Abstract
Sipuleucel-T (Provenge) is the first therapeutic cancer vaccine approved by the U.S. FDA. The approval heralds the long-awaited promise of improved patient survival with minimal toxicity by therapies designed to generate an active, specific anticancer immune response. The development of this first-in-class agent has also led to a better understanding of relevant patient populations and endpoints for clinical trials, findings that are relevant for other clinical trials of therapeutic vaccines in prostate cancer and other cancers. This article discusses the development and approval of sipuleucel-T in the context of other approved therapies for prostate cancer, as well as controversies and novel paradigms brought about by this new agent.
Keywords: prostate cancer, immunotherapy, cancer vaccine, cancer treatment
Introduction
Harnessing the power of the immune system and focusing that energy to eradicate cancer cells has been the objective of significant preclinical and clinical research for decades. Although there have been modest successes with cytokines such as IL-2 and IFN-γ, development of a targeted immune therapy with proven clinical benefit has been an elusive goal [1, 2]. Initially, peptide-based strategies were developed, but the clinical outcomes of these strategies were disappointing [3], spurring the search for additional approaches. One such approach involved selectively removing immune cells from a patient, priming these immune cells ex vivo to potentially allow for maximal immune activation, and then reinfusing these immune cells into the patient. Subsequently, these activated immune cells would induce significant targeted immune responses. This is the approach that led to the clinical development of sipuleucel-T (Provenge, Dendreon Corp., Seattle, WA, USA).
Rationale for development
Studies have suggested that a patient’s immune system is able to recognize tumor-associated antigens (TAAs) on cancer cells, but often they do not mount a significant immune response against these TAAs [4]. The rationale for developing therapeutic cancer vaccines such as sipuleucel-T is to enhance immune recognition and immune-mediated destruction of cancer cells, potentially prolonging survival.
Prostate cancer is a viable target for immunotherapy because, for most patients, the disease course is indolent, progressing over many years, as opposed to other cancers such as lung or pancreatic cancer where death often follows diagnosis within a year. Prostate cancer’s prolonged disease course allows time for the body to generate an immune response against the cancer cells [5]. In addition, many TAAs are uniquely associated with the prostate and are overexpressed in prostate cancer [6–8], including prostatic acid phosphatase (PAP, a 102-kD glycoprotein that may play a role in disease progression), prostate-specific antigen (PSA), prostate-specific membrane antigen (PSMA), and MUC-1 [9].
Although sipuleucel-T is designed to target PAP, the ensuing immune response may generate immune cells that target other TAAs unique to prostate cancer. In a phenomenon known as “antigen spreading” or “antigen cascade,” which has been observed in the setting of clinical trials, tumor-cell lysis generated by a vaccine targeting a single TAA may lead to immune-cell processing of additional TAAs, generating a broader immune response that targets multiple TAAs [10, 11]. Thus, targeting a single TAA with vaccine could ultimately lead to a diversified immune response with a greater antitumor effect.
Vaccine preparation
With a sound rationale for targeting TAAs, a strategy was developed to produce a therapeutic cancer vaccine to target PAP. Preparation of the sipuleucel-T vaccine involves obtaining peripheral blood mononuclear cells (PBMCs) by leukapheresis from prostate cancer patients. The total volume of blood processed is 8 to 14 liters, roughly 1 to 2 times the patient’s total blood volume. The collected PBMCs include cells that are CD54bright, CD3+, CD14+, and CD19+. These PBMCs are passed through 2 centrifuges, initially over a buoyant density solution of 1.077 g/mL and 320 mOsm, then washed twice to remove platelets. The second solution is 1.065 g/mL in density and 320 mOsm; cells in this second solution are then washed twice to remove granulocytes. The resulting concentration is composed primarily of antigen-presenting cells (APCs), including dendritic cells [12, 13].
PA2024 is a recombinant protein of PAP fused at its carboxyl terminus to the amine terminus of granulocyte-macrophage colony-stimulating factor (GM-CSF) via a glycine-serine link [14]. PAP is the ultimate target TAA; GM-CSF facilitates immune activation by enhancing APC recognition and uptake [12, 13]. After the PBMCs have been centrifuged and washed twice, the remaining cells are resuspended in AIM-V medium and incubated with 10 ug/mL of PA2024 at 37° C for 40 h. Once this process is complete, the activated cell product is then washed in Lactated Ringer’s and returned to the patient for reinfusion [12]. Vaccine potency is measured by the level of CD54+ cells, a marker associated with immune activation. Each infusion contains a minimum of 50 million CD54+ cells [101]. The entire process is repeated for each of the 3 infusions of sipuleucel-T.
Clinical development
Recurrent or newly diagnosed metastatic prostate cancer is treated with testosterone-lowering agents or orchiectomy. Although these treatments may initially cause regression of the disease by limiting testosterone-induced proliferation of malignant cells, the cancer generally will progress over time [15]. An initial phase I/II trial evaluated sipuleucel-T in men with rising PSA or progressive disease despite castrate levels of testosterone (castration-resistant prostate cancer; CRPC). Patients were treated with escalating numbers of activated cells at 0, 4, and 8 weeks, with an option for another dose at 24 weeks if the disease remained stable. Twelve men were treated in the phase I portion of the study and another 19 in the phase II portion at the maximum tolerated dose. Patients in the phase II portion had no radiographic or scintigraphic evidence of metastatic disease and were generally healthier than patients in the phase I group. The treatment was well tolerated at all dose levels, with mild (mostly grade 1 and 2) febrile reactions and myalgias. Three patients had a > 50% decline in PSA. Median time to progression (TTP) in the phase II portion was 29 weeks, with 7 patients having stable disease at 1 year. Median TTP was 31.7 weeks in patients treated at the highest dose level, compared to 12.1 weeks at the lower dose level given to the phase I cohort (P = 0.013) [16].
