Abstract
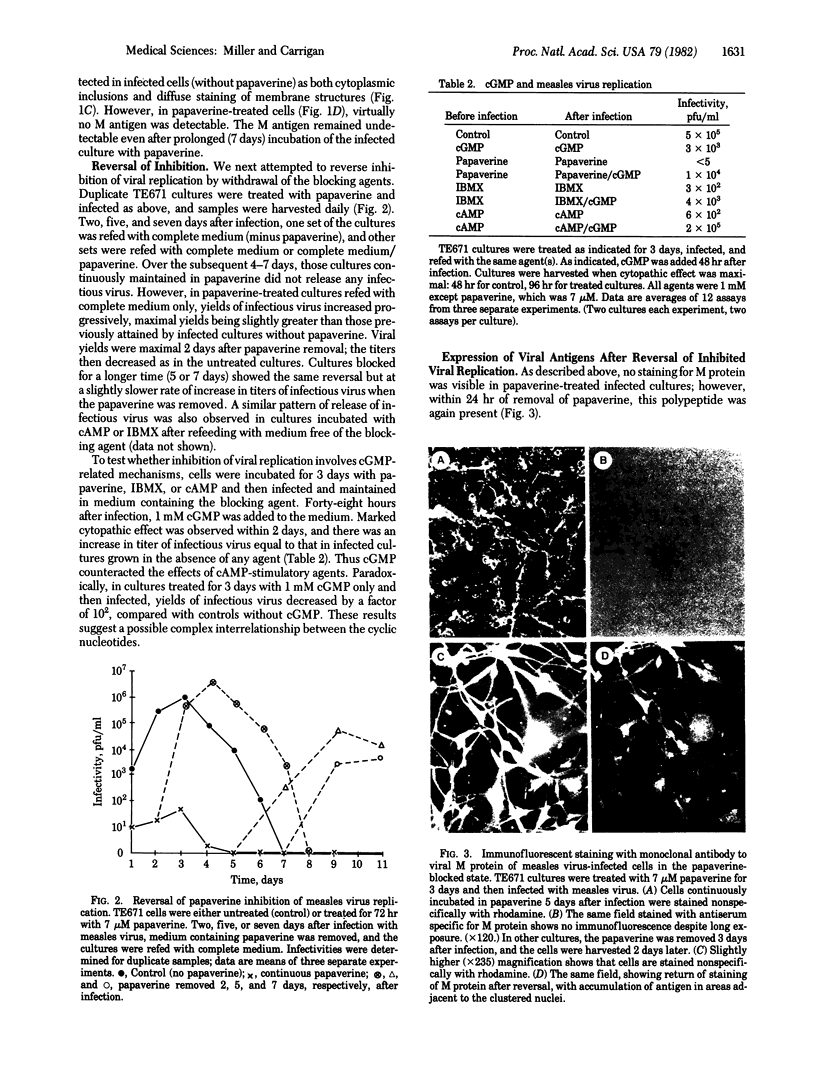
Conversion of acute measles virus infection to an indolent state has been achieved by treatment of infected cells of neural origin with agents that affect cyclic nucleotide metabolism. Striking results were obtained with papaverine, an inhibitor of cAMP phosphodiesterase that is capable of enhancing neural differentiation. In papaverine-treated cultures, decreased production of infectious virus was accompanied by selective disappearance of intracellular matrix proton, as detected by immunofluorescence. Viral nucleocapsid protein was enhanced in the cytoplasm while three other structural proteins--polymerase, hemagglutinin, and fusion protein--showed little change in distribution or intensity of staining. These results were specific for cells of neural origin and not observed in CV-1 or Vero cultures. cAMP, dibutyryl cAMP, 8-bromo-cAMP, and isobutylmethylxanthine all inhibited replication of virus but less so than did papaverine. Inhibition of virus replication by any of these agents was rapidly reversible, either by removal of the agent or by addition of cGMP to the culture medium and was accompanied by reappearance of the matrix protein. These results suggest that measles virus replication in neural cells depends on host factors, particularly those affecting endogenous cAMP and cGMP. Viral persistence may thus be related to the state of neural differentiation. This model system may yield information on mechanisms of recrudescence observed in some chronic diseases of the nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Matus A. I. Ultrastructural localization of cyclic GMP and cyclic AMP in rat striatum. J Cell Biol. 1981 Oct;91(1):287–292. doi: 10.1083/jcb.91.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholz C. M., Kiley M. P., Payne F. E. Isolation and characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Jul;16(1):192–202. doi: 10.1128/jvi.16.1.192-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan D. R., McKendall R. R., Johnson K. P. CNS disease following dissemination of SSPE measles virus from intraperitoneal inoculation of suckling hamsters. J Med Virol. 1978;2(4):347–357. doi: 10.1002/jmv.1890020408. [DOI] [PubMed] [Google Scholar]
- Ehrnst A. Growth phase related loss of measles virus surface-associated antigens and cytotoxic susceptibility in persistently infected cells. J Gen Virol. 1979 Dec;45(3):547–556. doi: 10.1099/0022-1317-45-3-547. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Gershey E. L. Simian virus 40-host cell interaction during lytic infection. J Virol. 1979 Apr;30(1):76–83. doi: 10.1128/jvi.30.1.76-83.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves M. C. Measles virus polypeptides in infected cells studied by immune precipitation and one-dimensional peptide mapping. J Virol. 1981 Apr;38(1):224–230. doi: 10.1128/jvi.38.1.224-230.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Measles-virus proteins in the brain tissue of patients with subacute sclerosing panencephalitis: absence of the M protein. N Engl J Med. 1981 May 7;304(19):1152–1155. doi: 10.1056/NEJM198105073041906. