Abstract
A suppressor mutation, D53, of the held-up2 allele of the Drosophila melanogaster Troponin I (wupA) gene is described. D53, a missense mutation, S185F, of the tropomyosin-2, Tm2, gene fully suppresses all the phenotypic effects of held-up2, including the destructive hypercontraction of the indirect flight muscles (IFMs), a lack of jumping, the progressive myopathy of the walking muscles, and reductions in larval crawling and feeding behavior. The suppressor restores normal function of the IFMs, but flight ability decreases with age and correlates with an unusual, progressive structural collapse of the myofibrillar lattice starting at the center. The S185F substitution in Tm2 is close to a troponin T binding site on tropomyosin. Models to explain suppression by D53, derived from current knowledge of the vertebrate troponin-tropomyosin complex structure and functions, are discussed. The effects of S185F are compared with those of two mutations in residues 175 and 180 of human α-tropomyosin 1 which cause familial hypertrophic cardiomyopathy (HCM).
INTRODUCTION
Muscle contraction is usually activated by a neurally stimulated intracellular release of Ca2+. In vertebrate striated muscle, released Ca2+ binds to the thin filament troponin-tropomyosin (Tn-Tm) complex, which consists of tropomyosin (Tm) and the troponins T, I and C (TnT, TnI, and TnC). Current models (see Farah and Reinach, 1995; Geeves and Lehrer, 1998; Squire and Morris, 1998) agree that Ca2+ binding to TnC changes the conformation of the Tn complex, releasing the inhibitory binding of TnI to F-actin and allowing Tm to move across the actin surface (Vibert et al., 1997). This movement cooperatively increases myosin accessibility to F-actin, activating the cross-bridge cycle.
Muscle protein interactions are generally well characterized, but the pathway from Ca2+ binding to contraction cannot yet be described in molecular detail. The amino acid sequences of vertebrate thin filament proteins (actin, Tm and Tn) are known and their general location in the Tn complex has been determined by low resolution structural studies (White et al., 1987; Al Khayat et al., 1995; Cabral-Lilly et al., 1997) and biochemical analyses. The atomic structure of part of the complex was recently determined (Vassylyev et al., 1998) and functional information derives from considerable biochemical research (see Farah and Reinach, 1995; Geeves and Lehrer, 1998).
Thin filament protein mutants with phenotypic effects are important in identifying residues with significant in vivo roles. Such mutants are known in humans through familial hypertrophic cardiomyopathies (HCM) (Redwood et al., 1999), in the nematode Caenorhabditis elegans (Kagawa et al., 1997; McArdle et al., 1998), and Drosophila melanogaster, where mutations affecting the indirect flight muscles (IFM) have been recovered (see Bernstein et al., 1993). The D. melanogaster IFMs are striated muscles and contain a Ca2+-regulated thin filament system but activation also requires applied strain (Peckham et al., 1990), as also found in vertebrate cardiac muscle.
We aim to study the function of the Drosophila Tm-Tn complex by isolating mutations that suppress the phenotypes of selected TnI mutants. held-up2 (hdp2) a missense mutation of the TnI gene, produces a fully penetrant raised wing phenotype. Six dominant suppressors of hdp2, including D53, were recovered (Prado et al., 1995). Here we show that D53 is a missense mutation, S185F, of the Tm2 tropomyosin gene and characterize the functional and structural effects of the suppression. We discuss these results with respect to known interactions within the vertebrate Tn-Tm complex. We observed that S185F occurs within a region of the Drosophila Tm which is homologous to that containing two human cardiac α-tropomyosin mutations which cause HCM and compare the effects of D53 with these mutations.