Immunologic parameters were also evaluated in these patients. All treated patients had increased T-cell proliferation in response to PA2024, and the majority of patients generated increased antibody to PAP and GM-CSF. Only two patients were evaluated for antigen-specific responses via ELISPOT assay, but both demonstrated T cell-specific responses to PA2024, with increased IFN-γ production. The investigators reported that those with better immune responses had improved TTP [16].
A second phase II trial evaluated sipuleucel-T in patients with CRPC and documented metastatic disease. In this study, patients were infused with the prepared cellular product at weeks 0 and 2, then treated at weeks 4, 8, and 12 with a subcutaneous injection of PA2024. Again, the treatment was well tolerated, with grade 1 to 2 fatigue and fever/chills as the most common adverse events. Nineteen of 21 patients were evaluable for response after receiving both cellular infusions and at least 1 subcutaneous PA2024 injection. Median TTP was 118 days, and 2 patients had PSA declines of 25 to 50%. A third patient had a baseline PSA of 221 ng/mL that rose transiently then became undetectable, along with complete resolution of lymph node metastasis. This patient maintained a complete response during the entire 52 months of follow-up. No PSA declines or radiographic improvements were noted in any other patients. T-cell proliferation increased in response to PA2024 in 15 patients; however, only transient antibody responses were noted. The patient with the complete response did have a sustained T-cell proliferation response to PA2024.[17].
Based on these initial demonstrations of safety and efficacy, 2 concurrent randomized, placebo-controlled phase III trials were initiated. The placebo consisted of APCs prepared identically to the vaccine, minus the culture with PA2024 fusion protein (PAP/GM-CSF). Both trials randomized patients with minimally symptomatic CRPC 2:1 in favor of sipuleucel-T, and treated them with the maximum number of cells that could be prepared from leukapheresis at 0, 2, and 4 weeks. TTP was the primary endpoint of both trials [18, 19]. In the first trial, which enrolled 127 patients, TTP favored sipuleucel-T (n = 82), with a median of 11.7 weeks compared to 10 weeks for placebo (n = 45). However, these results did not reach statistical significance (P = 0.052) [18]. A subset analysis demonstrated a statistically significant improvement in TTP for patients with a Gleason score of ≤ 7 (P = 0.002) [20]. Based on these findings, the second phase III trial terminated accrual early at 98 patients [19].
A subsequent analysis of the first trial showed an overall survival (OS) advantage in favor of sipuleucel-T (25.9 months vs. 21.4 months for placebo; P = 0.01). OS at 36 months of follow-up was 34% for sipuleucel-T vs. 11% for placebo (P = 0.005). An immunologic analysis performed in a subset of patients (31 treated with sipuleucel-T and 18 treated with placebo) demonstrated increased T-cell proliferation to PA2024 in response to sipuleucel-T compared to placebo [18].
When data from the prematurely closed second phase III trial were evaluated, there was again no significant difference in TTP between the 2 groups (10.9 weeks vs. 9.9 weeks; P = 0.719), but the OS analysis favored sipuleucel-T (n = 65) at 19 months compared to 15.7 months for placebo (n = 33; P = 0.331). When data from the 2 studies were combined, sipuleucel-T-treated patients (n = 147) had better outcomes than placebo-treated patients (n = 78), with a median TTP improvement of 11.1 vs. 9.7 weeks (P = 0.111) and a median OS advantage of 23.2 vs. 18.9 months (P = 0.011) [19].
Although these data presented compelling support for an OS advantage for patients treated with sipuleucel-T, OS was not the primary endpoint of either phase III trial. Based largely on that premise, the U.S. Food and Drug Administration (FDA) did not approve sipuleucel-T. Therefore, a larger phase III trial (IMmunotherapy for Prostate AdenoCarcinoma Treatment; IMPACT), already underway at the time of the FDA decision, was completed with OS as the primary endpoint.
