on the metabolism of an organism, and thus concerning its health status. In the present study, 11 metabolites were found to vary with the differential response of the early RA patients receiving MTX treatment, which suggest they could be used to predict the therapeutic effects of MTX. It appeared that nucleic acid metabolism may be highly interrelated to the therapeutic effects, with uric acid and uracil being two of the most prominent metabolites noted. In addition, the changes in levels of TMAO and hypoxanthine suggest an alteration in purine and pyrimidine ratios, and in turn may affect cell metabolism, energy conservation and biosynthetic pathways, even signal transduction and translation (23). Glycine has been suggested to have anti-inflammatory properties by acting on macrophage chloride channels to blunt cytokine release, and methionine is associated with SAM synthesis that may influence metabolism of nucleic acid (24,25). Both glycine and methionine are important metabolites in homocysteine metabolism, and higher levels of glycine and methionine in the effective group implied a favorable response to MTX in the present study. As far as we know, the main physiological function of the one-carbon unit is to synthesize purines and pyrimidines, and it is closely linked to amino acid metabolism and nucleic acid metabolism (26). The one-carbon unit mainly arises from serine, glycine, histidine and tryptophan, and the three compounds were found to be altered in the serum of the effective group (27). Increased levels of hypoxanthine in the serum of the effective group may be attributed to a decrease in the levels of oxidative stress following MTX therapy. Taurine is also known to have antioxidant effects which appear to downregulate pro-inflammatory cytokine production, and was present at increased levels in the effective group (28). Furthermore, the Krebs cycle is also likely to play an important role in the course of treatment since the concentrations of α-oxoglutarate and aspartate were decreased in the effective group when compared with those of the non-effective group (29,30).
The potential metabolic pathways highlight the complexity of the metabolic response to MTX treatment. Although conclusively defining roles for each metabolite were not feasible based on the data from this study, 11 metabolites presented here may be considered useful biomarkers for their discriminatory power to distinguish the effectiveness of MTX treatment in patients with early RA. We therefore hypothesized that several metabolic pathways are related to the therapeutic effects of MTX, such as nucleic acid metabolism, homocysteine metabolism, one-carbon metabolism, and metabolite analysis may be used to predict the effects of MTX treatment in RA patients.
One of the ultimate goals of the current method was to enable comparisons between RA patients with a favorable response to MTX and those who respond poorly in clinical settings. According to the different situations, we could rapidly adjust the therapy regimen in the clinic. But one problem faced in clinical investigations is the inherently greater variability in a human population compared with that seen in our study. Thus, the study factors such as age, gender, diet, individual difference and other environmental influences, can be more extensively controlled by investigators than when the enrolled human study populations are investigated. Although the exact metabolites identified as potential ‘biomarkers’ may vary among different individuals, more studies could use the methodology applied here to investigate the efficacy of MTX treatment in RA patients. Metabolomic analysis is undoubtedly applicable to the search for biological predictors of response to drug treatment in RA, but future studies should employ larger patient cohorts, more discriminatory analyses, and a less equivocal clinical phenotype.
In conclusion, the monitoring of entire sets of metabolic features is critical to evaluate the efficacy of drug treatments. A metabolic ‘bioprofile’, consisting of predictive serum metabolite features from 1H NMR spectral data of early RA patients receiving MTX treatment are presented in this study. Significantly, our study demonstrated that various serum biomarkers can be used to evaluate MTX treatment in patients with early RA, and the metabolomic approach including 1H NMR spectroscopy coupled with multivariate statistical analysis may be useful to predict the outcome of drug response and treatment.
Acknowledgments
The authors specially thank Dr Ming Jiang of the School of Pharmacy, Tongji Medical College for his technical assistance.
Abbreviations:
- 1H NMR
1H nuclear magnetic resonance
- RA
rheumatoid arthritis
- MTX
methotrexate
- DAS
disease activity score
- PCA
principal component analysis
- PLS-DA
partial least squares discriminant analysis
- DMARDs
disease-modifying anti-rheumatic drugs
- NSAIDs
non-steroid anti-inflammatory drugs
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Rath T, Rubbert A. Drug combinations with methotrexate to treat rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:S52–S57. [PubMed] [Google Scholar]
- 3.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 4.Braun J. Optimal administration and dosage of methotrexate. Clin Exp Rheumatol. 2010;28:46–51. [PubMed] [Google Scholar]
- 5.Ranganathan P, McLeod HL. Methotrexate pharmacogenetics: the first step toward individualized therapy in rheumatoid arthritis. Arthritis Rheum. 2006;54:1366–1377. doi: 10.1002/art.21762. [DOI] [PubMed] [Google Scholar]
- 6.Dervieux T, Furst D, Lein DO, Capps R, Smith K, Walsh M, Kremer J. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum. 2004;50:2766–2774. doi: 10.1002/art.20460. [DOI] [PubMed] [Google Scholar]