MATERIALS AND METHODS
Stocks and Fly Strains
Flies were maintained at 25°C on a yeast-sugar-agar medium. Unless otherwise stated, strains were obtained from the European Stock Center (Umeå) or the MidAmerica Drosophila Stock Center (Bowling Green). All chromosome and gene symbols unless specifically described are as in Flybase on http://flybase.bio.indiana.edu/. For the two Tm genes, the symbols Tm1 (was TmII - Bautch et al., 1982) and Tm2 (was TmI) are used as proposed in Karlik et al. (1984). The hdp and up alleles are in genes which encode respectively TnI, wupA (wings-up A) (Beall and Fyrberg, 1991; Barbas et al., 1991) and TnT, up (upheld) (Fyrberg et al., 1990). The wupA gene is the sole TnI-encoding gene in Drosophila (Barbas et al., 1991). The hdp2 mutation is in constitutive exon 5 (Beall and Fyrberg, 1991; Prado et al., 1995) where it causes a substitution of alanine116 by valine, A116V (using the numbering system of Prado et al., 1995 for Drosophila TnI residues which includes the IFM-specific exon 3 which introduces a 61-residue N-terminal extension).
Microscopy Procedures
For polarised light photomicroscopy thoraces were prepared and mounted as described in Nongthomba and Ramachandra (1999). Fly half-thoraces were prepared for transmission electron microscopy (TEM) as described in Kronert et al. (1995).
PCR and Sequencing
A 3334 bp fragment including the complete Act88F coding sequence was PCR amplified with Taq DNA polymerase from genomic DNA of hdp2 and hdp2; D53 flies using the 5′TGTAGGTGGAGCTAACCGTGTGC (sense) and 5′GCTGCCTTTGAAGAGCTTTCGG (antisense) primers. The amplified product was gel-purified (Geneclean, BIO101), ligated into the pGEM-T vector system (Promega), and transformed into TG1 reco cells. DNA from three separate clones was extracted, purified, sequenced with internal oligonucleotide primers using an ABI Prism Dye Cycle Sequencing Kit containing AmpliTaq DNA polymerase (Perkin Elmer-Cetus), and analyzed using an in-house automated sequencer.
RT-PCR was used to amplify the Tm1 gene exons that are spliced to form the ‘mTm’ (muscle tropomyosin) mRNA. This mRNA is derived by splicing together exons 1–3, 5, 7, 8, 10, 11, 13 and 17 (Karlik and Fyrberg, 1986; Hanke and Storti, 1988). Total RNA was isolated from newly emerged hdp2 and hdp2; D53 flies and reverse transcribed using random nucleotide hexamers and Moloney murine leukemia virus reverse transcriptase (Life Technologies). The cDNA was used in PCR reactions with Pfu DNA polymerase (Stratagene) using 5′GTTCAAGTCGCGGATAACTCCGAATAAAAGTT (sense) and 5′CAGTGCGCCTACGATTATGC (antisense) primers to amplify specifically the coding region. The PCR products were gel purified, ligated into pGEM-T, and transformed into TG1 reco cells. Three clones from two separate RT-PCR reactions were sequenced as above.
DNA extracted from Oregon-R, hdp2, and D53 flies was used for PCR amplifications with Pfu DNA polymerase (either native or cloned) of overlapping Tm2 genomic fragments containing exons 2, 3 and the coding sequence of exon 4. The primers used were 5′AGGATTCAGTTATTCAGCATAC, 5′TCCTCAATCTGTTGCACCTT, 5′TTGGTATCGGCATCCTCAGC, 5′GGAGGAGGAGCTGAAGGTGG, 5′GGGAGTTGCCGACAACCTAGT, 5′GGGTGGTCAAGGGCATTGTGTG, and 5′CAAACTGAACGAGGAGGTGC. Amplified products from a minimum of two PCR reactions for each fragment were purified and sequenced as described above.
Adult and Larval Muscle Performance Tests
Flight testing was done as previously described (Drummond et al., 1991). Individual flies were scored for flight upwards (U), horizontal (H), downwards (D), or not at all (N). The mean flight score, [U + H]/Total flies, was arcsine transformed to give a Normal distribution. Five samples, > 50 flies, were scored. Wing beat records were obtained and analyzed as described in Schmitz et al. (2000) using 6 flies per genotype, each sampled 3 times.
Adult walking ability was measured in a vertical 100-ml glass measuring cylinder, internal diameter 30 mm, with a line marked 80 mm above the base. For each test 8–30 flies were introduced into the cylinder. Flies were knocked to the cylinder bottom and the time taken for 50% of the flies to walk past the marked line was scored.