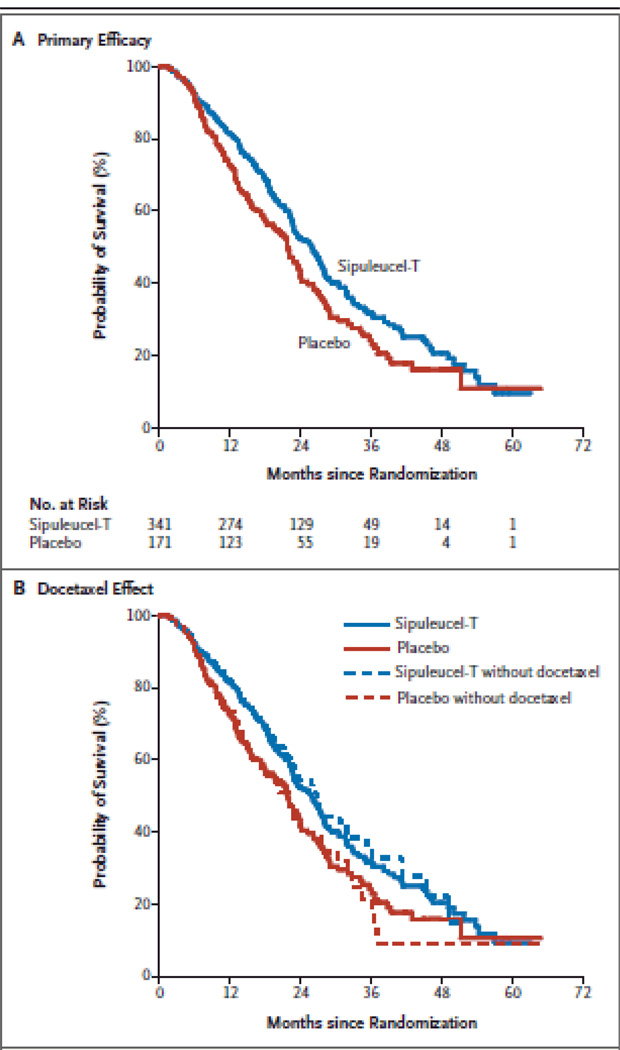
As in the previous phase III trials, patients were required to have minimally symptomatic metastatic CRPC, no previous chemotherapy within 3 months, no long bone pathological fractures, and no spinal cord compression. Initially, patients were required to have Gleason scores of ≤ 7; however, when the final OS data from the first 2 phase III trials suggested improvement in OS irrespective of Gleason score, the trial was amended to include men with any Gleason score. The study enrolled 512 patients, treating 341 with sipuleucel-T at weeks 0, 2, and 4 and treating 171 with placebo at the same time points. Placebo was produced by using a third of the APCs obtained from leukapheresis and culturing the cells without PA2024. As in the earlier trials, at progression placebo-treated patients were allowed, at their physicians’ discretion, to be treated with the remainder of their cryopreserved immune cells incubated with PA2024. TTP was again unchanged by treatment with sipuleucel-T (14.6 weeks) relative to placebo (14.4 weeks; P = 0.63). However, OS analysis again favored sipuleucel-T, with a median survival of 25.8 months compared to 21.7 months for patients in the placebo arm (Figure 1, panel A). The 36-month survival probability was 31.7% in the sipuleucel-T arm compared with 23.0% in the placebo arm. A post hoc analysis indicated that the improved OS could not be explained by the timing of subsequent docetaxel or the proportion of patients in each arm who subsequently received docetaxel (Figure 1, panel B) [21].
Figure 1.
Sipuleucel-T improves survival but not progression in metastatic castration-resistant prostate cancer. A: primary efficacy analysis of sipuleucel-T from the IMPACT study (HR 0.78; P = 0.03). B: overall survival analysis with and without censoring at initiation of docetaxel therapy. A consistent treatment effect was observed after censoring at time of docetaxel treatment (HR 0.65; P = 0.009)
Adverse events reported more frequently in the sipuleucel-T group included a transient flu-like syndrome (fever, chills, headache, muscle aches) and grade 1 or 2 fatigue that resolved within 1 to 2 days. Grade 3 adverse events within 1 day of infusion were seen in 6.8% of sipuleucel-T-treated patients. Overall, < 1% of patients were unable to receive all 3 infusions due to adverse events, a level that compares very favorably to chemotherapy approved for prostate cancer. Based on the results of this study, the FDA approved sipuleucel-T for patients with metastatic CRPC, making it the first FDA-approved therapeutic cancer vaccine [102].
A subset of selected patients from this study was evaluated for immune responses, including antibody response and T-cell proliferation. Antibody responses (defined as titers > 400 via ELISA) to PA2024 were detected in 66.2% of the 151 patients evaluated from the sipuleucel-T arm, compared with 2.9% of 70 patients from the placebo arm. Antibodies to PAP were found in 28.5% of patients evaluated from the sipuleucel-T arm, compared to 1.4% of patients evaluated from the placebo arm. For patients with higher antibody titers, survival outcomes were improved compared to patients with titers < 400 to PA2024 (P < 0.001) and PAP (P = 0.08). Increased T-cell proliferation was seen with both of these antigens, but there was no association with improved survival [21].
Further analysis of immune data from several trials with sipuleucel-T suggested that the magnitude of CD54 cells mobilized by the patient may be an indicator of improved clinical outcome. Immunologic data suggest that CD54 is a possible marker of APC activation. Therefore, it may be possible to evaluate CD54 expression after cell product preparation as a means to indirectly assess APC engagement, which likely would improve the efficacy of the vaccine [19, 22]. Recent analysis from selected patients enrolled in the IMPACT trial found an association between increased CD54-expressing cells and improved survival outcomes [23]. A further integrated analysis of all 3 phase III studies of sipuleucel-T (n = 476) found correlations between OS and the product parameters of cumulative CD54+ cell counts (P = 0.016), total nucleated cell counts (P < 0.001), and CD54 upregulation (P = 0.002), which persisted after adjustment for the baseline prognostic factors PSA and LDH [24].
While these analyses are intriguing, the ultimate clinical role of biomarkers in patient care remains unclear; they do not yet represent surrogates for response or survival. Furthermore, the role of CD54-expressing cells in other immune therapies has not been fully evaluated, and additional prospective analyses are warranted.
Overview of the market
Until recently, despite advances in the treatment of other malignancies, development of therapies for metastatic prostate cancer appeared to be mired in failed attempts to improve on the standard-of-care regimen of docetaxel and prednisone. This regimen was approved by the FDA in 2004, based on a 2- to 3-month improvement in survival over mitoxantrone and prednisone, the previous standard that had provided only palliative benefits [25–27]. A recent example of a failed attempt to improve on the standard of care was a trial evaluating bevacizumab with docetaxel and prednisone vs. docetaxel and prednisone alone. The results of this study showed no significant changes in survival, the primary endpoint [28]. In spite of these difficulties, the market for metastatic prostate cancer treatments has become crowded (Table 1).
Table 1.
