Abstract
To explore an effective combination therapy for malignant ascites, the therapeutic value of the combination of Endostar, a modified recombinant human endostatin, and β-elemene, an active component of a traditional Chinese herb, in an H22 mouse malignant ascites model was investigated. The optimal dose combination of Endostar and β-elemene was determined by evaluating the inhibition of ascites volume and increase in the survival rate of the mice. Other therapeutic effects and the underlying mechanisms were investigated under the optimal dose combination (8 mg/kg Endostar plus 100 mg/kg β-elemene). The mice were randomly divided into four treatment groups and received intraperitoneal injection once a day for eight days: control (0.9% normal saline), Endostar (8 mg/kg), β-elemene (100 mg/kg) or optimal dose combination (8 mg/kg Endostar plus 100 mg/kg β-elemene), respectively. The results of this study revealed that the combination therapy had significant synergistic effects on the inhibition of ascites formation and a deceased number of tumor cells and protein levels in ascites compared with the results of treatment with a single agent. A decreased peritoneal microvascular permeability and reduction in VEGF, MMP-2 and hypoxia inducible factor 1α (HIF1α) was noted in the combination group, when compared with single agent treatment. These studies found that in the ascitic tumor cells, the protein levels of VEGF and MMP-2, as well as levels of VEGF mRNA, were significantly inhibited by the combination therapy. The potentiating effects of the combination of Endostar with β-elemene suggest that this novel therapy may yield an effective therapy for the treatment of malignant ascites.
Keywords: Endostar, recombinant human endostatin, β-elemene, malignant ascites, antiangiogenesis, vascular endothelial growth factor
Introduction
Malignant ascites, an abnormal accumulation of fluid in the peritoneal cavity caused by tumor infiltration or secretion, is a recognized indication of end-stage events in various types of cancer. The local fluid accumulation of ascites may cause abdominal pain, bowel obstruction, loss of appetite, nausea, fatigue, anorexia and cachexia, which deteriorate the quality of life of patients. The mean survival rate of patients following the diagnosis of malignant ascites is approximately 20 weeks (1). Therefore, palliating the symptoms and prolonging survival have become the foremost goal. The treatment of malignant ascites remains a difficult problem to solve. The established approaches include salt restriction, diuresis, peritoneovenous shunts, paracentesis and chemotherapy via intraperitoneal injection in selected cases. However, these conventional methods occasionally relieve symptoms but only for a short while, and do not prolong survival. Their effects are variable and unreliable. Since there are no generally accepted and evidence-based guidelines for the management of malignant ascites (2), novel methods are required for its treatment in the clinic.
The increased peritoneal microvascular permeability is one of the main factors which contributes to the development of malignant ascites. Vascular endothelial growth factor (VEGF) is believed to be a primary influence in stimulating vessel formation and increasing vascular permeability, which is related to the formation of malignant metastases (3). Therefore, anti-VEGF is a promising candidate for the clinical treatment of malignant ascites.
Endostatin, a 20-kDa C-terminal fragment of collagen XVIII identified by O'Reilly in 1997, significantly inhibits VEGF-induced endothelial cell proliferation and decreases angiogenesis and tumor growth (4). However, the poor stability, low biochemical activity and high production costs limit the clinical application of collagen XVIII. The Chinese scientist Luo Yongzhang developed Endostar, a novel recombinant human endostatin containing an additional 9-amino acid sequence at the N-terminus to improve its stability and biological activity, and produced Endostar in E. coli to reduce the production costs (5). Endostar was approved in 2005 by the State Food and Drug Administration of China (SFDA) for the treatment of non-small cell lung cancer (NSCLC) following phase I–III clinical trials in China (6). Currently, the drug is used to treat different types of tumors combined with chemotherapy in the clinic (7). Endostar is provided to treat malignant ascites and significantly improve quality of life with high efficiency and low toxicity (8).
β-elemene, extracted from Chinese medicinal herbs, including Curcuma wenyujin YH Chen & C Ling, has broad-spectrum antitumor activity by inhibiting proliferation and inducing apoptosis in several types of solid tumor cells (9). Curcuma wenyujin inhibits tumor growth and metastasis by suppressing VEGF-mediated angiogenesis (10), and demonstrates synergistic effects on lung cancer cells when combined with taxanes (11). In phase III clinical trials, it was observed that intraperitoneal injection of β-elemene relieved the symptoms of malignant ascites and improved quality of life with minor side effects (12).
Although Endostar and β-elemene are effective agents for the treatment of malignant ascites, it is always difficult to control refractory and recurrent malignant ascites by administration of a single drug. Therefore, a combination therapy for obstinate ascites is likely to be valuable in the clinic. In the present study, we evaluated the effects of Endostar and β-elemene, alone and combined, on malignant ascites in an H22 ascitic mouse model. The peritoneal microvascular permeability and the underlying mechanism was also investigated.
Materials and methods
Cell culture and animal model
H22 hepatoma ascitic cells were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). H22 cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum in a Forma Series II water-jacketed incubator (Thermo Fisher Scientific, Rockford, IL, USA) at 37°C under a humidified atmosphere of 5% CO2. The expression of VEGF mRNA and protein in H22 cells was verified by quantitative real-time polymerase chain reaction (RT-qPCR) and western blot analysis, respectively. Female ICR mice (6 weeks old, 18–20 g) were purchased from Shanghai Laboratory Animal Center, CAS (SLACCAS, Shanghai, China) and maintained in pathogen-free cages at the Animal Experimental Center. Animal quality was controlled (Certification #2007000523797).