Late third instar larval crawling was measured on 1.5% (wt/vol) agar-sucrose medium in plates marked with a 0.5-cm grid, as total gridlines crossed in 5 min. Larval feeding movements were quantified by counting nonlocomotory, pharyngeal movements in a 2-min period on agar plates thinly coated with yeast. Larvae which did not feed continuously were discarded.
RESULTS
Suppressor D53 Is a Tropomyosin 2 Mutation
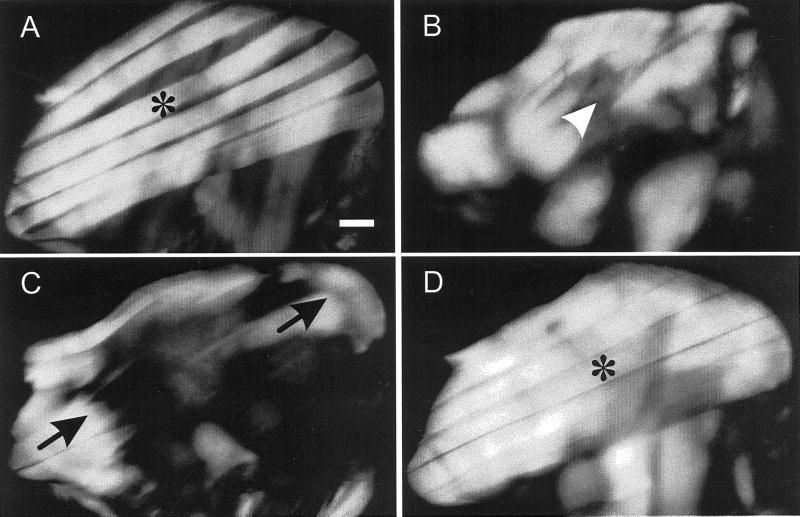
In hdp2 flies the IFMs hypercontract (Figure 1C) so that myofibrillar material remains (arrows) only near the muscle attachment sites. This effect is completely suppressed in hdp2; D53/+ (Figure 1D) and hdp2; D53/53 flies (not shown), which are indistinguishable from wild-type (Figure 1A). Even in pupae at 78 h APF (after puparium formation), ∼ 20 h before adult emergence, the hdp2 muscles (Figure 1B) begin to hypercontract. IFMs cannot be imaged by birefringence before 78 h, but using a lacZ gene promoter-reporter construct and β-galactosidase staining we have shown that developing hdp2 IFMs have a normal structure at 75 h APF (Nongthomba and Sparrow, in preparation). D53 suppression mapped to a region on chromosome 3 (Prado et al., 1995) that contains the IFM-specific actin gene, Act88F, and two tropomyosin genes, Tm1 and Tm2. Due to the very tight linkage of these genes D53 was located by sequencing the relevant coding regions of all three genes. The Act88F sequence was identical to the GenBank sequence (M18830) except for seven silent codon changes.
Figure 1.
Polarized light micrographs of dissected thoraces to show the progressive hypercontraction of hdp2 IFMs and suppression of this phenotype by D53. Each thorax is shown with the anterior-posterior axis running right to left. (A) Wild-type fly dorso-longitudinal IFMs (asterisk). (B) hdp2 pupa at 78 h APF to show the initiation of hypercontraction (arrowhead). (C) hdp2 fly just after emergence showing IFM remnants (arrowheads) after hypercontraction and (D) a hdp2; D53/+ fly showing complete suppression of hdp2 hypercontraction. Bar = 0.120 mm.
The Tm1 gene produces four alternatively spliced products (Hanke and Storti, 1988). Only one, the mTm mRNA, has an expression pattern coincident with the muscles in which hdp2 phenotypes are suppressed by D53 (see below). The Tm1 coding sequences from hdp2 and hdp2; D53 flies, obtained by RT-PCR, and the wild-type mTm isoform sequence (Swissprot ID:TPM1_DROME) generated by splicing appropriate exons from EMBL sequences L00355-L00363 (Hanke and Storti, 1986, 1988) were identical.