Summary of agents demonstrating an overall survival advantage in phase III trials in metastatic castration-resistant prostate cancer.
| Treatment | Comments |
|---|---|
| Docetaxel and prednisone | FDA-approved in 2004, this regimen is most likely to bring symptomatic relief for patients with metastatic disease. In spite of its therapeutic benefits, many patients are reluctant to receive this regimen because its side effects are more severe than those of hormonal therapies. |
| Sipuleucel-T | FDA-approved in 2010, this first-in-class therapeutic cancer vaccine has demonstrated an overall survival advantage relative to placebo in patients with minimally symptomatic disease. Its brief course of therapy (3 infusions over 1 month) and minimal side effect profile make it appealing. Issues restricting use include initial limited availability and lack of intermediate biomarkers indicative of clinical benefit. |
| Cabazitaxel and prednisone | FDA-approved in 2010, this was the first regimen to demonstrate an overall survival advantage in metastatic prostate cancer patients who progressed on docetaxel-based therapies. Its side effect profile, including significant myelosuppression, may limit its utility in chemorefractory patients. Residual side effects include marrow toxicity. |
| Abiraterone | In late 2010, this CYP-17 lyase inhibitor demonstrated overall survival benefits in patients with metastatic disease who had already been treated with chemotherapy. Its side effect profile is favorable relative to chemotherapy. Ongoing studies are evaluating this agent in chemotherapy-naïve patients with metastatic prostate cancer. |
In recent years, strategies for the development of prostate cancer therapeutics have changed significantly. Although initially described decades ago, the androgen receptor has been rediscovered as a viable target, even in patients with progressive metastatic disease following testosterone-lowering therapy [29]. After the last FDA-approved androgen receptor-targeting agent was developed more than 20 years ago, the focus of investigation shifted to cytotoxic chemotherapy [30]. The prevailing dogma had suggested that in metastatic disease, sequential hormonal-based therapies would yield little clinical benefit, and that the disease was likely fueled by a mutated androgen receptor, making hormonal control of the disease infeasible. However, data from studies of 2 promising agents have radically changed this perspective.
Abiraterone was designed to inhibit the CYP17A pathway and thereby decrease androgen synthesis [31]. Abiraterone acts by decreasing androgen synthesis, similar to ketoconazole, an older agent plagued by substantial drug interactions and toxicities, that is frequently used as a second-line hormonal agent [15]. Early clinical trials with abiraterone demonstrated striking efficacy in terms of PSA declines and radiographic and biomarker responses. Significant responses were seen even in patients who had been previously treated with chemotherapy, a population thought to be refractory to hormonal treatments [32–34]. A phase III trial of abiraterone vs. placebo in patients previously treated with chemotherapy was stopped early because of an observed survival advantage for patients in the abiraterone arm [103]. It is widely believed that this outcome will lead to FDA approval for patients who have progressed on docetaxel and prednisone. Another phase III trial of abiraterone in chemotherapy-naïve patients with metastatic prostate cancer has completed accrual and is in the follow-up period, awaiting final data [104].
Another promising agent in prostate cancer treatment is MDV3100. This compound is a modern androgen receptor antagonist that has higher binding affinity and greater effects downstream from the androgen receptor, making it more potent than currently approved androgen receptor antagonists [35]. Phase I/II data with this agent were also promising, showing significant declines in PSA and delayed progression in both chemotherapy-naïve and chemotherapy-refractory patients [36]. Based on these findings, MDV3100 is in phase III testing in patients with progressive metastatic disease [105]. It is quite possible that within 1 to 3 years, MDV3100 and/or abiraterone will be available as alternatives to chemotherapy for patients with minimally symptomatic metastatic disease no longer contained by testosterone-lowering therapy.
Not all chemotherapy trials since 2004 have been failures. In a phase III trial, cabazitaxel and prednisone in docetaxel-refractory patients demonstrated an OS advantage over mitoxantrone and prednisone [37]. This led to FDA approval of cabazitaxel in June 2010 as a treatment for docetaxel-refractory patients [106].
The appropriate patient population for sipuleucel-T can be clarified by evaluating its anticipated benefits in the context of prostate cancer patients. As in the phase III trial described above, the logical course is to treat minimally symptomatic patients with sipuleucel-T, since no short-term changes in disease progression are expected with this vaccine-based therapy. Symptomatic patients are more likely to benefit from chemotherapy, which is more likely than any immunotherapy to be cytoreductive in the short term.
Another consideration when deciding if sipuleucel-T is appropriate is life expectancy. Since there is no change in initial disease progression with this agent, but improvements are seen in OS, it is evident that time is required for this treatment to yield clinical benefit (note the overlapping Kaplan-Meier curves for the first 6 to 8 months, illustrated in Figure 1). Therefore, patients with longer life expectancy are more likely to benefit from vaccine therapy.
Clinical assessment will be valuable in determining who should recieve vaccine therapy. Studies of patients with metastatic prostate cancer have demonstrated that patients who experience more severe pain are likely to succumb sooner to their disease. A retrospective study evaluated 599 patients from large Cancer and Leukemia Group B trials. The results showed that a greater magnitude of pain resulted in a median OS of 10.2 months, while a lesser magnitude of pain yielded a median OS of 17.6 months (P < 0.001) [38]. These data indicate that patients with high levels of pain are less likely to survive long enough to derive benefit from sipuleucel-T, and are more likely to derive short-term clinical benefit from chemotherapy. This concept is further supported by a smaller phase II study with another therapeutic cancer vaccine, which demonstrated that patients with more indolent disease characteristics at time of therapy derived greatest benefit from the vaccine in terms of prolonged survival [39].