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J., Gould E. Defective interfering particles produced during the replication of measles virus. Med Microbiol Immunol. 1974;160(2-3):155–164. doi: 10.1007/BF02121722. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Sahgal V., Harter D. H., Choppin P. W. Abnormal levels of antibodies to measles-virus proteins in patient with mental retardation and seizures 24 years after measles encephalitis. Lancet. 1979 Nov 3;2(8149):967–968. doi: 10.1016/s0140-6736(79)92669-2. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Rapp F. Measles virus: an unwanted variant causing hydrocephalus. Science. 1975 Feb 7;187(4175):450–451. doi: 10.1126/science.1111114. [DOI] [PubMed] [Google Scholar]
- Herndon R. M., Rena-Descalzi L., Griffin D. E., Coyle P. K. Age dependence of viral expression. Electron microscopic and immunoperoxidase studies of Measles virus replication in mice. Lab Invest. 1975 Nov;33(5):544–553. [PubMed] [Google Scholar]
- Iwasaki Y., Clark H. F. Rabies virus infection in mouse neuroblastoma cells. Lab Invest. 1977 Jun;36(6):578–574. [PubMed] [Google Scholar]
- Jabbour J. T., Duenas D. A., Sever J. L., Krebs H. M., Horta-Barbosa L. Epidemiology of subacute sclerosing panencephalitis (SSPE). A report of the SSPE registry. JAMA. 1972 May 15;220(7):959–962. [PubMed] [Google Scholar]
- Johnson K. P., Norrby E. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. 3. Induction of defective measles infection in hamster brain. Exp Mol Pathol. 1974 Oct;21(2):166–178. doi: 10.1016/0014-4800(74)90087-2. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Norrby E., Swoveland P., Carrigan D. R. Experimental subacute sclerosing panencephalitis: selective disappearance of measles virus matrix protein from the Central nervous system. J Infect Dis. 1981 Aug;144(2):161–169. doi: 10.1093/infdis/144.2.161. [DOI] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Absence of M protein in a cell-associated subacute sclerosing panencephalitis virus. Nature. 1980 Jun 12;285(5765):490–492. doi: 10.1038/285490a0. [DOI] [PubMed] [Google Scholar]
- Lucas C. J., Ubels-Postma J. C., Rezee A., Galama J. M. Activation of measles virus from silently infected human lymphocytes. J Exp Med. 1978 Oct 1;148(4):940–952. doi: 10.1084/jem.148.4.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Hayes E. C., Zweerink H. J. Cells infected with a cell-associated subacute sclerosing panencephalitis virus do not express M protein. Virology. 1981 Jan 30;108(2):515–520. doi: 10.1016/0042-6822(81)90460-8. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Isaacs H., Rongey R., Peer M., Au W., Soukup S. W., Gardner M. B. Establishment of a human medulloblastoma cell line. Int J Cancer. 1977 Aug 15;20(2):206–212. doi: 10.1002/ijc.2910200207. [DOI] [PubMed] [Google Scholar]
- Miller C. A. Intranuclear polypeptides of measles and subacute sclerosing panencephalitis virus-infected cultures. Virology. 1980 Feb;101(1):272–276. doi: 10.1016/0042-6822(80)90502-4. [DOI] [PubMed] [Google Scholar]