Larval muscles express a Tm2 mRNA, containing exons 1–4; IFM and leg muscles express a Tm2 mRNA consisting of exons 1–3 and 5 (Basi et al., 1986; Karlik and Fyrberg, 1986). As D53 suppresses hdp2 effects in IFMs, legs, and larval muscles (see below), the common coding exons (2 and 3) were sequenced by PCR of genomic DNA from hdp2 and hdp2; D53 flies. In hdp2 flies Tm2 exons 2 and 3 were identical to the wild-type sequence (EMBL AC: K03277; Swissprot TPM2_DROME; Basi and Storti, 1986). However, exon 2 in hdp2; D53 flies contained a TCC to TTC mutation in codon 185, which will replace serine by phenylalanine (S185F); exon 3 was wild-type.
Suppression of IFM Defects
Flies hemi- or homozygous for hdp2 hold their wings vertically, but when D53 was present in one (+/D53) or two (D53/D53) gene doses, their wings were in the resting position. Young (2–3 days) hdp2; +/D53 or hdp2; D53/D53 flies flew, but less well than wild-type (Table 1A). By 6–7 days old the flight ability of hdp2; D53/+ flies, but not hdp2; D53/D53, had been dramatically reduced. These data show that D53 suppression of the hdp2 flightless phenotype is dominant and almost complete. The age effect suggests that incomplete suppression in hdp2; D53/+ heterozygotes causes a progressive deterioration in muscle function. Does this correlate with structural changes to the myofibrils?
Table 1.
Flight abilities of adult flies of hdp2 and D53 genotypes aged 2 to 3 (A) and 6 to 7 (B) days post-eclosion
| Chromosome III | X-chromosome
|
||||
|---|---|---|---|---|---|
| +/+ | +/hdp2 | hdp2/hdp2 | |||
| A) 2–3 d | |||||
| +/+ | 81.4 ± 5.0 | * | 64.9 ± 7.0 | * | 0.8 ± 0.3 |
| * | * | ||||
| +/D53 | 66.9 ± 3.5 | 75.6 ± 11.2 | 62.3 ± 16.4 | ||
| (*) | |||||
| D53/D53 | 68.1 ± 4.9 | 63.8 ± 10.1 | * | 55.2 ± 6.8 | |
| Chromosome III | X-chromosome
|
||||
|---|---|---|---|---|---|
| +/+ | +/hdp2 | hdp2/hdp2 | |||
| B) 6–7 d | |||||
| +/+ | 81.9 ± 6.9 | 74.5 ± 7.1 | * | 0.4 ± 0.03 | |
| * | |||||
| +/D53 | 70.8 ± 6.5 | 60.2 ± 6.2 | * | 0.4 ± 0.07 | |
| (*) | * | ||||
| D53/D53 | 62.8 ± 6.9 | 59.3 ± 7.2 | 42.7 ± 11.3 | ||
Figures are in degrees (± 1 SD) due to the arcsine transformation. Ninety degrees would indicate that all flies flew upwards. * between genotypes indicates a difference in flight ability at the 5% level in pairwise Student's t-tests; (*) indicates the same level of significance in difference between the D53/D53 row and that of +/+. The +/+ and hdp2/+ columns contain female data; the hdp2/hdp2 data are from homozygous females and hemizygous males.