Although sipuleucel-T is the first therapeutic cancer vaccine approved by the FDA for any cancer, another therapeutic cancer vaccine is about to enter phase III testing in patients with minimally symptomatic metastatic CRPC. PSA-TRICOM (Prostvac™; developed by the National Cancer Institute [NCI] and licensed to BN Immunotherapeutics, Mountain View, CA) is an experimental, off-the-shelf vaccine that targets PSA by employing genetically altered poxviruses to deliver PSA and T-cell costimulatory molecules, resulting in an immune response against the TAA [40]. The agent is administered subcutaneously and delivers the targeting information to APCs through cellular infection. Ultimately, these infected APCs express a PSA peptide to T cells in an immune context, resulting in PSA-specific activation [41, 42].
After initial studies demonstrated the safety of poxviral vaccines targeting PSA, transgenes for 3 T-cell costimulatory molecules (designated TRICOM) were added to the second generation of the vaccine (PSA-TRICOM), which demonstrated enhanced T-cell activation [43–49]. A placebo-controlled, multicenter trial in metastatic CRPC randomized 125 patients 2:1 in favor of PSA-TRICOM. Patients on the placebo arm received an empty poxviral vector containing no transgenes for PSA and no costimulatory molecules. As in the sipuleucel-T trials, patients receiving vaccine showed no change in TTP (the primary endpoint), but demonstrated an OS benefit of 25.1 months vs. 16.6 months (P = 0.0061) [50].
A concurrent, single-arm phase II study with PSA-TRICOM was conducted at the NCI in 32 patients with metastatic CRPC, resulting in a similar median OS of 26.6 months. Furthermore, correlative immunologic evaluation established an increase in activated, PSA-specific T cells in many patients. There was also a trend suggesting that patients with the greatest magnitude of immune response had the greatest improvement in OS (P = 0.055) [39]. A global phase III trial of PSA-TRICOM in metastatic CRPC is expected to launch in 2011, with OS as the primary endpoint [102]. Although it will be several years before results of this phase III trial are known, the fact that PSA-TRICOM does not require leukapheresis and off-site cellular processing will be an advantage should it enter the market in competition with sipuleucel-T [40].
Another important consideration and potential obstacle for sipuleucel-T is cost. The cost of one month of therapy exceeds 90,000 dollars USD, in part because of the logistics of producing this individualized therapy. It is estimated that about 75% of sipuleucel-T recipients will be Medicare beneficiaries, so there was widespread concern when the Center for Medicare Services initiated a national coverage analysis to determine whether the use of sipuleucel-T is “reasonable and necessary”[51]. Concerns about the expense of new cancer therapeutics are neither new nor limited to sipuleucel-T [52]. Traditional chemotherapy agents are also costly. The average cost of a docetaxel-based regimen for prostate cancer (including treatment of side effects) is around 60,000 USD, and regimens using bevacizumab and lenalidomide are even more expensive [51, 53]. These direct costs must be balanced against quality of life and side effects of treatment. An agreement concerning appropriate Medicare reimbursement for sipuleucel-T will likely be reached by the time the agent is available for wider distribution in early to mid-2011.
In sum, apart from chemotherapy, sipuleucel-T is the only FDA-approved agent demonstrating a survival advantage for patients with metastatic CRPC. This immunotherapy would be most appropriate for patients with indolent disease characteristics and minimal symptoms, typical of patients in the prechemotherapy setting. Treatments such as MDV3100, abiraterone, and PSA-TRICOM may challenge sipuleucel-T in this niche in coming years, but no current competitor has demonstrated improved OS in patients with asymptomatic metastatic CRPC.
Expert commentary
FDA approval of sipuleucel-T is an important milestone in cancer immunotherapy. This first-in-class agent demonstrated an OS benefit in multiple trials and has a favorable side effect profile, but has several significant obstacles to overcome before it can be a major player in prostate cancer therapeutics. Foremost among these obstacles is supply. Given the labor-intensive and proprietary nature of the vaccine preparation, sipuleucel-T currently can only be prepared at Dendreon’s facilities on the East and West coasts of the U.S. Large-scale production of the vaccine is not anticipated until mid-2011.
A second obstacle may persist after issues of production and supply are overcome. Sipuleucel-T has many unique aspects, none more vexing to clinicians than that it improves survival without improving median TTP. This fact may make some physicians reluctant to use the agent. However, this finding has been seen with other emerging immunotherapies such as ipilimumab in metastatic melanoma and Prostvac in prostate cancer [50, 54]. There is an immunologic rationale why this may be a class effect of these agents [55]. Nonetheless, the absence of any intermediate indicator of response such as an immunologic biomarker creates a degree of uncertainty not present with hormonal therapy, where PSA may be useful, or chemotherapy, where imaging can determine response. Development of intermediate biomarkers of response may be vital to the clinical implementation of new immunologic therapies such as sipuleucel-T.
Without intermediate endpoints, clinicians will have to determine how to monitor patients who have been treated with sipuleucel-T. A helpful recommendation from the PSA Working Group II is that PSA level alone should not be a determinant for when to assess disease status for patients with metastatic CRPC [56]. This is especially true for vaccine-based therapy, where PSA declines are rarely seen [21]. Clinicians could employ second-line hormonal therapies in these patients after completing the one-month course of sipuleucel-T therapy. Chemotherapy with docetaxel and prednisone would also be appropriate. A greater challenge for clinicians may be to withhold treatment from these patients and simply monitor slowly rising PSA over time. During this observation period, sharp rises in PSA or development of new symptoms may serve as signals to re-image patients to evaluate for disease progression, which may indicate the need for chemotherapy. This surveillance approach is a somewhat unconventional way of dealing with metastatic cancer and would thus require the consent of both patient and clinician. Ultimately, the role of sipuleucel-T in cancer therapy will be determined by clinicians willing to take on the challenges of this novel immune-based therapy.