To establish the mouse model of malignant ascites, cultured H22 cells were harvested and prepared as a cell suspension at a concentration of 1×107 cells/ml in phosphate-buffered saline (PBS). Mice were intraperitoneally injected with 0.2 ml of cell suspension (total, 2×106 cells/mouse). The procedure was not associated with mortality or morbidity.
All possible steps were taken to avoid animal suffering at each stage of the experiment. The procedures used in this study had the approval of the Institutional Animal Care and Use Committee of Jiangsu Simcere Pharmaceutical Research Institute.
Reagents
Endostar solution (10 g/l, batch #YY2011008) was provided by Simcere Pharmaceutical Research (Nanjing, China). β-elemene (l-methyl-l-vinyl-2,4-diisopropenyl-cyclohexane) solution (5 g/l, batch #0909221) was purchased from Jingang Pharmaceutical (Dalian, China).
RPMI-1640 culture medium and fetal bovine sera were purchased from Gibco (Grand Island, NY, USA). Primary antibodies for VEGF, MMP-2 and β-actin were obtained from Abcam (Cambridge, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for VEGF, matrix metalloproteinase-2 (MMP-2) and hypoxia inducible factor 1α (HIF1α) were purchased from R&D (Minneapolis, MN, USA). Evans blue dye was purchased from Sigma (St. Louis, MO, USA).
Animal treatment
The doses of Endostar and β-elemene used to treat malignant ascites in the clinic are 30 mg/m2 (8) and 200–300 mg/m2 (12) via intraperitoneal injection, and the translated dose from human to mouse is approximately 8 mg/kg and 50–75 mg/m2, respectively. Therefore, the dose range of Endostar and β-elemene used in this research was 4, 8 and 16 mg/kg and 50, 75 and 100 mg/kg, respectively. The safety of these doses (Endostar 16 mg/kg, β-elemene 100 mg/ kg and their combination) was evaluated in our previous study, and no drug-induced hematological toxicity, hepatotoxicity or liver and kidney injury were observed (unpublished data).
To determine the most effective dose of Endostar and β-elemene, we evaluated the effects of different doses on malignant ascites in the mouse model. The mice were randomly divided into seven treatment groups (n=20, each) 24 h after intraperitoneal injection of H22 cells. The control group received the vehicle used in all treatments, i.e., 0.9% normal saline (NS); the Endostar treatment groups received 4, 8 and 16 mg/kg of Endostar in NS; and the β-elemene treatment groups received 50, 75 and 100 mg/kg of β-elemene in NS. Mice were administered the respective drug treatment once a day for eight days via intraperitoneal injection. A total of 10 mice from each group were euthanized by CO2 inhalation 24 h after the final drug administration and ascites were collected to measure the volume. The remaining 10 mice were maintained until death to determine the survival rate.
To determine the optimal dose combination of Endostar and β-elemene, we combined the most effective dose of Endostar with the other doses of β-elemene, and combined the most effective dose of β-elemene with the other doses of Endostar. Different dose combinations were used to treat ascitic mice. The treatments were the same as those previously mentioned.
Other therapeutic effects and the underlying mechanisms were investigated with the optimal dose combination. The mice were randomly divided into four treatment groups (n=20, each) 24 h after intraperitoneal injection of H22 cells. The control group received NS, the Endostar group and β-elemene group received their single doses which were used in the combination group and the combination group received the optimal dose combination. The treatments were the same as previously mentioned and 10 mice of each group were euthanized to collect ascites samples for further detection. A remaining 10 mice per group were used for Evans blue extravasation detection.
Physical, cellular and biochemical examinations
To measure the ascites volume, ascites fluid was collected and measured via syringe from the opened abdominal wall following euthanasia.
The ascites fluid samples were diluted 10-fold prior to analysis. The tumor cells were counted under a microscope (CKX41, Olympus, Tokyo, Japan). Each sample was counted three times and the average was calculated.
The ascitic protein was examined by clinical biochemical analyzer (7020, Hitachi, Tokyo, Japan).
Evans blue extravasation detection
To determine whether the drugs decreased the peritoneal microvascular permeability, the leakage of Evans blue dye into the ascites fluid was analyzed (13). A total of 10 mice from each group were injected with 0.2 ml of 5% Evans blue solution via the caudal vein. The Evans blue solution was prepared using 5 g Evans blue dye dissolved in 100 ml 0.9% NS. Ascites fluid was collected 2 h post-injection and the samples were centrifuged at 2,000 rpm for 5 min. The extravasated Evans blue dye in the supernatants was assessed at 540 nm by a spectrophotometer (UV-2450, Shimadsu, Columbia, MD, USA).
ELISA
To evaluate the influence of drugs on cytokines in the ascites fluid, VEGF, MMP-2 and HIF1α in the ascites fluid were analyzed using an ELISA assay. The ascites samples were collected and centrifuged at 2,000 rpm for 5 min and the supernatants were analyzed.
