Abstract
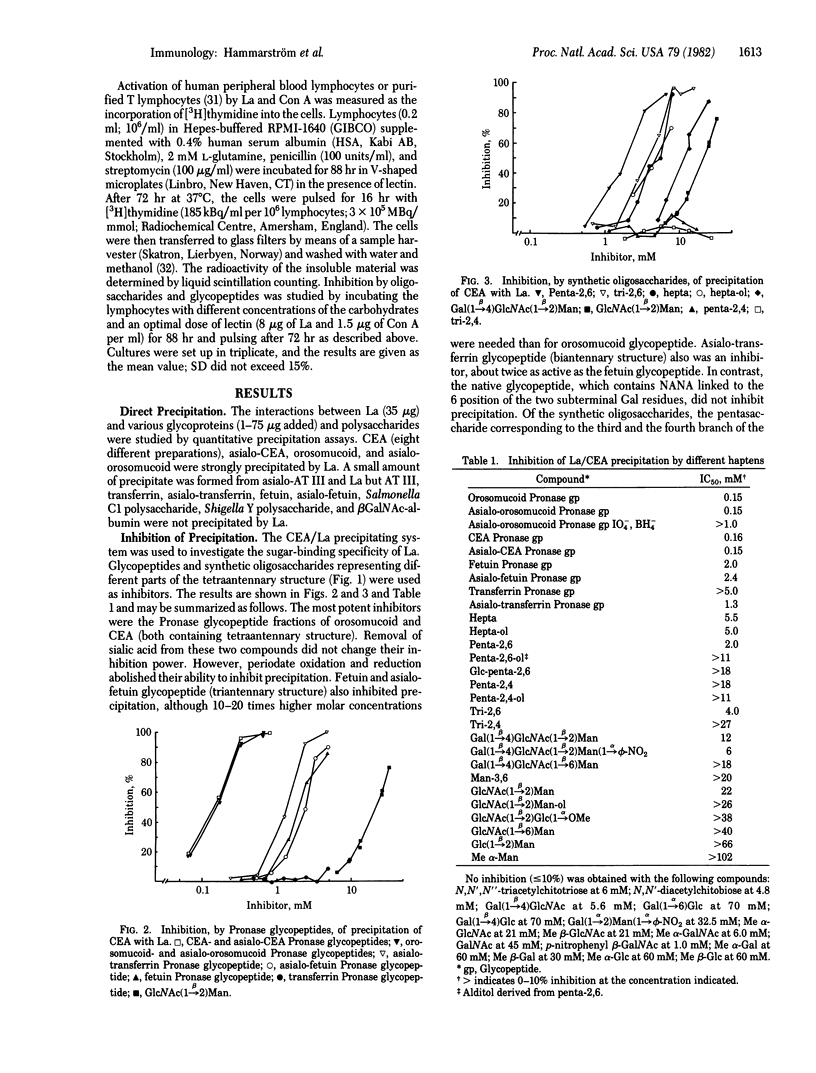
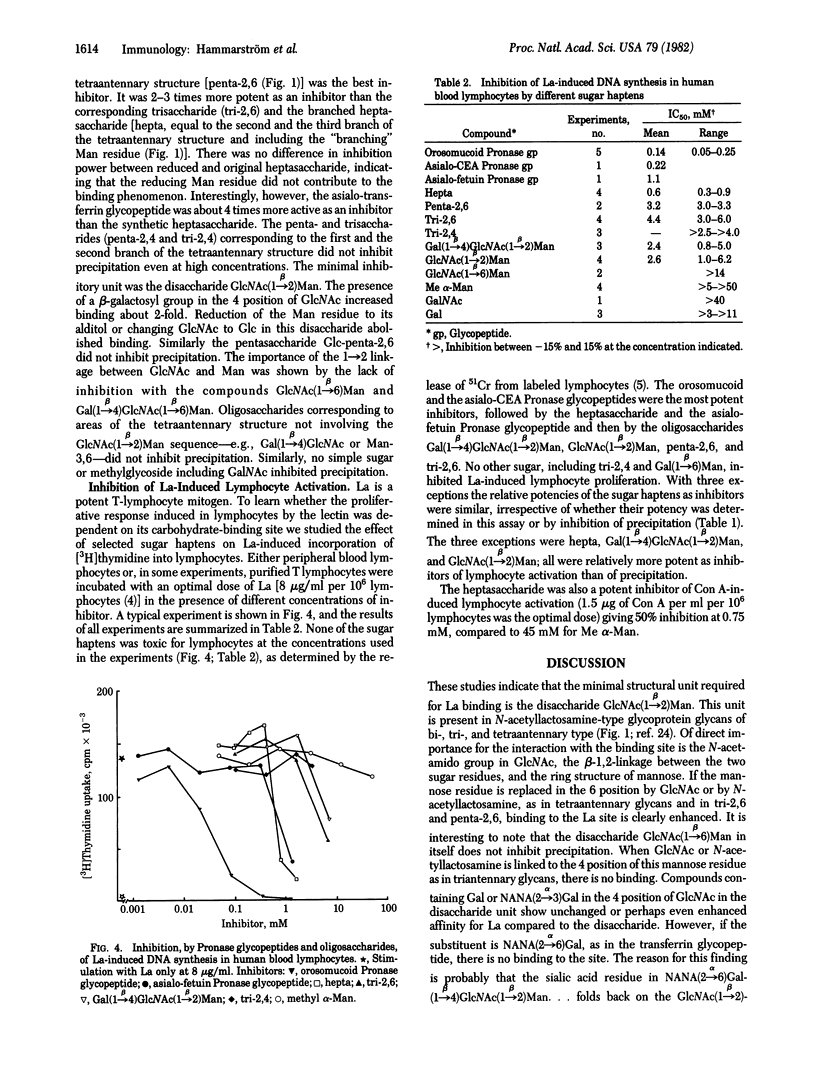
The carbohydrate binding specificity of leukoagglutinin (La; Phaseolus vulgaris isolectin L4) was studied by using quantitative precipitation and precipitation-inhibition. A series of purified glycopeptides and synthetic oligosaccharides were used as inhibitors. The minimal structural unit required for La binding was the disaccharide GlcNac(1 leads to beta 2)Man. Additions for this basic unit of different sugar residues gave a positive or negative contribution to binding. The most complementary structure was the pentasaccharide (formula: see text). This pentasaccharide units occurs in tetraantennary N-acetyllactosamine-type glycoprotein glycans. Glycoproteins containing such structures were accordingly precipitated by La. Selected glycopeptides and oligosaccharides were also tested as inhibitors of La-induced DNA synthesis in human lymphocytes. The pattern of inhibition was essentially the same as that obtained by precipitation-inhibition, indicating that binding to lymphocytes via the carbohydrate binding site of the lectin is an essential step in the activation process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbondanza A., Franceschi C., Licastro F., Serafini-Cessi F. Properties of a glycopeptide isolated from human Tamm-Horsfall glycoprotein. Interaction with leucoagglutinin and anti-(human Tamm-Horsfall glycoprotein) antibodies. Biochem J. 1980 May 1;187(2):525–528. doi: 10.1042/bj1870525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnarp J., Haraldsson M., Lönngren J. Synthesis of three oligosaccharides that form part of the complex type of carbohydrate moiety of glycoproteins. Carbohydr Res. 1981 Nov 16;97(2):307–313. doi: 10.1016/s0008-6215(00)80676-x. [DOI] [PubMed] [Google Scholar]
- Bretting H., Kabat E. A., Liao J., Pereira M. E. Purification and characterization of the agglutinins from the sponge Aaptos papillata and a study of their combining sites. Biochemistry. 1976 Nov 16;15(23):5029–5038. doi: 10.1021/bi00668a013. [DOI] [PubMed] [Google Scholar]