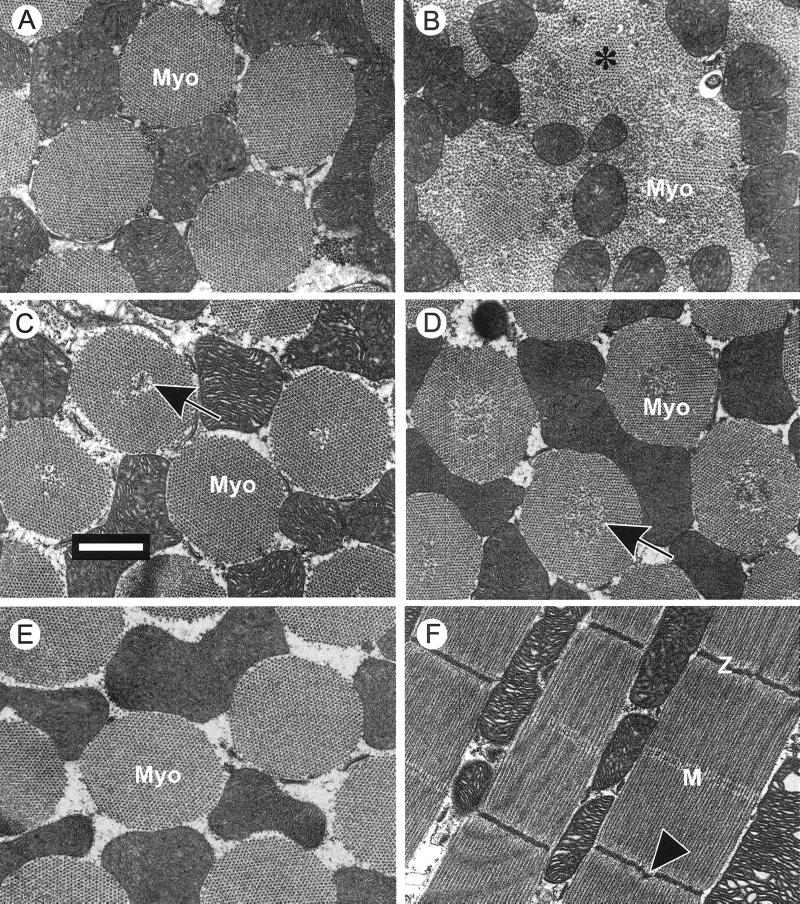
Electron micrographs of IFMs from 2–3 d-old hdp2 flies showed (Figure 2B) an almost complete absence of wild-type myofibrillar structure (Figure 2A). The fibers contained disorganized thick and thin filaments with only small islands of myofibrillar lattice (asterisk) remaining. IFMs of 2–3 d-old hdp2; D53/+ flies (Figure 2C) showed an almost complete restoration of wild-type myofibrillar structure. However, some myofibrils showed small areas of disorganized filament lattice at their centers which in longitudinal sections (Figure 2F) were visible as Z-disk dislocations. By 6–7 days, all the myofibrils of hdp2; D53/+ flies (Figure 2D) contain extensive disorganized centers, an effect never seen in hdp2; D53 (Figure 2E) or D53 homozygotes (not shown). These structural changes correlate with the reduced flight ability of hdp2; D53/+ flies with increasing age (Table 1). Since neither occur in D53 homozygotes, complete D53 suppression is a recessive character.
Figure 2.
Transmission electron micrographs of hdp2; D53/+ IFMs to show age effects. (A-C) Transverse sections of IFM myofibrils (Myo) from 2- to 3-day-old flies: (A) Wild-type. (B) hdp2 showing the almost complete disruption of the myofibrils as a result of hypercontraction and (C) hdp2; D53/+ showing suppression but with some myofibrillar lattice disorder at the centers. (D, E) Transverse sections of myofibrils from 6- to 7-day-old flies: (D) hdp2; D53/+ flies demonstrating the increased central disruption of all myofibrils and (E) hdp2; D53/D53 myofibrils showing the complete suppression of hdp2 myofibrillar defects. (F) Longitudinal section of 6- to 7-day-old hdp2; D53/+ myofibrils, with disruptions at some of the Z-disk centers. Bar = 1 μm.