Five-year view
As medical oncologists move into the age of modern immunotherapeutics, they will need to clearly understand how these agents can best be employed. Therapeutic cancer vaccines pose new questions as they open many opportunities. Once optimal patient populations are identified (those with indolent disease and lower tumor burden), it is reasonable to expect that cancer vaccines will be of clinical benefit in malignancies other than prostate cancer [57]. For instance, therapeutic cancer vaccines are currently being evaluated in metastatic lung cancer [107].
Given the time required to develop an immune response and the potential for sustained effects after vaccine administration, vaccine therapy may be best employed earlier in the disease process, before patients develop metastases. This potential has been evaluated in prostate cancer in patients with rising PSA but no overt evidence of metastatic disease [58–60]. An even more appealing option biologically may be to move therapeutic cancer vaccines to the adjuvant setting, since vaccines are not likely to introduce additional toxicities to adjuvant chemotherapy or radiation [61].
It is possible that treatments combining vaccine with standard therapies could enhance the clinical benefits of both treatments. It has been demonstrated in prostate cancer patients that chemotherapy does not diminish the immune response generated by vaccine immunotherapy [62]. Furthermore, preclinical data suggest that vaccine combined with chemotherapy may yield superior antitumor effects compared to either treatment alone [11]. Radiation, even in low doses, may alter the phenotypic expression of antigens and ligands on the surface of cancer cells, which may enhance the ability of the immune system to recognize and destroy cancer cells [63–65]. Clinical data from a trial combining a therapeutic cancer vaccine with hormonal therapy also suggested a clinical benefit [66]. Increasing knowledge concerning how testosterone-lowering therapy enhances immune response has gone a long way toward explaining these clinical benefits [67–69].
In the next several years, combination immunotherapy regimens may also demonstrate clinical efficacy. Ipilimumab is a fully humanized antibody that helps to prevent moderation and diminution of immune response by interfering with binding to cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). Ordinarily APC/T-cell interactions via CTLA-4 downregulate immune responses as the body’s natural mechanism to minimize overzealous immune and autoimmune responses. This is demonstrated in CTLA-4 knockout mice, who rapidly develop multi-organ failure due to infiltrating autoreactive T cells, resulting in death [70]. Ipilimumab has recently demonstrated an OS advantage in metastatic melanoma [54], and has shown promise in combination with therapeutic cancer vaccines in preclinical models, perhaps by enhancing the immune stimulatory effects of the vaccines [71–73].
In addition to combinations of vaccines and immune-regulating agents, preclinical data support the concurrent use of multiple vaccines to generate a greater immune response than is generated by a single vaccine [74]. A multivalent immune response may have more clinical impact than an immune response initiated by a single agent. Combinations of vaccine therapies in clinical studies may determine methods to induce maximum immune response, and may also provide important insight into the potential antitumor effects of the immune system.
Sipuleucel-T has helped to usher in a new age in medical oncology. For years, clinicians have employed toxic regimens in an attempt to control the growth of human malignancies. This past decade has seen the advent of more targeted therapeutics; however, cancer cells are still able to mutate and overcome even these more focused approaches. Modern immunotherapeutics, including therapeutic cancer vaccines, may be able to initiate a clinically relevant immune response to help limit the growth of cancer cells, even as they mutate. An immune response is dynamic, with the potential to modulate and evolve along with its adversaries. If initiated early in the disease course, these immune-stimulating therapies might generate a strong enough immune response to suppress tumor growth for some time, keeping up with mutations through an evolving immune response, as demonstrated by the phenomenon of antigen cascade. There is also the potential to enhance standard and emerging therapeutics. Therapeutic vaccines have been in clinical development for a long time. Their ultimate impact will depend on a broader understanding of clinical expectations for this type of therapy, improved availability of the drugs, and rational combinations with other agents.
Key issues.
Sipuleucel-T is the first FDA-approved therapeutic cancer vaccine, with a statistically significant 4.1-month improvement in median overall survival (HR 0.78).
Sipuleucel-T has minimal toxicity compared to chemotherapy.
Sipuleucel-T requires apheresis at 3 time points and is individualized for each patient.
Sipuleucel-T is controversial due to its cost of 93,000 USD for a one-month treatment course of 3 infusions, and because improvements in overall survival are seen with no improvement in time to progression (apparently a class effect).
Sipuleucel-T is approved for patients with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer.