- Fournet B., Montreuil J., Strecker G., Dorland L., Haverkamp J., Vliegenthart F. G., Binette J. P., Schmid K. Determination of the primary structures of 16 asialo-carbohydrate units derived from human plasma alpha 1-acid glycoprotein by 360-MHZ 1H NMR spectroscopy and permethylation analysis. Biochemistry. 1978 Nov 28;17(24):5206–5214. doi: 10.1021/bi00617a021. [DOI] [PubMed] [Google Scholar]
- Franzén L. E., Svensson S., Larm O. Structural studies on the carbohydrate portion of human antithrombin III. J Biol Chem. 1980 Jun 10;255(11):5090–5093. [PubMed] [Google Scholar]
- Goldstein I. J., Hammarström S., Sundblad G. Precipitation and carbohydrate-binding specificity studies on wheat germ agglutinin. Biochim Biophys Acta. 1975 Sep 9;405(1):53–61. doi: 10.1016/0005-2795(75)90313-x. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Engvall E., Johansson B. G., Svensson S., Sundblad G., Goldstein I. J. Nature of the tumor-associated determinant(s) of carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1528–1532. doi: 10.1073/pnas.72.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaifu R., Osawa T. Synthesis of O-beta-D-galactopyranosyl-(1 linked to 4)-O-(2acetamido-2-deoxy-beta-D-glucopyranosyl)-(1 linked to 2)-D-mannose and its interaction with various lectins. Carbohydr Res. 1976 Dec;52:179–185. doi: 10.1016/s0008-6215(00)85958-3. [DOI] [PubMed] [Google Scholar]
- Kenne L., Lindberg B., Petersson K. Basic structure of the oligosaccharide repeating-unit of the Shigella flexneri O-antigens. Carbohydr Res. 1977 Jul;56(2):363–370. doi: 10.1016/s0008-6215(00)83357-1. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Structure of membrane receptors for plant lectins. Ann N Y Acad Sci. 1974;234(0):276–282. doi: 10.1111/j.1749-6632.1974.tb53039.x. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The structural basis of the different affinities of two types of acidic N-glycosidic glycopeptides for concanavalin A--sepharose. FEBS Lett. 1976 Nov 15;72(1):117–120. doi: 10.1016/0014-5793(76)80911-8. [DOI] [PubMed] [Google Scholar]
- Leavitt R. D., Felsted R. L., Bachur N. R. Biological and biochemical properties of Phaseolus vulgaris isolectins. J Biol Chem. 1977 May 10;252(9):2961–2966. [PubMed] [Google Scholar]