Western blot analyses
Western blotting was performed to analyze the expression of VEGF and MMP-2 in tumor cells. Tumor cells were lysed in 1X lysis buffer and lysates centrifuged (Centrifuge 5415R, Eppendorf, Cambridge, UK) at 13,000 x g for 15 min at 4°C. The protein concentration was determined by bicinchoninic acid (BCA) assay (Pierce BCA Protein Assay kit, Thermo Fisher Scientific). The 20–50 μg samples were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The blots were blocked with PBS containing 5% powdered milk at room temperature for 1 h, then sequentially incubated with primary antibodies overnight at 4°C, followed by secondary antibodies for 1 h at 37°C. Finally, the blots were detected using electrochemiluminescence (ECL) reagents and Bio-Rad Molecular Imager ChemiDoc XRS + imaging system (Bio-Rad, Hercules, CA, USA). The optical densities of protein bands were analyzed with Sigma Scan Pro 5 and normalized with a loading control.
Reverse transcription and real-time quantitative PCR (RT-qPCR)
The RT-qPCR assay was used to analyze VEGF mRNA in tumor cells. Total RNA was isolated from tumor cells for reverse transcription and RT-qPCR with the appropriate primers designed by Primer software (version 5.0). The specific primer pairs for VEGF were: forward, 5′-AGACACACCCACCCACATACA-3′; reverse, 5′-ACATCCTCCTCCCAACACAAG-3′. The RT-qPCR assay was performed following the manufacturer's instructions (MultiGene Gradient, Labnet International, Inc., Edison, NJ, USA). All samples were subjected to 40 cycles of PCR, which included triplicate samples and controls with no template. β-actin was used as the reference.
Statistical analyses
Data are presented as mean ± standard deviation (SD). SPSS software (version 16.0, SPSS, Chicago, IL, USA) was used for data analysis. For multiple mean comparisons, the one-way ANOVA least significant difference (LSD) method was performed to determine the homogeneity of variance or the Games-Howell method for heterogeneity of variance. The Kaplan-Meier method was used to analyze survival rate and draw survival curves. The log-rank test was applied to compare survival curves. P<0.05 was considered to indicate a statistically significant result.
To analyze the effects of the drugs in combination, the coefficient of drug interaction (CDI) was calculated. The CDI was calculated as follows: CDI = AB/(A x B) (14). According to the ascites volumes of each group, AB is the ratio of the combination group to the control group, A or B is the ratio of the single agent group to control group. A CDI value <1, =1 or >1 indicates the synergistic, additive or antagonistic effect, respectively. CDI<0.7 indicates that the drug combination is significantly synergistic.
Results
Optimal effective dose of Endostar and β-elemene is 8 and 100 mg/kg, respectively
In the Endostar treatment groups, the mean ascites volume in the 8 mg/kg group was significantly less than that of the other two groups (P<0.05; Fig. 1A), and the survival of the 8 mg/kg group was also longest (P<0.05; Fig. 2A). In the β-elemene treatment groups, the mean ascites volume in 100 mg/kg group was the lowest (P<0.05; Fig. 1B), and it improved the survival rate to a greater extent (P<0.05; Fig. 2B). There was no difference in the mean ascites volumes between the 4 mg/kg Endostar and control group (P>0.05); this was also observed in the 50 mg/kg β-elemene group and the control groups (P>0.05). Therefore, we combined 8 and 16 mg/kg Endostar with 75 and 100 mg/kg β-elemene separately, to produce four combination groups as follows: 8 mg/ kg Endostar plus 75 mg/kg β-elemene, 8 mg/kg Endostar plus 100 mg/kg β-elemene, 16 mg/kg Endostar plus 75 mg/kg β-elemene and 16 mg/kg Endostar plus 100 mg/kg β-elemene.
Figure 1.
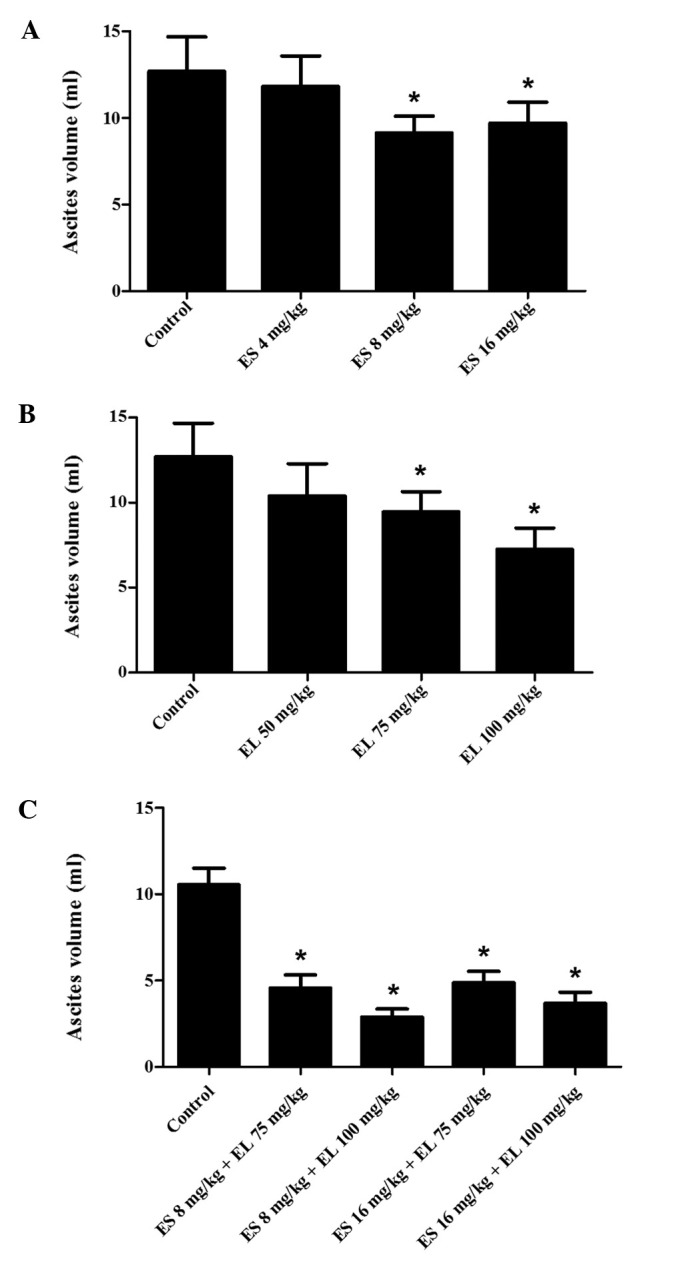
Effects of Endostar, β-elemene and their combination on the formation of ascites fluid. Mice were treated with different doses of (A) Endostar, (B) β-elemene and (C) their combination for eight days. The mice were euthanized to collect ascites fluid via syringe and the mean ascites volumes were measured. *P<0.05 vs. control group, n=10. ES, Endostar; EL, β-elemene.
