Abstract
Hedyotis diffusa Willd (HDW) has long been used as an important component in several Chinese medicine formulae to clinically treat various types of cancer, including colorectal cancer (CRC). Previously, we reported that HDW inhibits CRC growth via the induction of cancer cell apoptosis and the inhibition of tumor angiogenesis. In the present study, to further elucidate the mechanism of HDW-mediated antitumor activity, we investigated the effect of HDW ethanol extract (EEHDW) on the proliferation of HT-29 human colon carcinoma cells. We found that EEHDW reduced HT-29 cell viability and survival in a dose- and time-dependent manner. We also observed that EEHDW treatment blocked the cell cycle, preventing G1 to S progression, and reduced mRNA expression of pro-proliferative PCNA, Cyclin D1 and CDK4, but increased that of anti-proliferative p21. Our findings suggest that Hedyotis diffusa Willd may be an effective treatment for CRC via the suppression of cancer cell proliferation.
Keywords: proliferation, cell cycle, Chinese herbal medicine, colorectal cancer, Hedyotis diffusa Willd
Introduction
Cancer cells are characterized by uncontrolled proliferation (1), therefore inhibiting the excessive proliferation of tumor cells is one of the key approaches for the development of anti-cancer drugs. Eukaryotic cell proliferation is regulated by the cell cycle, which is divided into a series of phases. G1/S transition is one of the two main checkpoints that control cell cycle progression (2). G1/S progression is mainly regulated by Cyclin D1 and Cyclin-dependent kinase 4 (CDK4) (3,4). PCNA is an acidic nuclear protein that has been recognized as a histological marker for the G1/S phase in the cell cycle (5). p21 is a CDK inhibitor, which can bind to CDK-Cyclin complexes and alter their function in order to suppress cell proliferation (6).
Drug resistance and toxicity against normal cells limit the effectiveness of current chemotherapies for the treatment of colorectal cancer (CRC) (7–10), which is a serious public health problem worldwide (11). These problems highlight the urgent need for the development of novel cancer chemotherapies. Recently, natural products have received great interest since they have relatively few side effects compared with modern chemotherapeutics and have been used clinically for thousands of years as significant alternative remedies for a variety of diseases including cancer (12–17). One promising medicinal plant is Hedyotis diffusa Willd (HDW) that belongs to the Rubiaceae family and is widely distributed throughout Northeast Asia. As a well-known traditional Chinese folk-medicine, it is used for heat-clearing, detoxification, promotion of blood circulation and the removal of blood stasis (18). HDW has also long been used as an significant component in several Chinese medicine formulae to treat various types of cancer, including CRC (18–20). Previously, we reported that Hedyotis diffusa Willd inhibits the growth of CRC, likely via the induction of cancer cell apoptosis and the inhibition of tumor angiogenesis (21,22). To further elucidate the mechanism of the tumoricidal activity of HDW we investigated its effect on the proliferation of human colon carcinoma HT-29 cells.
Materials and methods
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin, Trypsin-EDTA and TRIzol reagent were purchased from Invitrogen (Carlsbad, CA, USA). SuperScript II reverse transcriptase was obtained from Promega (Madison, WI, USA). All other chemicals, unless otherwise stated, were obtained from Sigma Chemicals (St. Louis, MO, USA).
Preparation of ethanol extract of Hedyotis diffusa Willd (EEHDW)
EEHDW was prepared as described previously (20,21). Briefly, stock solutions of EEHDW were prepared by dissolving the EEHDW powder in 40% DMSO to a final concentration of 400 mg/ml and stored at −20°C. The working concentrations of EEHDW were made by diluting the stock solution in the culture medium. The final concentrations of DMSO in the medium were <0.5%.
Cell culture
Human colon carcinoma HT-29 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). HT-29 cells were grown in DMEM. DMEM was supplemented with 10% (v/v) FBS, 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were cultured at 37°C and 5% CO2 in a humidified environment.