- Miller J. B., Hsu R., Heinrikson R., Yachnin S. Extensive homology between the subunits of the phytohemagglutinin mitogenic proteins derived from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1388–1391. doi: 10.1073/pnas.72.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Noyes C., Heinrikson R., Kingdon H. S., Yachnin S. Phytohemagglutinin mitogenic proteins. Structural evidence for a family of isomitogenic proteins. J Exp Med. 1973 Oct 1;138(4):939–951. doi: 10.1084/jem.138.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montreuil J. Primary structure of glycoprotein glycans: basis for the molecular biology of glycoproteins. Adv Carbohydr Chem Biochem. 1980;37:157–223. doi: 10.1016/s0065-2318(08)60021-9. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Nordén N. E., Svensson S. Structural studies on the carbohydrate portion of fetuin. J Biol Chem. 1979 Jun 10;254(11):4545–4553. [PubMed] [Google Scholar]
- Räsänen V., Weber T. H., Gräsbeck R. Crystalline kidney-bean leucoagglutinin. Eur J Biochem. 1973 Sep 21;38(1):193–200. doi: 10.1111/j.1432-1033.1973.tb03050.x. [DOI] [PubMed] [Google Scholar]
- SCHIFFMAN G., KABAT E. A., THOMPSON W. IMMUNOCHEMICAL STUDIES ON BLOOD GROUPS. XXX. CLEAVAGE OF A, B, AND H BLOOD-GROUP SUBSTANCES BY ALKALI. Biochemistry. 1964 Jan;3:113–120. doi: 10.1021/bi00889a018. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Franceschi C., Sperti S. Specific interaction of human Tamm-Horsfall gylcoprotein with leucoagglutinin, a lectin from Phaseolus vulgaris (red kidney bean). Biochem J. 1979 Nov 1;183(2):381–388. doi: 10.1042/bj1830381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman G. B., Strong D. M., Ahmed A., Green S. S., Sell K. W., Hartzman R. J., Bach F. H. Human mixed lymphocyte cultures. Evaluation of a microculture technique utilizing the Multiple Automated Sample Harvester (MASH). Clin Exp Immunol. 1973 Oct;15(2):289–302. [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber T. H. Isolation and characterization of a lymphocyte-stimulating leucoagglutinin from red kidney beans. (Phaseolus vulgaris). Scand J Clin Lab Invest Suppl. 1969;111:1–80. [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]
- Yachnin S., Allen L. W., Baron J. M., Svenson R. H. The potentiation of phytohemagglutinin-induced lymphocyte transformation by cell-cell interaction; a matrix hypothesis. Cell Immunol. 1972 Apr;3(4):569–589. doi: 10.1016/0008-8749(72)90120-7. [DOI] [PubMed] [Google Scholar]