Figure 2.
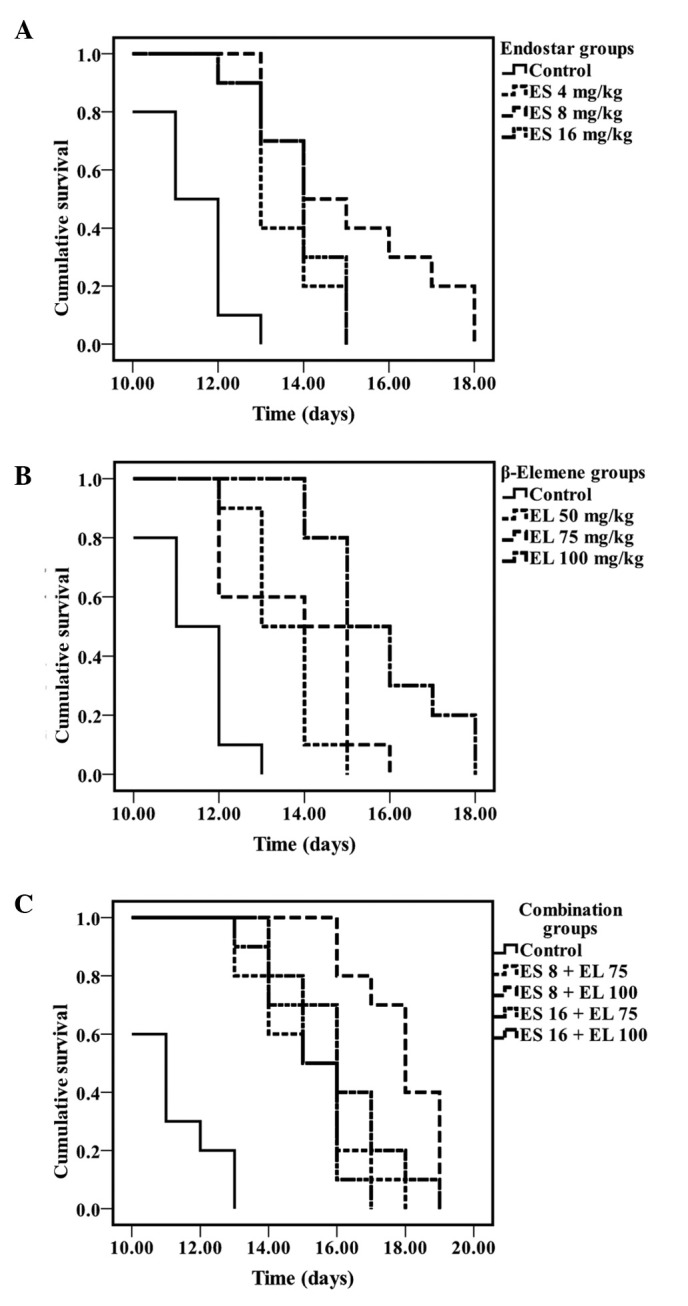
Kaplan-Meier survival analysis of mice treated with Endostar, β-elemene and their combination (log-rank test). The survival curves of mice treated with different doses of (A) Endostar, (B) β-elemene, (C) a combination of Endostar and β-elemene. (P<0.05, n=10, each). ES, Endostar; EL, β-elemene.
Optimal dose combination is 8 mg/kg Endostar plus 100 mg/ kg β-elemene
All treatment groups decreased the ascites formation (P<0.05; Fig. 1C) and also improved the survival of the mice (P<0.05; Fig. 2C). Among the four combination groups, the mean ascites volume in the 8 mg/kg Endostar plus 100 mg/ kg β-elemene group was the lowest (P<0.05) and the survival rate of mice was also the longest (P<0.05). Hence, 8 mg/kg Endostar plus 100 mg/kg β-elemene was the optimal dose combination in the present study.