Cell viability evaluation
Cell viability was assessed by MTT colorimetric assay. HT-29 cells were seeded into 96-well plates at a density of 1x104 cells/well in 0.1 ml medium. The cells were treated with various concentrations (0, 1, 3 and 5 mg/ml) of EEHDW for various periods of time. At the end of the treatment, 10 μl MTT (5 mg/ml in phosphate-buffered saline, PBS) was added to each well and the samples were incubated for an additional 4 h at 37°C. The purple-blue MTT formazan precipitate was dissolved in 100 μl DMSO. The absorbance was measured at 570 nm using an ELISA reader (BioTek, Model ELX800, Winooski, VT, USA).
Colony formation assay
The HT-29 cells were seeded into 6-well plates at a density of 2x105 cells/well and treated with various concentrations (0, 1, 3 and 5 mg/ml) of EEHDW for 24 h. The cells were then diluted in fresh medium in the absence of EEHDW and reseeded into 6-well plates at a density of 1.5x103 cells/well. Following incubation for 7 or 8 days in a 37°C humidified incubator with 5% CO2, the colonies were counted under a light microscope. Cell survival was calculated by normalizing the survival of the control cells as 100%.
Cell cycle analysis
The cell cycle analysis was carried out by flow cytometry using a fluorescence-activated cell sorting (FACS) MoFlo XDP (Beckman Coulter, Miami, FL, USA) and propidium iodide (PI) staining. Following treatment with the indicated concentrations (0, 1, 3 and 5 mg/ml) of EEHDW for 24 h, HT-29 cells were harvested and adjusted to a concentration of 1x106 cells/ml, then fixed in 70% ethanol at 4°C overnight. The fixed cells were washed twice with cold PBS and then incubated for 30 min with RNase (8 μg/ml) and PI (10 μg/ml). The fluorescent signal was detected through the FL2 channel and the proportion of DNA in various phases was analyzed using ModfitLT Version 3.0 (Verity Software House, Topsham, ME, USA).
RNA extraction and RT-PCR analysis
A total of 2x105 HT-29 cells were seeded into 6-well plates in 2 ml medium and treated with indicated concentrations (0, 1, 3 and 5 mg/ml) of EEHDW for 24 h. Total RNA was isolated using TRIzol reagent. Oligo(dT)-primed RNA (1 μg) was reverse-transcribed with SuperScript II reverse transcriptase (Promega) according to the manufacturer’s instructions. The obtained cDNA was used to determine the mRNA levels of Cyclin D1, CDK4, PCNA and p21 by PCR. GAPDH was used as an internal control. The sequences of the primers used for amplification of Cyclin D1, CDK4, PCNA, p21 and GAPDH transcripts are as follows: Cyclin D1 forward 5′-TGGATGCTGGAGGTCTGCGAG GAA-3′ and reverse 5′-GGCTTCGATCTGCTCCTGGCA GGC-3′ [Temperature (Tm), 55°C; 573 bp]; CDK4 forward 5′-CATGTAGACCAGGACCTAAGC-3′ and reverse 5′-AAC TGGCGCATCAGATCCTAG-3′ (Tm, 58°C; 206 bp); PCNA forward 5′-GCTGACATGGGACACTTA-3′, and reverse 5′-CTCAGGTACAAACTTGGTG-3′ (Tm, 56°C; 610 bp); p21 forward 5′-GCGACTGTGATGCGCTAATGG-3′, and reverse 5′-TAGAAATCTGTCATGCTGGTCTGC-3′ (Tm=55°C, 358 bp); GAPDH forward 5′-CGACCACTTTGTCAAG CTCA-3′, and reverse 5′-AGGGGTCTACATGGCAACTG-3′ (Tm, 58°C; 240 bp). Samples were analyzed by gel electrophoresis (1.5% agarose). The DNA bands were examined using a Gel Documentation system (BioRad, Model Gel Doc 2000, USA).
Statistical analysis
All data were expressed as the means of three independent experiments and data was analyzed using the SPSS package for Windows (Version 11.5; Chicago, IL, USA). Statistical analysis of the data was performed using a Student’s t-test and ANOVA. P<0.05 was considered to indicate a statistically significant result.