References
- 1.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210(4):474–484. doi: 10.1097/00000658-198910000-00008. discussion 484-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holdener EE, Emmons RP, Brunda M, Evans L, Levitt D. Interferon-alpha and interleukin-2 in the treatment of renal cell cancer. Prog Clin Biol Res. 1990;348:61–69. [PubMed] [Google Scholar]
- 3.Ressing ME, Offringa R, Toes RE, Ossendorp F, de Jong JH, Brandt RM, et al. Immunotherapy of cancer by peptide-based vaccines for the induction of tumor-specific T cell immunity. Immunotechnology. 1996;2(4):241–251. doi: 10.1016/s1380-2933(96)00057-7. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty NG, Stevens RL, Mehrotra S, Laska E, Taxel P, Sporn JR, et al. Recognition of PSA-derived peptide antigens by T cells from prostate cancer patients without any prior stimulation. Cancer Immunol Immunother. 2003;52(8):497–505. doi: 10.1007/s00262-003-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffey DS, Isaacs JT. Prostate tumor biology and cell kinetics--theory. Urology. 1981;17(Suppl 3):40–53. [PubMed] [Google Scholar]
- 6.Rhodes DR, Barrette TR, Rubin MA, Ghosh D, Chinnaiyan AM. Meta-analysis of microarrays: interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res. 2002;62(15):4427–4433. [PubMed] [Google Scholar]
- 7.Fong L, Small EJ. Immunotherapy for prostate cancer. Semin Oncol. 2003;30(5):649–658. doi: 10.1016/s0093-7754(03)00350-6. [DOI] [PubMed] [Google Scholar]
- 8.Cereda V, Poole DJ, Palena C, Das S, Bera TK, Remondo C, et al. New gene expressed in prostate: a potential target for T cell-mediated prostate cancer immunotherapy. Cancer Immunol Immunother. 2010;59(1):63–71. doi: 10.1007/s00262-009-0723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145(5):907–923. doi: 10.1016/s0022-5347(17)38491-4. [DOI] [PubMed] [Google Scholar]
- 10.Sharifi N, Dahut WL, Steinberg SM, Figg WD, Tarassoff C, Arlen P, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96(7):985–989. doi: 10.1111/j.1464-410X.2005.05798.x. [DOI] [PubMed] [Google Scholar]
- 11.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14(11):3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burch PA, Breen JK, Buckner JC, Gastineau DA, Kaur JA, Laus RL, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6(6):2175–2182. [PubMed] [Google Scholar]
- 13.So-Rosillo R, Small EJ. Sipuleucel-T (APC8015) for prostate cancer. Expert Rev Anticancer Ther. 2006;6(9):1163–1167. doi: 10.1586/14737140.6.9.1163. [DOI] [PubMed] [Google Scholar]
- 14.Tao MH, Levy R. Idiotype/granulocyte-macrophage colony-stimulating factor fusion protein as a vaccine for B-cell lymphoma. Nature. 1993;362(6422):755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- 15.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 16.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18(23):3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 17.Burch PA, Croghan GA, Gastineau DA, Jones LA, Kaur JS, Kylstra JW, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a Phase 2 trial. Prostate. 2004;60(3):197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 18.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 19.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 20.Small E, Rini B, Higano C, Redfern C, Nemunaitis J, Valone F, et al. A randomized, placebo-controlled phase III trial of APC8015 in patients with androgen-independent prostate cancer (AiPCa) [abstract] Proc Am Soc Clin Oncol. 2003;22:1534. [Google Scholar]
- 21.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 22.Sheikh N, Jones L. CD54 is a surrogate marker of antigen presenting cell activation. Cancer Immunol Immunother. 2008;57(9):1381–1390. doi: 10.1007/s00262-008-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart F, dela Rosa C, Sheikh N, McNeel D, Frohlich M, Urdal D, et al. Correlation between product parameters and overall survival in three trials of sipuleucel-T, an autologous active cellular immunotherapy for the treatment of prostate cancer [abstract] J Clin Oncol. 2010;28(15s):4552. [Google Scholar]
- 24.Frohlich M, Sheikh N, Xu Y, Whitmore J, Poehlein C, Urdal D. Sipuleucel-T immune parameters and correlation with overall survival. Presented at: iSBTC 25th Annual Scientific Meeting; 30 September–4 October 2010; Washington, DC, USA. [Google Scholar]
- 25.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 26.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 27.Moore MJ, Osoba D, Murphy K, Tannock IF, Armitage A, Findlay B, et al. Use of palliative end points to evaluate the effects of mitoxantrone and low-dose prednisone in patients with hormonally resistant prostate cancer. J Clin Oncol. 1994;12(4):689–694. doi: 10.1200/JCO.1994.12.4.689. [DOI] [PubMed] [Google Scholar]
- 28.Kelly W, Halabi S, Carducci M, George D, Mahoney J, Stadler W, et al. A randomized, double-blind, placebo-controlled phase III trial comparing docetaxel, prednisone, and placebo with docetaxel, prednisone, and bevacizumab in men with metastatic castration-resistant prostate cancer (mCRPC): Survival results of CALGB 90401 [abstract] J Clin Oncol. 2010;28(18s) doi: 10.1200/JCO.2011.39.4767. LBA4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huggins C, Bergenstal DM. Inhibition of human mammary and prostatic cancers by adrenalectomy. Cancer Res. 1952;12(2):134–141. [PubMed] [Google Scholar]
- 30.Furr BJ. "Casodex" (ICI 176,334)--a new, pure, peripherally-selective anti-androgen: preclinical studies. Horm Res. 1989;32(Suppl 1):69–76. doi: 10.1159/000181315. [DOI] [PubMed] [Google Scholar]
- 31.Madan RA, Arlen PM. Abiraterone. Cougar Biotechnology. IDrugs. 2006;9(1):49–55. [PubMed] [Google Scholar]
- 32.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 33.Attard G, Reid AH, A'Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27(23):3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28(9):1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sartor AO, Tangen CM, Hussain MH, Eisenberger MA, Parab M, Fontana JA, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group trial (SWOG 9426) Cancer. 2008;112(11):2393–2400. doi: 10.1002/cncr.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375(9724):1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sartor A, Oudard S, Ozguroglu M, Hansen S, Machiels J, Shen L, et al. Cabazitaxel or mitoxantrone with prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel: Final results of a multinational phase III trial (TROPIC). Presented at: ASCO, Genitourinary Cancers Symposium; San Francisco, CA, USA. 2010. Mar 5–7, [Google Scholar]