Suppression in Adult, Non-IFM Muscles
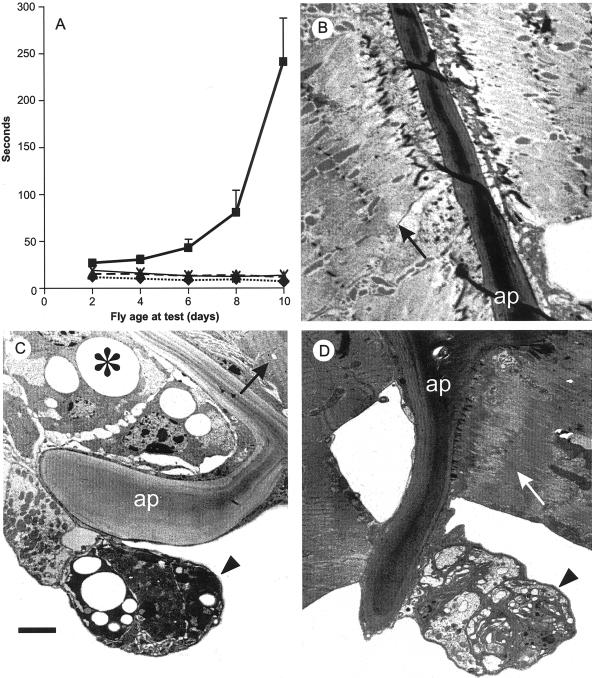
Since the hdp2 mutation occurs in a constitutive exon of the sole Drosophila TnI gene (Beall and Fyrberg, 1991), its dysfunction in non-IFM muscles should affect behavior. hdp2 flies cannot jump (Deak et al., 1982) but hdp2; D53/+ and hdp2; D53/D53 flies jumped readily. Since jumping is powered by the tergal depressor of trochanter (TDT) muscles, D53 must be expressed there. The walking speed of hdp2 flies was reduced even in young flies (Figure 3A) compared with wild-type, as reported previously (Deak, 1977) and was further reduced as flies aged (Figure 3A). Older flies appeared crippled, continually fell over, or showed a propensity to fall off vertical surfaces. One or two gene doses of D53 restored walking ability of hdp2 flies to almost wild-type levels, indicating that D53 is expressed and suppresses the hdp2 phenotype in the muscle groups required for walking. Neither hdp2; D53/D53 nor hdp2; D53/+ flies showed a reduced walking ability with age (Figure 3A). Leg muscles from 12-d wild-type flies (Figure 3B) had a normal fibrillar structure, but in hdp2 flies of similar age (Figure 3C), although a few muscles showed a normal muscle structure (arrow) most muscles (asterisk and arrowhead) lacked myofilaments and exhibited large intracellular vacuoles (asterisk) and electron dense staining of cells and nuclei (arrowhead). One muscle (arrowhead) has detached from one of its apodemes. These effects correlate with the behavioral studies. Aged hdp2; D53/+ flies showed (Figure 3D) an intermediate phenotype in which some muscles were clearly damaged (arrowhead) but most appeared normal (arrow). Although the behavioral studies suggested a complete dominant suppression of hdp2 by D53, these structural data indicate that dominant suppression is incomplete.
Figure 3.
The effects of age on wild-type and hdp2 leg muscles. (A) Walking ability of hdp2 (▪), Oregon-R (♦), hdp2; D53/+ (×) and hdp2; D53/D53 (▴). ‘Seconds’ is mean time (10 tests/sample) for 50% of flies to walk upwards > 80 mm. Error bars ± 1 SD. (B-D) Electron micrographs of the proximal apodeme (ap) of femur from 12-day-old (B) wild-type, (C) hdp2, and (D) hdp2; D53/+ males. In hdp2 flies a few muscles were structurally normal (compare arrows in B and C), but some were detached (arrowhead) and with most of the others showed degenerative changes with intracellular vacuoles (*) and electron dense staining of cells and nuclei (arrowhead); in hdp2; D53/+ flies (D) although some muscles were collapsed (arrowhead), others were attached and had normal structure (compare arrows B and D). Bar = 2 μm.
Suppression of Larval Muscle Defects
As wupA must be expressed in larval muscles (see above), hdp2 should affect larval behaviors. Crawling of hdp2 larvae was very different from wild-type (Table 2). Many appeared paralyzed in their posterior segments; frequently the posterior was raised, reminiscent of the ‘hook’ phenotype of kinesin or kinesin-related mutants (Hurd and Saxton, 1996). As hdp2 larvae crawl, they ‘roll’. Locomotory and feeding behaviors of hdp2; D53 larvae were not significantly different from those of wild-type confirming a) that the Tm2 gene is expressed in larval muscles and, b) that D53 suppresses the effects of hdp2 (Table 2) in these muscles.
Table 2.
Larval behavior
| Behavior | Genotype
|
|||
|---|---|---|---|---|
| hdp2 | hdp2; D53 | Wild-type | ||
| Crawling (grid squares/5 min) | 7.7 ± 2.1 | 12.1 ± 2.6** | 13.3 ± 5.7** | |
| n = | 19 | 19 | 19 | |
| Feeding (movements/min) | 100.5 ± 11.1 | 155.4 ± 23.2** | 148.4 ± 27.2** | |
| n = | 113 | 116 | 119 | |
The behavior of wild type (Oregon-R) and hdp2; D53 larvae were not significantly different.
indicates significant difference from hdp2 (P < 0.01).