Results
EEHDW inhibits the proliferation of HT-29 cells
We examined the effect of EEHDW on HT-29 cell viability by MTT assay. As shown in Fig. 1, EEHDW treatment reduced cell viability in a dose- and time-dependent manner compared to untreated control cells (P<0.05). The cell viability was decreased to 36% at the highest concentration of EEHDW (5 mg/ml) at 24 h in this study. To further verify these results, we examined the effect of EEHDW on HT-29 cell survival using a colony formation assay. As shown in Fig. 2, treatment with 1, 3 and 5 mg/ml of EEHDW for 24 h reduced the cell survival rate in a dose-dependent manner by 34, 70 and 84% compared to untreated control cells (P<0.05). These data suggest that EEHDW inhibits HT-29 cell proliferation.
Figure 1.
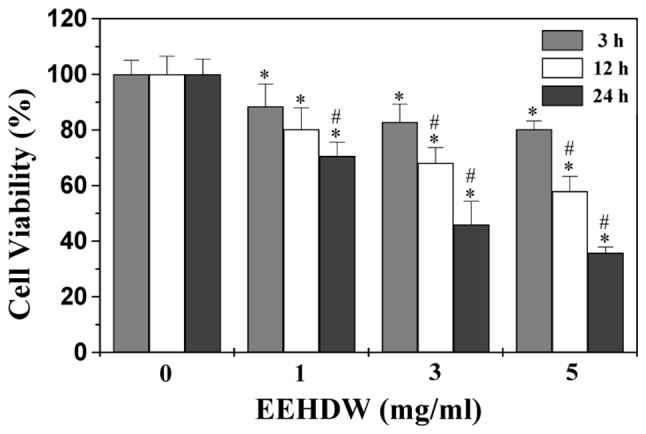
Effect of EEHDW on HT-29 cell viability. Cells were treated with various concentrations of EEHDW for the indicated time periods. Cell viability was determined by MTT assay. The data were normalized to the viability of control cells (100%, treated with 0.5% DMSO vehicle). Data are means with SD (standard deviation; error bars) from at least three independent experiments. *P<0.05, versus untreated control cells; #P<0.05, versus cells treated for 3 h with the same dose of EEHDW. EEHDW, ethanol extract of Hedyotis Diffusa Willd.
Figure 2.
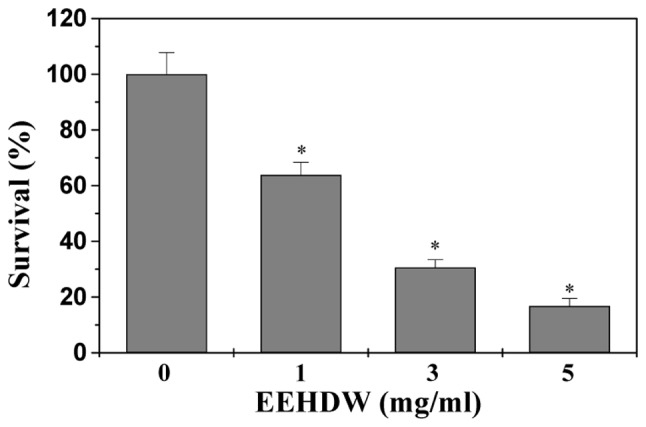
Effect of EEHDW on HT-29 cell survival. Cells were treated with indicated concentrations of EEHDW for 24 h. Cell survival was determined by colony formation analysis. The data were normalized to the survival of control cells. Data are averages with SD (standard deviation; error bars) from at least three independent experiments. *P<0.05, versus control cells. EEHDW, ethanol extract of Hedyotis diffusa Willd.
EEHDW blocks G1/S progression of HT-29 cells
The G1/S transition is one of the two main checkpoints that regulate cell cycle progression and thus the cell proliferation. We, therefore, investigated the effect of EEHDW on the G1 to S progression in HT-29 cells via PI staining followed by FACS analysis. As shown in Fig. 3A and B, the percentage of S-phase cells following treatment with 0, 1, 3 and 5 mg/ml of EEHDW was 39.13, 34.14, 28.42 and 26.91%, respectively (P<0.05), indicating that EEHDW inhibits HT-29 cell proliferation by blocking the cell cycle at the G1 to S progression.