Formation of malignant ascites is synergistically suppressed
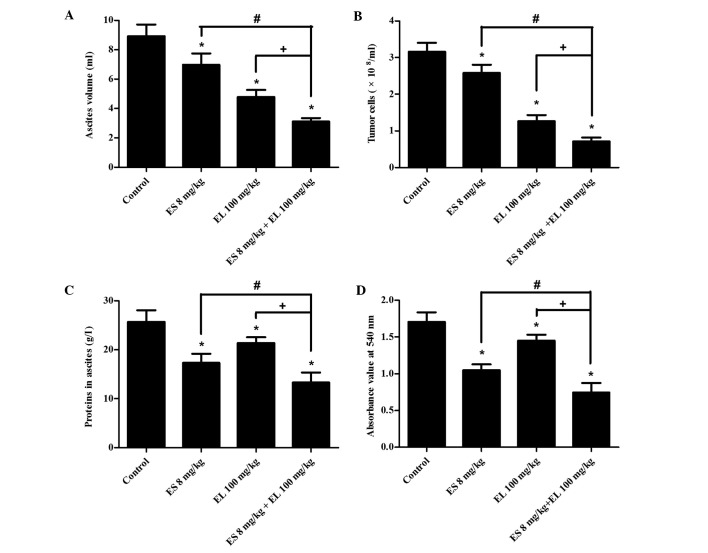
The results of the malignant ascites fluid assays included mean ascites volume, tumor cell number and ascitic protein levels. The mean ascites volumes in the control, Endostar, β-elemene and combination groups were 8.93±0.79, 7.34±0.76, 4.79±0.47 and 3.12±0.23 ml. The ascites volume in the combination group was less than that of the other three groups (P<0.05; Fig. 3A) and the CDI value was 0.79, which indicated that 8 mg/kg Endostar plus 100 mg/kg β-elemene treatment synergistically suppressed the formation of malignant ascites. Compared with Endostar and β-elemene groups, the tumor cell number and ascitic protein in the combination group were also significantly decreased (P<0.05; Fig. 3B and C).
Figure 3.
Effects of Endostar, β-elemene and their optimal dose combination on the ascites volume, number of ascitic tumor cells, ascitic protein levels and peritoneal vascular permeability. (A) The ascites volume of mouse was measured by syringe. (B) The ascites fluid samples were diluted 10-fold prior to analysis, and the number of tumor cells was counted under a microscope. (C) The ascitic protein was examined using a clinical biochemical analyzer. (D) The absorbance value of ascites was assessed at 540 nm by a spectrophotometer. *P<0.05 vs. control group; #P<0.05 vs. ES (Endostar) 8 mg/kg group; +P<0.05 vs. EL (β-elemene) 100 mg/kg group, n=10.
Peritoneal microvascular permeability is reduced
The extravasation of Evans blue dye into the peritoneal cavity is an indirect indicator of peritoneal vascular permeability. The amount of Evans blue in the peritoneal cavity was assessed from absorbance readings (540 nm) of supernatants prepared from ascites fluid samples. The mean absorbance value of the control, Endostar, β-elemene and combination groups was 1.71±0.13, 1.05±0.08, 1.45±0.09 and 0.88±0.07, respectively. In the Endostar and β-elemene groups, the mean absorbance value was significantly lower than that of the control group (P<0.05; Fig. 3D). Among the four groups, the mean absorbance in the combination group was the lowest (P<0.05), suggesting that the 8 mg/kg Endostar plus 100 mg/kg β-elemene effectively reduced peritoneal microvascular permeability.
VEGF, MMP-2 and HIF1α are downregulated
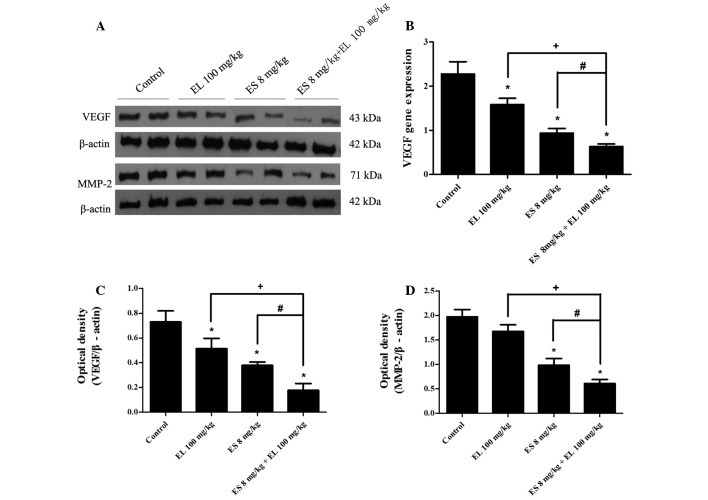
The ELISA results revealed that the levels of VEGF, MMP-2, and HIF1α in ascites were significantly lower in the combination group than in either the Endostar or β-elemene single treatment group (P<0.05; Fig. 4). Western blotting showed that the levels of VEGF in ascitic tumor cells were downregulated in both the Endostar and β-elemene groups, and were lowest in the combination group (P<0.05; Fig. 5A and C). There was no difference in the levels of MMP-2 between the β-elemene group and control group (P>0.05), but it was downregulated in the Endostar group, and the effects were enhanced in the combination group (P<0.05; Fig. 5A and D).