- 38.Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26(15):2544–2549. doi: 10.1200/JCO.2007.15.0367. [DOI] [PubMed] [Google Scholar]
- 39.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59(5):663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):479–481. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 41.Arlen PM, Mohebtash M, Madan RA, Gulley JL. Promising novel immunotherapies and combinations for prostate cancer. Future Oncol. 2009;5(2):187–196. doi: 10.2217/14796694.5.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Essajee S, Kaufman HL. Poxvirus vaccines for cancer and HIV therapy. Expert Opin Biol Ther. 2004;4(4):575–588. doi: 10.1517/14712598.4.4.575. [DOI] [PubMed] [Google Scholar]
- 43.Gulley J, Chen AP, Dahut W, Arlen PM, Bastian A, Steinberg SM, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53(2):109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 44.Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22(11):2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 45.Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, Grosenbach DW, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178(4 Pt 1):1515–1520. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 46.DiPaola R, Chen Y, Bubley G, Do D, Re R, Mi M, et al. A phase II study of PROSTVAC-V (vaccinia)/TRICOM and PROSTVAC-F (fowlpox)/TRICOM with GM-CSF in patients with PSA progression after local therapy for prostate cancer: Results of ECOG 9802. Presented at: ASCO Genitourinary Cancers Symposium; Orlando, FL, USA. 2009. Feb 26–28, [Google Scholar]
- 47.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59(22):5800–5807. [PubMed] [Google Scholar]
- 48.Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62(20):5770–5777. [PubMed] [Google Scholar]
- 49.Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001;61(11):4497–4505. [PubMed] [Google Scholar]
- 50.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baghdadi R. Dendreon vs CMS: why the Provenge coverage controversy is bigger than just one product. Oncology. 2010;24(10):881–883. [PubMed] [Google Scholar]
- 52.Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101(15):1044–1048. doi: 10.1093/jnci/djp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cancer Patients Receiving Chemotherapy: Opportunities for Better Management. Milliman Client Report. 2010 Mar 30;:1–30. [Google Scholar]
- 54.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15(9):969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madan RA, Mohebtash M, Schlom J, Gulley JL. Therapeutic vaccines in metastatic castration-resistant prostate cancer: principles in clinical trial design. Expert Opin Biol Ther. 2010;10(1):19–28. doi: 10.1517/14712590903321421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27(25):4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zlotocha S, Staab MJ, Horvath D, Straus J, Dobratz J, Oliver K, et al. A phase I study of a DNA vaccine targeting prostatic Acid phosphatase in patients with stage D0 prostate cancer. Clin Genitourin Cancer. 2005;4(3):215–218. doi: 10.3816/CGC.2005.n.036. [DOI] [PubMed] [Google Scholar]
- 60.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174(2):539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 61.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 62.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12(4):1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tseng CW, Trimble C, Zeng Q, Monie A, Alvarez RD, Huh WK, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother. 2009;58(5):737–748. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newcomb EW, Demaria S, Lukyanov Y, Shao Y, Schnee T, Kawashima N, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12(15):4730–4737. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 65.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 66.Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14(14):4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–4971. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 69.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 71.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3(7):611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 72.Allison JP, Chambers C, Hurwitz A, Sullivan T, Boitel B, Fournier S, et al. A role for CTLA-4-mediated inhibitory signals in peripheral T cell tolerance? Novartis Found Symp. 1998;215:92–98. doi: 10.1002/9780470515525.ch7. discussion 98-102, 186-190. [DOI] [PubMed] [Google Scholar]
- 73.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174(10):5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boehm AL, Higgins J, Franzusoff A, Schlom J, Hodge JW. Concurrent vaccination with two distinct vaccine platforms targeting the same antigen generates phenotypically and functionally distinct T-cell populations. Cancer Immunol Immunother. 2010;59(3):397–408. doi: 10.1007/s00262-009-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.FDA Prescribing Information for Provenge. PROVENGE Package Insert. http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM210031.pdf.
- 102.FDA Press Release; 2010. Apr 29, FDA Approves a Cellular Immunotherapy for Men with Advanced Prostate Cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm210174.htm. [Google Scholar]
- 103.Cougar Biotechnology Press Release; 2010. Sep 9, Study of Investigational Agent Abiraterone Acetate for Metastatic Advanced Prostate Cancer Unblinded After Meeting Pre-Determined Criteria. http://www.prnewswire.com/news-releases/study-of-investigational-agent-abiraterone-acetate-for-metastatic-advanced-prostate-cancer-unblinded-after-meeting-pre-determined-criteria-102576469.html. [Google Scholar]
- 104.NIH Clinical Trials; [updated 21 May 2010]. Abiraterone Acetate in Asymptomatic or Mildly Symptomatic Patients With Metastatic Castration-Resistant Prostate Cancer. http://clinicaltrials.gov/ct2/show/NCT00887198?term=abiraterone&rank=10. [Google Scholar]
- 105.NIH Clinical Trials; [updated 22 September 2010]. Safety and Efficacy Study of MDV3100 in Patients With Castration-Resistant Prostate Cancer Who Have Been Previously Treated With Docetaxel-based Chemotherapy (AFFIRM) http://clinicaltrials.gov/ct2/show/NCT00974311?term=MDV3100&rank=3. [Google Scholar]
- 106.FDA Press Release; 2010. Jun 17, Cabazitaxel. http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm216214.htm. [Google Scholar]
- 107.NIH Clinical Trials; [updated 21 September 2010]. Cancer Vaccine Study for Unresectable Stage III Non-small Cell Lung Cancer. http://clinicaltrials.gov/ct2/show/NCT00409188?term=stimuvax&rank=6. [Google Scholar]