Figure 3.
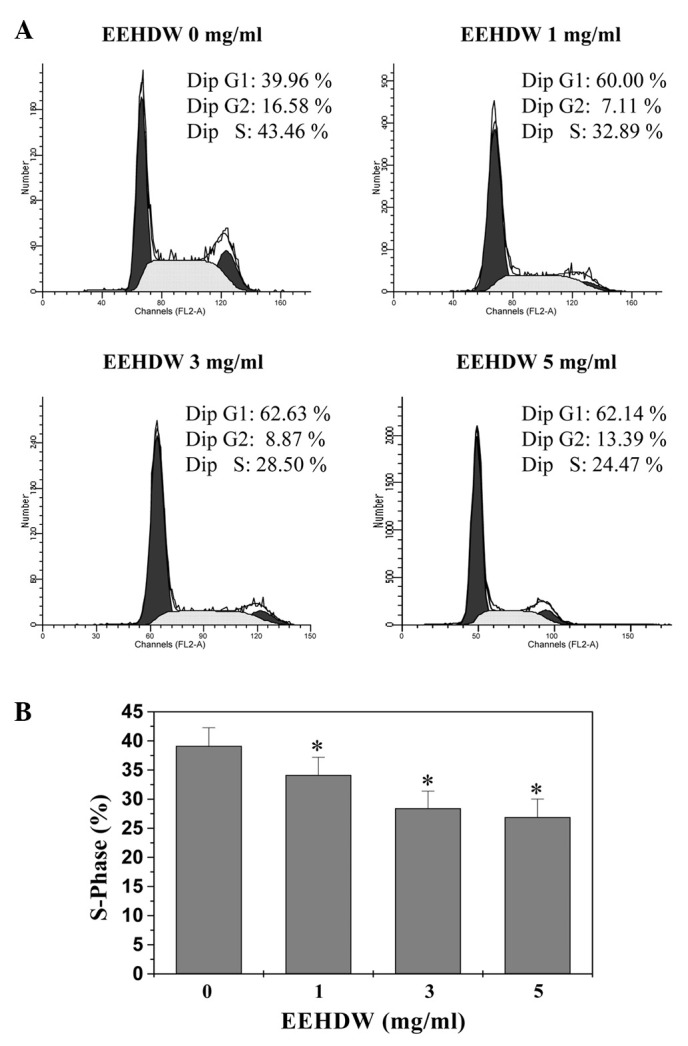
Effect of EEHDW on the cell cycle progression of HT-29 cells. (A) Cells were treated with indicated concentrations of EEHDW for 24 h, stained with PI and analyzed by FACS. Images are representative of three independent experiments. (B) The proportion of DNA in S phase was calculated using ModfitLT Version 3.0 Software. Data shown are averages with SD (standard deviation; error bars) from three independent experiments. *P<0.05, versus control cells. FACS, fluorescence-activated cell sorting; EEHDW, ethanol extract of Hedyotis diffusa Willd; PI, propidium iodide.
EEHDW regulates mRNA expression of PCNA, Cyclin D1, CDK4 and p21
We examined the effect of EEHDW on the mRNA expression of the pro-proliferative PCNA, Cyclin D1, CDK4, and the anti-proliferative p21 using RT-PCR. As shown in Fig. 4, EEHDW treatment markedly reduced the mRNA expression of PCNA, Cyclin D1 and CDK4, but increased that of p21.
Figure 4.