- 7.Kawabata T, Nishida K, Takasugi K, et al. Increased activity and expression of histone deacetylase 1 in relation to tumor necrosis factor-alpha in synovial tissue of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R133–R145. doi: 10.1186/ar3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roybal JL, Endo M, Radu A, Zoltick PW, Flake AW. Early gestational gene transfer of IL-10 by systemic administration of lentiviral vector can prevent arthritis in a murine model. Gene Ther. 2011;18:719–726. doi: 10.1038/gt.2011.23. [DOI] [PubMed] [Google Scholar]
- 9.Drouin J, Haraoui B, 3e Initiative Group Predictors of clinical response and radiographic progression in patients with rheumatoid arthritis treated with methotrexate monotherapy. J Rheumatol. 2010;37:1405–1410. doi: 10.3899/jrheum.090838. [DOI] [PubMed] [Google Scholar]
- 10.Rudwaleit M, Yin Z, Siegert S, Grolms M, Radbruch A, Braun J, Sieper J. Response to methotrexate in early rheumatoid arthritis is associated with a decrease of T cell derived tumour necrosis factor alpha, increase of interleukin 10, and predicted by the initial concentration of interleukin 4. Ann Rheum Dis. 2000;59:311–314. doi: 10.1136/ard.59.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson JK, Lindon JC. Systems biology: metabolomics. Nature. 2008;455:1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 12.Malet-Martino M, Holzgrabe U. NMR techniques in biomedical and pharmaceutical analysis. J Pharm Biomed Anal. 2011;55:1–15. doi: 10.1016/j.jpba.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Inoue S, Hashiguchi M, Takagi K, Kawai S, Mochizuki M. Preliminary study to identify the predictive factors for the response to methotrexate therapy in patients with rheumatoid arthritis. Yakugaku Zasshi. 2009;129:843–849. doi: 10.1248/yakushi.129.843. [DOI] [PubMed] [Google Scholar]
- 14.Um SY, Chung MW, Kim KB, et al. Pattern recognition analysis for the prediction of adverse effects by non-steroidal anti-inflammatory drugs using 1H NMR-based metabolomics in rats. Anal Chem. 2009;81:4734–4741. doi: 10.1021/ac9000282. [DOI] [PubMed] [Google Scholar]
- 15.Lauridsen MB, Bliddal H, Christensen R, et al. 1H NMR spectroscopy-based interventional metabolic phenotyping: a cohort study of rheumatoid arthritis patients. Proteome Res. 2010;9:4545–4553. doi: 10.1021/pr1002774. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Fransen J, Creemers MC, van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology. 2004;43:1252–1255. doi: 10.1093/rheumatology/keh297. [DOI] [PubMed] [Google Scholar]
- 18.MacIntyre DA, Jiménez B, Lewintre EJ, et al. Serum metabolome analysis by 1H-NMR reveals differences between chronic lymphocytic leukaemia molecular subgroups. Leukemia. 2010;24:788–797. doi: 10.1038/leu.2009.295. [DOI] [PubMed] [Google Scholar]
- 19.Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, Nicholson JK. Metabolic profiling, metabolomic and metabolomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 20.Okada T, Afendi FM, Altaf-Ul-Amin M, Takahashi H, Nakamura K, Kanaya S. Metabolomics of medicinal plants: the importance of multivariate analysis of analytical chemistry data. Curr Comput Aided Drug Des. 2010;6:179–196. doi: 10.2174/157340910791760055. [DOI] [PubMed] [Google Scholar]
- 21.Bourré-Tessier J, Haraoui B. Methotrexate drug interactions in the treatment of rheumatoid arthritis: a systematic review. J Rheumatol. 2010;37:1416–1421. doi: 10.3899/jrheum.090153. [DOI] [PubMed] [Google Scholar]
- 22.Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 23.Simoni RE, Gomes LN, Scalco FB, Oliveira CP, Aquino Neto FR, de Oliveira ML. Uric acid changes in urine and plasma: an effective tool in screening for purine inborn errors of metabolism and other pathological conditions. J Inherit Metab Dis. 2007;30:295–309. doi: 10.1007/s10545-007-0455-8. [DOI] [PubMed] [Google Scholar]
- 24.Weljie AM, Dowlatabadi R, Miller BJ, Vogel HJ, Jirik FR. An inflammatory arthritis-associated metabolite biomarker pattern revealed by 1H NMR spectroscopy. J Proteome Res. 2007;6:3456–3464. doi: 10.1021/pr070123j. [DOI] [PubMed] [Google Scholar]
- 25.Rees WD, Wilson FA, Maloney CA. Sulfur amino acid metabolism in pregnancy: the impact of methionine in the maternal diet. J Nutr. 2006;136:S1701–S1705. doi: 10.1093/jn/136.6.1701S. [DOI] [PubMed] [Google Scholar]
- 26.Jámbor A, Molnár-Perl I. Amino acid analysis by high-performance liquid chromatography after derivatization with 9-fluorenylmethyloxycarbonyl chloride. Literature overview and further study. J Chromatogr A. 2009;1216:3064–3077. doi: 10.1016/j.chroma.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 27.Carr DF, Whiteley G, Alfirevic A, Pirmohamed M, FolATED study team Investigation of inter-individual variability of the one-carbon folate pathway: a bioinformatic and genetic review. Pharmacogenomics J. 2009;9:291–305. doi: 10.1038/tpj.2009.29. [DOI] [PubMed] [Google Scholar]
- 28.Schirra HJ, Anderson CG, Wilson WJ, Kerr L, Craik DJ, Waters MJ, Lichanska AM. Altered metabolism of growth hormone receptor mutant mice: a combined NMR metabolomics and microarray study. PLoS One. 2008;3:e2764. doi: 10.1371/journal.pone.0002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadykov MR, Zhang B, Halouska S, et al. Using NMR metabolomics to investigate tricarboxylic acid cycle-dependent signal transduction in Staphylococcus epidermidis. J Biol Chem. 2010;285:36616–36624. doi: 10.1074/jbc.M110.152843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenz EM, Bright J, Wilson ID, Morgan SR, Nash AF. A 1H NMR-based metabolomic study of urine and plasma samples obtained from healthy human subjects. J Pharm Biomed Anal. 2003;33:1103–1115. doi: 10.1016/s0731-7085(03)00410-2. [DOI] [PubMed] [Google Scholar]