Figure 4.
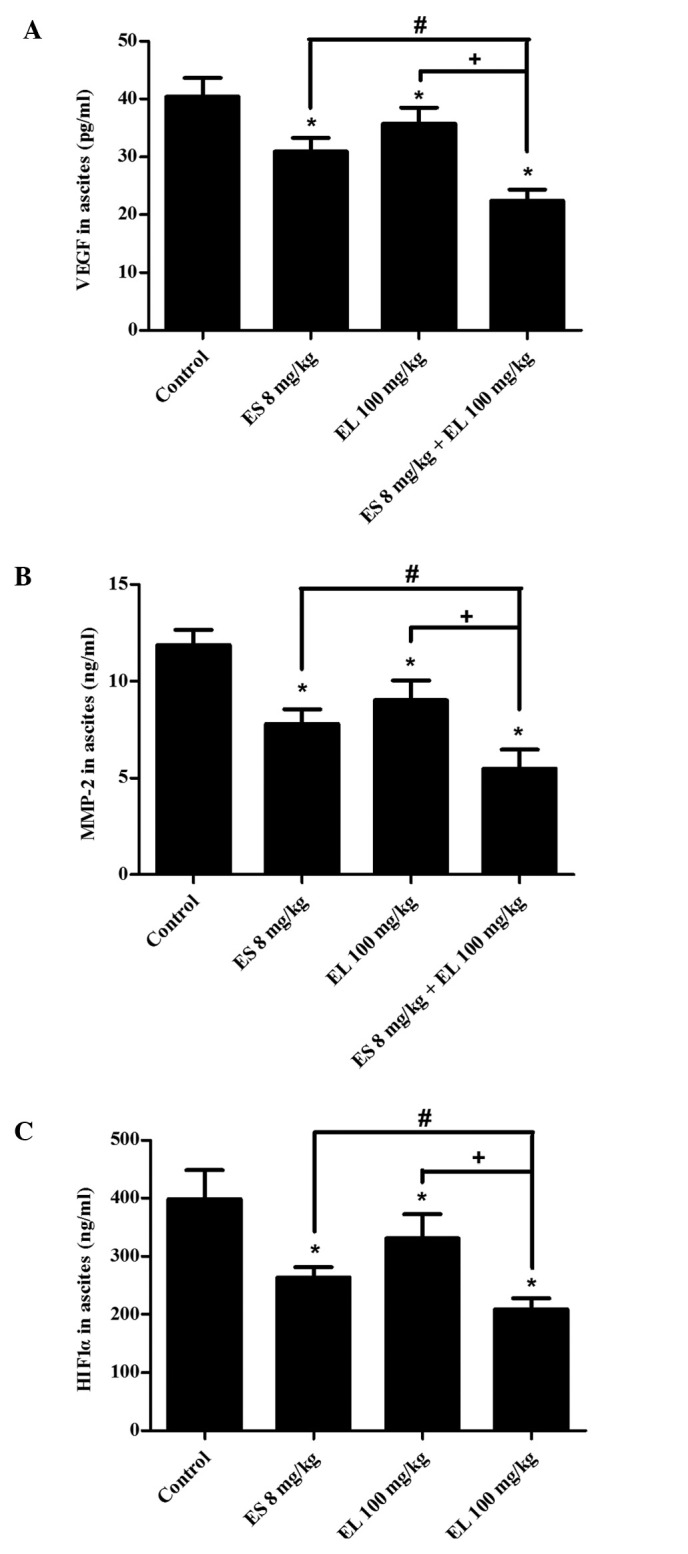
Levels of related cytokines in ascites fluid. Ascites fluid of each mouse was collected to detect (A) VEGF, (B) MMP-2 and (C) HIF1α by ELISA assay. *P<0.05 vs. control group; #P<0.05 vs. ES (Endostar) 8 mg/kg group; +P<0.05 vs. EL (β-elemene) 100 mg/kg group, n=6. HIF1α, hypoxia inducible factor 1α.
Figure 5.
Effects of Endostar and β-elemene on the expression of VEGF and MMP-2 proteins, as well as VEGF mRNA in ascites tumor cells. (A) Cell lysates were collected for western blot analysis of VEGF and MMP-2, and β-actin was used as a loading control. (C and D) Optical densities of VEGF, MMP-2 and β-actin were analyzed and normalized with loading control. (B) VEGF mRNA levels were analyzed by real time qPCR. *P<0.05 vs. control group; #P<0.05 vs. ES (Endostar) 8 mg/kg group; +P<0.05 vs. EL (β-elemene) 100 mg/kg group, n=3. ES, Endostar; EL, β-elemene.
Furthermore, the relative expression of VEGF mRNA was 2.28±0.28, 0.94±0.11, 1.58±0.15 and 0.63±0.06 in the control, Endostar, β-elemene and combination groups, respectively. The results showed that VEGF mRNA of tumor cells in Endostar, β-elemene and combination groups was also suppressed, and it was lowest in the combination group (P<0.05; Fig. 5B).
Discussion
It is generally believed that antiangiogenic therapy mainly stabilizes tumor growth by cutting off the blood supply, but its objective response rate (ORR) is limited. For example, the ORR of Endostar is only 3–5%. Therefore, antiangiogenesis is usually combined with cytotoxic agents to improve the efficiency. While β-elemene is considered to be a powerful antineoplastic agent, it is reasonable to apply the combination therapy of Endostar and β-elemene in the treatment of malignant ascites and to verify the treatment efficacy via the H22 mouse ascites model (15).