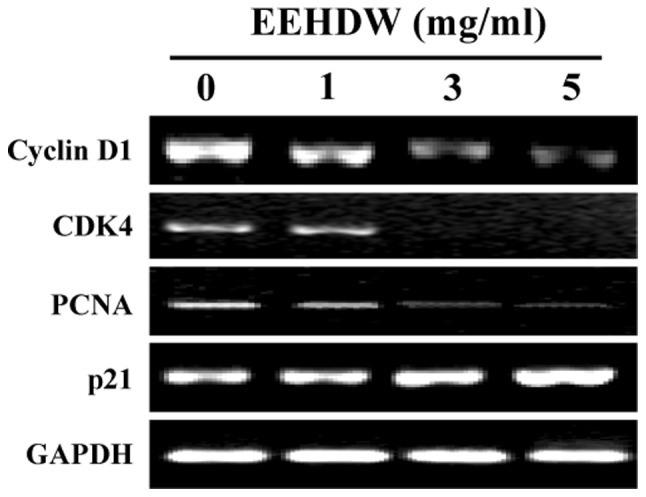
Effect of EEHDW on the mRNA expression of Cyclin D1, CDK4, PCNA and p21 in HT-29 cells. Cells were treated with the indicated concentrations of EEHDW for 24 h. The mRNA levels of Cyclin D1, CDK4, PCNA and p21 were determined by RT-PCR. GAPDH was used as an internal control. Data are representative of three independent experiments. EEHDW, ethanol extract of Hedyotis diffusa Willd; CDK4, Cyclin-dependent kinase 4.
Discussion
Hedyotis diffusa Willd (HDW) is a Chinese medicinal herb with numerous reported pharmacological applications. HDW is clinically effective in the treatment of various types of cancer, including CRC (18–20). Recently, we reported that the direct cytotoxic effect of HDW on colon cancer cells is partially due to the induction of mitochondrion-dependent apoptosis (21). However, the effect of HDW on cancer cell proliferation is still unclear.
Using MTT and colony formation assays, in the present study, we demonstrated that the ethanol extract of Hedyotis diffusa Willd (EEHDW) inhibited the proliferation of human colon carcinoma HT-29 cells. Cell proliferation is regulated by the cell cycle, which consists of four periods; S phase (DNA synthesis), M phase (mitosis), G1 and G2 phase. The G1/S transition is one of the two main checkpoints of the cell cycle (2), and is responsible for the initiation and completion of DNA replication. By using FACS analysis and PI staining we observed that the inhibitory effect of EEHDW on HT-29 cell proliferation was associated with the prevention of G1 to S transition. The G1/S progression is strongly regulated by Cyclin D1 which forms an active complex with its CDK major catalytic partners (CDK4/6) (3,4). An unchecked or hyperactivated Cyclin D1/CDK4 complex may be responsible for enhanced cellular proliferation. PCNA, a 36-kDa DNA polymerase delta auxiliary protein that is involved in cell proliferation and is specifically expressed in proliferating cell nuclei, has been recognized as a histological marker for the G1/S phase in the cell cycle (5). As a proliferation inhibitor, p21 protein plays a role in G1 arrest by binding to and inhibiting the activity of Cyclin-CDK complexes and PCNA (6). Therefore, the expression of PCNA, CDK4, Cyclin D1 and p21 reflects the proliferation state of HT-29 cells to some extent. As predicted, following EEHDW treatment the mRNA expression of Cyclin D1, CDK4 and PCNA in HT-29 cells was downregulated but that of p21 was upregulated.
In conclusion, we report for the first time that EEHDW inhibits the proliferation of HT-29 cells via cell cycle arrest. Together with our previous study, these results suggest that Hedyotis diffusa Willd inhibits cancer progression via multiple mechanisms, including the induction of cancer cell apoptosis, inhibition of cell proliferation and tumor angiogenesis.
Acknowledgments
This work was sponsored by the Natural Science Foundation of Fujian Province of China (2010J01195), the Research Foundation of the Education Bureau of Fujian Province of China (JA10162) and the Developmental Fund of Chen Keji Integrative Medicine (CKJ 2010030).