- Osunkoya B. O., Oyediran A. B., Cooke A. Multinucleated giant cells in PHA-stimulated leucocyte cultures of children with measles. Immunology. 1973 Oct;25(4):737–742. [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Merigan T. C. A useful quantitative semimicromethod for viral plaque assay. Proc Soc Exp Biol Med. 1973 Apr;142(4):1174–1179. doi: 10.3181/00379727-142-37202. [DOI] [PubMed] [Google Scholar]
- Rammohan K. W., McFarland H. F., McFarlin D. E. Induction of subacute murine measles encephalitis by monoclonal antibody to virus haemagglutinin. Nature. 1981 Apr 16;290(5807):588–589. doi: 10.1038/290588a0. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Martin S. J., Gould E. A. A comparison of polypeptides in measles and SSPE virus strains. J Gen Virol. 1979 Mar;42(3):603–608. doi: 10.1099/0022-1317-42-3-603. [DOI] [PubMed] [Google Scholar]
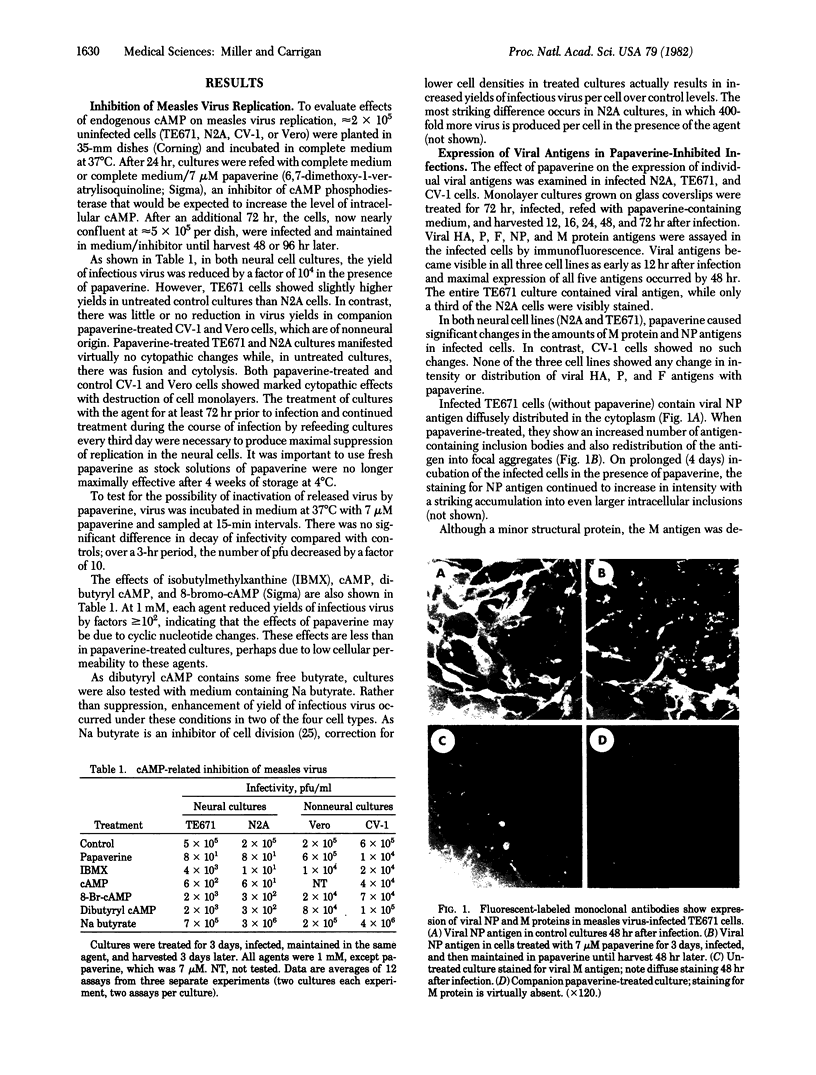
- Robbins S. J., Rapp F. Inhibition of measles virus replication by cyclic AMP. Virology. 1980 Oct 30;106(2):317–326. doi: 10.1016/0042-6822(80)90255-x. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Defendi V., Wigglesworth N. M. The effect of dibutyryl cyclic AMP on transformation by oncogenic viruses. Virology. 1973 Jan;51(1):230–232. doi: 10.1016/0042-6822(73)90383-8. [DOI] [PubMed] [Google Scholar]
- Stanwick T. L., Anderson R. W., Nahmias A. J. Interaction between cyclic nucleotides and herpes simplex viruses: productive infection. Infect Immun. 1977 Nov;18(2):342–347. doi: 10.1128/iai.18.2.342-347.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Lucas S. J., Albrecht P. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975 Sep 1;142(3):773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormar H., Mehta P. D., Brown H. R. Comparison of wild-type and subacute sclerosing panencephalitis strains of measles virus. Neurovirulence in ferrets and biological properties in cell cultures. J Exp Med. 1978 Sep 1;148(3):674–691. doi: 10.1084/jem.148.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi T., Orvell C., Vartdal F., Norrby E. Production of antibodies against measles virions by use of the mouse hybridoma technique. Arch Virol. 1981;67(2):149–157. doi: 10.1007/BF01318598. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Differences between the intracellular polypeptides of measles and subacute sclerosing panencephalitis virus. Nature. 1978 Mar 30;272(5652):458–460. doi: 10.1038/272458a0. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Intracellular synthesis of measles virus-specified polypeptides. J Virol. 1978 Jan;25(1):285–297. doi: 10.1128/jvi.25.1.285-297.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Rustigian R., Stallcup K. C., Byers K. B., Winston S. H., Fields B. N. Measles virus-specified polypeptide synthesis in two persistently infected HeLa cell lines. J Virol. 1979 Sep;31(3):677–684. doi: 10.1128/jvi.31.3.677-684.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. E., Jr, Glaser R., Rapp F. Effect of dibutyryl cyclic AMP on the induction of Epstein-Barr virus in hybrid cells. J Virol. 1973 Dec;12(6):1442–1445. doi: 10.1128/jvi.12.6.1442-1445.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]