We evaluated the effects of Endostar and β-elemene on inhibiting ascites formation and prolonging survival, and determined their optimal dose combination. The results of this study showed that the mice with a smaller ascites volume survived longer, which indicates that inhibiting the ascites fluid may be an effective method to prolong lifespan for patients with malignant ascites in the clinic.
The formation of malignant ascites is the result of tumor peritoneal cavity metastasis, thus suppression of tumor cells is the most direct and effective therapy. In this study, the combination of Endostar and β-elemene enhanced the effects of the suppression of tumor cell growth, which may be mainly due to β-elemene. As mentioned previously, the high peritoneal microvascular permeability plays a significant role in the formation of malignant ascites (3), which increases the leakage of fluid from peritoneal microvessels to the peritoneal cavity and accelerates the accumulation of ascites fluid. In the present study, the peritoneal microvascular permeability was markedly decreased by Endostar and β-elemene, and proteins in the ascites fluid leaked from peritoneal microvessels were also reduced, particularly with combination treatment. Thus, Endostar and β-elemene synergistically inhibit the formation of ascites, and the survival rate of mice was also prolonged.
The formation of malignant ascites involves increased peritoneal microvasculature, invasion and metastasis of tumor cells and the anoxic environment of the peritoneal cavity, which are associated with VEGF, MMP-2 and HIF1α. Therefore, we determined the levels of the related cytokines and factors.
Firstly, the results of the present study indicate that combining Endostar with β-elemene causes a greater significant reduction in VEGF mRNA and protein in ascites fluid and tumor cells than treatment with either Endostar or β-elemene alone, and that the levels of VEGF are correlated with the peritoneal microvascular permeability. VEGF is a multi-functional cytokine that contributes to stimulating angiogenesis and augmenting the permeability of the pre-existing microvasculature. The VEGF-mediated increase in vessel permeability for plasma proteins is 10,000 times higher than that induced by histamine (16). Markedly elevated levels of VEGF have been observed in malignant pleural effusions and ascites fluid (17), and VEGF levels correlate with its ability to induce vascular leakage, which can be blocked by anti-VEGF therapy (18). Therefore, blocking the high peritoneal microvascular permeability induced by VEGF may have been the most significant reason for the inhibition of malignant ascites in this study.
Secondly, a greater reduction of MMP-2 in ascites fluid and tumor cells was observed in 8 mg/kg Endostar plus 100 mg/kg β-elemene treated mice than either 8 mg/kg Endostar or 100 mg/kg β-elemene treatments alone. MMPs are able to degrade regulatory proteins associated with the tumor microenvironment and promote the invasion of tumor cells through normal tissues and blood vessel walls resulting in metastasis and the expression and activities of MMPs are increased in almost all kinds of human cancers compared with normal tissues (19). As an indication of tumor metastasis, the formation of malignant ascites is a complex process that includes cell adhesion, invasion and migration, while MMPs, mainly MMP-2 and MMP-9, have been shown to be multi-functional promoters attributing to malignant ascites formation. In a study on type IV collagenase (MMP-2 and MMP-9) activity in benign and malignant ascites, MMP-2 and MMP-9 were detectable only in malignant ascites (20). Therefore, combination treatment may suppress the invasion and metastasis of tumor cells in the peritoneal cavity by the strong downregulation of MMP-2, resulting in the reduction of ascites formation.
Finally, the more marked inhibition of HIF1α in ascites fluid was observed in mice treated with 8 mg/kg Endostar plus 100 mg/kg β-elemene, and the levels of VEGF and MMP-2 were simultaneously decreased by treatment. HIF1α is a transcription factor that plays an essential role in cellular and systemic responses to hypoxia. Under hypoxic conditions, the hypoxia signalling pathway is activated through a series of steps involving the upregulation of HIF1α, nuclear translocation, dimerization with HIF1β, binding to DNA and induction of gene transcription; VEGF, erythropoietin (EPO) and nitric oxide synthase (NOS) are induced for angiogenesis, erythropoiesis and glycolysis that improve cellular adaption to hypoxia and the restoration of oxygen homeostasis (21). The upregulated MMP-2 under hypoxic conditions, a common phenomenon in tumors, was decreased by the inhibition of HIF1α in malignant gliomas (22). Tumor cells growing in ascites suffer from a severe anoxic environment, and the rapid proliferation of tumor cells aggravates hypoxia, which activates HIF1α and induces the transcription of VEGF and MMP-2. Elevated levels of VEGF increase high peritoneal microvascular permeability, and over-expressed MMP-2 promotes tumor invasion and metastasis. Consequently, VEGF and MMP-2 cooperatively facilitate the formation of malignant ascites. The results of the present study suggest that the inhibition of HIF1α caused by Endostar and β-elemene may contribute to the decrease of hypoxia-induced VEGF and MMP-2, at least in part.
In conclusion, the mechanism related to the combination therapy may involve two mechanisms. The first is that β-elemene inhibits the proliferation of tumor cells, and decreases the secretion of cytokines by reducing tumor burden; while Endostar inhibits tumor growth by its effect of antiangiogenesis and downregulation of MMP-2 and HIF1α, which enhances the suppression of tumor cells. The second is that Endostar decreases the peritoneal microvascular permeability by anti-VEGF effects that are enhanced by β-elemene. As a result, they cooperatively inhibit the formation of malignant ascites and prolong the lifespan of mice.