Abbreviations:
- EEHDW
ethanol extract of Hedyotis diffusa Willd
- CRC
colorectal cancer
- DMSO
dimethyl sulfoxide
- MTT
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
References
- 1.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 2.Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994;79:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Robles AI, Martinez LA, Liu F, Gimenez-Conti IB, Conti CJ. Expression of G1 cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in androgen-induced prostate proliferation in castrated rats. Cell Growth Differ. 1996;7:1571–1578. [PubMed] [Google Scholar]
- 4.Graña X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 5.Zhong W, Peng J, He H, Wu D, Han Z, Bi X, Dai Q. Ki-67 and PCNA expression in prostate cancer and benign prostatic hyperplasia. Clin Invest Med. 2008;31:E8–E15. doi: 10.25011/cim.v31i1.3136. [DOI] [PubMed] [Google Scholar]
- 6.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 7.Gustin DM, Brenner DE. Chemoprevention of colon cancer: current status and future prospects. Cancer Metast Rev. 2002;21:323–348. doi: 10.1023/a:1021271229476. [DOI] [PubMed] [Google Scholar]
- 8.Gorlick R, Bertino JR. Drug resistance in colon cancer. Semin Oncol. 1999;26:606–611. [PubMed] [Google Scholar]
- 9.Longley DB, Allen WL, Johnston PG. Drug resistance, predictive markers and pharmacogenomics in colorectal cancer. Biochim Biophys Acta. 2006;1766:184–196. doi: 10.1016/j.bbcan.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Boose G, Stopper H. Genotoxicity of several clinically used topoisomerase II inhibitors. Toxicol Lett. 2000;116:7–16. doi: 10.1016/s0378-4274(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 12.Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199–334. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi AK, Singh RP, Agarwal C, Chan DC, Agarwal C. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G2-M arrest, and apoptosis. Clin Cancer Res. 2002;8:3512–3519. [PubMed] [Google Scholar]
- 14.Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 15.Jeune MA, Kumi-Diaka J, Brown J. Anticancer activities of pomegranate extracts and genistein in human breast cancer cells. J Med Food. 2005;8:469–475. doi: 10.1089/jmf.2005.8.469. [DOI] [PubMed] [Google Scholar]
- 16.Sausville EA. Versipelostatin: unfolding an unsweetened death. J Natl Cancer Inst. 2004;96:1266–1267. doi: 10.1093/jnci/djh276. [DOI] [PubMed] [Google Scholar]
- 17.Won HJ, Han CH, Kim YH, Kwon HJ, Kim BW, Choi JS, Kim KH. Induction of apoptosis in human acute leukemia Jurkat T cells by Albizzia julibrissin extract is mediated via mitochondria-dependent caspase-3 activation. J Ethnopharmacol. 2006;106:383–389. doi: 10.1016/j.jep.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Song LR. Zhonghuabencao. Vol. 61. Shanghai Science and Technology Press; Shanghai: 1999. p. 433. [Google Scholar]
- 19.Yang JJ, Hsu HY, Ho YH, Lin CC. Comparative study on the immunocompetent activity of three different kinds of Peh-Hue-Juwa-Chi-Cao, Hedyotis diffusa, H. corymbosa and Mollugo pentaphylla after sublethal whole body X-irradiation. Phytother Res. 1997;11:428–432. [Google Scholar]
- 20.Li R, Zhao HR, Lin YM. Anti-tumor effect and protective effect on chemotherapeutic damage of water soluble extracts from Hedyotis diffusa. J Chin Pharm Sci. 2002;11:54–58. [Google Scholar]
- 21.Lin JM, Chen YQ, Wei LH, Chen XZ, Xu W, Hong ZF, Sferra TJ, Peng J. Hedyotis Diffusa Willd extract induces apoptosis via activation of the mitochondrion-dependent pathway in human colon carcinoma cells. Int J Oncol. 2010;37:1331–1338. doi: 10.3892/ijo_00000785. [DOI] [PubMed] [Google Scholar]
- 22.Lin JM, Wei LH, Xu W, Hong ZF, Liu XX, Peng J. Effect of Hedyotis Diffusa Willd extract on tumor angiogenesis. Mol Med Rep. 2011;4:1283–1288. doi: 10.3892/mmr.2011.577. [DOI] [PubMed] [Google Scholar]