Although the decrease in VEGF, MMP-2 and HIF1α was considered to be correlated with the inhibition of malignant ascites in this study, the underlying mechanism remains unclear. A variety of signaling pathways play essential roles in the development of tumors, and further studies are needed to determine whether signaling pathways, including PI3K/Akt, ERK1/2 or p38 MAPK, are involved in the treatment of malignant ascites by Endostar and β-elemene.
Our study initially demonstrated that combined therapy with Endostar and β-elemene synergistically inhibited ascites formation and prolonged the survival rate in a mouse model of H22 ascitic hepatoma cells. The underlying mechanism may be related to the enhanced effects of suppressing tumor cells and decreasing peritoneal microvascular permeability, as well as the inhibition of VEGF, MMP-2 and HIF1α. This study provided substantial experimental evidence to support this combination therapy regimen in the clinic.
Acknowledgments
The study was financially supported by Jiangsu Simcere Pharmaceutical Research Co., Ltd. This manuscript was edited by Medjaden Bioscience Limited. We thank members of the laboratory and our collaborators for the numerous useful suggestions.
References
- 1.Garrison RN, Kaelin LD, Galloway RH, Heuser LS. Malignant ascites: clinical and experimental observations. Ann Surg. 1986;203:644–651. doi: 10.1097/00000658-198606000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker G, Galandi D, Blum HE. Malignant ascites: systematic review and guideline for treatment. Eur J Cancer. 2006;42:589–597. doi: 10.1016/j.ejca.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Tamsma JT, Keizer HJ, Meinders AE. Pathogenesis of malignant ascites: Starling's law of capillary hemodynamics revisited. Ann Oncol. 2001;12:1353–1357. doi: 10.1023/a:1012504904713. [DOI] [PubMed] [Google Scholar]
- 4.O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 5.Ling Y, Yang Y, Lu N, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79–84. doi: 10.1016/j.bbrc.2007.06.155. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Sun Y, Liu Y, et al. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi. 2005;8:283–290. doi: 10.3779/j.issn.1009-3419.2005.04.07. (In Chinese). [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Huang XE, Yan PW, et al. Efficacy and safety of endostar combined with chemotherapy in patients with advanced solid tumors. Asian Pac J Cancer Prev. 2010;11:1119–1123. [PubMed] [Google Scholar]
- 8.Jiang Z, Qin S. Study progression of recombinant human endostatin (Endostar) for the treatment of malignant serous effusion. Chinese-German J Clin Oncol. 2011;10:435–441. [Google Scholar]
- 9.Li QQ, Wang G, Huang F, et al. Antineoplastic effect of beta-elemene on prostate cancer cells and other types of solid tumor cells. J Pharm Pharmacol. 2010;62:1018–1027. doi: 10.1111/j.2042-7158.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Lu Y, Wu J, et al. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother Pharmacol. 2011;67:799–808. doi: 10.1007/s00280-010-1378-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Li QQ, Zou B, et al. In vitro combination characterization of the new anticancer plant drug β-elemene with taxanes against human lung carcinoma. Int J Oncol. 2007;31:241–252. [PubMed] [Google Scholar]
- 12.Wang J, Zhang H, Sun Y. Phase III clinical trial of elemenum emulsion in the management of malignant pleural and peritoneal effusions. Zhonghua Zhong Liu Za Zhi. 1996;18:464–467. (In Chinese). [PubMed] [Google Scholar]
- 13.Kanaoka Y, Maekawa A, Penrose JF, et al. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J Biol Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 14.Cao SS, Zhen YS. Potentiation of antimetabolite antitumor activity in vivo by dipyridamole and amphotericin B. Cancer Chemother Pharmacol. 1989;24:181–186. doi: 10.1007/BF00300240. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Wang X, Lu H. Amifostine increases cure rate of cisplatin on ascites hepatoma 22 via selectively protecting renal thioredoxin reductase. Cancer Lett. 2008;260:127–136. doi: 10.1016/j.canlet.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 17.Zebrowski BK, Liu W, Ramirez K, et al. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6:373–378. doi: 10.1007/s10434-999-0373-0. [DOI] [PubMed] [Google Scholar]
- 18.Luo JC, Toyoda M, Shibuya M. Differential inhibition of fluid accumulation and tumor growth in two mouse ascites tumors by an antivascular endothelial growth factor/permeability factor neutralizing antibody. Cancer Res. 1998;58:2594–2600. [PubMed] [Google Scholar]
- 19.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 20.Sun XM, Dong WG, Yu BP, et al. Detection of type IV collagenase activity in malignant ascites. World J Gastroenterol. 2003;9:2592–2595. doi: 10.3748/wjg.v9.i11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hewitson KS, McNeill LA, Schofield CJ. Modulating the hypoxia-inducible factor signaling pathway: applications from cardiovascular disease to cancer. Curr Pharm Des. 2004;10:821–833. doi: 10.2174/1381612043452884. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara S, Nakagawa K, Harada H, et al. Silencing hypoxiainducible factor-1α inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol. 2007;30:793–802. [PubMed] [Google Scholar]