Muscle Effects due to D53 Mutation Per Se
Often genetic suppressors have their own mutant phenotype. IFMs from D53 homozygotes have a wild-type structure (Figure 2E). To look for more subtle effects the flight ability of D53/+ and D53/D53 flies was measured (Table 1). Both genotypes showed a small, significant reduction in flight ability that was age-independent. Oregon-R flies gave a wing beat frequency of 240.6 ± 23.4 Hz (n = 6 flies) while D53 homozygotes produced 214.7 ± 18.6 Hz, a modest but significant reduction (Student's t test p = 0.041). The walking speed of D53 and wild-type flies was very similar and did not change with age (data not shown). Thus, D53 has a mild IFM phenotype, which is not detected in leg muscles.
Specificity of the D53 Suppression
Suppression could result from altered stoichiometry or from specific amino acid alterations affecting protein interactions. Gene dosage studies should reproduce the former type of suppression, whereas the latter should be allele-specific. Df(3R)ea5022rxl lacks both the Tm1 and Tm2 genes and Tm2C10 lacks a functional Tm2 copy (Kreuz et al., 1996). Males that had hdp2/Y; Df(3R)ea5022rxl/+ or hdp2/Y; Tm2C10/+ genotypes showed partial suppression of IFM hypercontraction but did not fly; hdp2/Y; Df(3R)ea5022rxl/+ flies had the wings-up phenotype (Table 3). We define partial IFM suppression as thoraces in which muscle fibers often span the complete thorax, but where some do not show continuous birefringence (cf. Figure 4B) from one attachment site to the other. The hdp2/Y; Df(3R)ea5022rxl/+ and Tm2C10/+ results suggest that the reduction in Tm1 gene copy number did not contribute to suppression. This was confirmed by a lack of hdp2 dominant suppression (data not shown) by recessive lethal P-element insertion Tm1 alleles (gift of Dr. C. O'Kane). Other recessive lethal Tm2 mutations partially suppressed IFM hypercontraction, but all the flies were flightless (Table 3).
Table 3.
Effects of Tm2 null alleles on the hdp2 phenotype
| Genotype | n | Wing position
(%)
|
Muscle fiber phenotype (%)
|
||||
|---|---|---|---|---|---|---|---|
| U | D | N | H | P | N | ||
| hdp2/Y; +/+ | 25 | 100 | 0 | 0 | 100 | 0 | 0 |
| hdp2/Y; ea5022rxl/+ | 26 | 100 | 0 | 0 | 50 | 50 | 0 |
| hdp2/Y; DL2/+ | 12 | 42 | 58 | 0 | 58 | 25 | 17 |
| hdp2/Y; C10/+ | 36 | 11 | 89 | 0 | 50 | 50 | 0 |
| hdp2/Y; J8/+ | 26 | 100 | 0 | 0 | 58 | 35 | 7 |
| hdp2/Y; L2/+ | 22 | 100 | 0 | 0 | 100 | 0 | 0 |
n = number of flies examined. Wing position: ‘N’ is wild type (normal), ‘U’ is wings raised (up) position typical of hdp2, and ‘D’ is wings held alongside (down) the abdomen. Muscle fiber phenotypes were assessed by polarized light microscopy of bisected thoraces. H, hypercontracted' (cf. Figure 1C); P, partially suppressed (see text and Figure 4B); N, wild type/fully suppressed (Figure 1A/1D). Df(3R) ea5022rxl is a deficiency that completely deletes easter, Tm1 (Erdelyi et al., 1995), and Tm2 (Kreuz et al., 1996); DL2 is a γ-ray induced dominant flightless, recessive lethal mutation of Tm2 associated with a translocation breakpoint within 88F (Sparrow, unpublished) and C10, J8, and L2 are recessive lethal, null Tm2 mutations (Kreuz et al., 1996). All females of genotypes, hdp2/+; Tm2x/+ (not shown; x = different alleles) hold their wings in the normal position, are flightless, and show a normal IFM pattern.
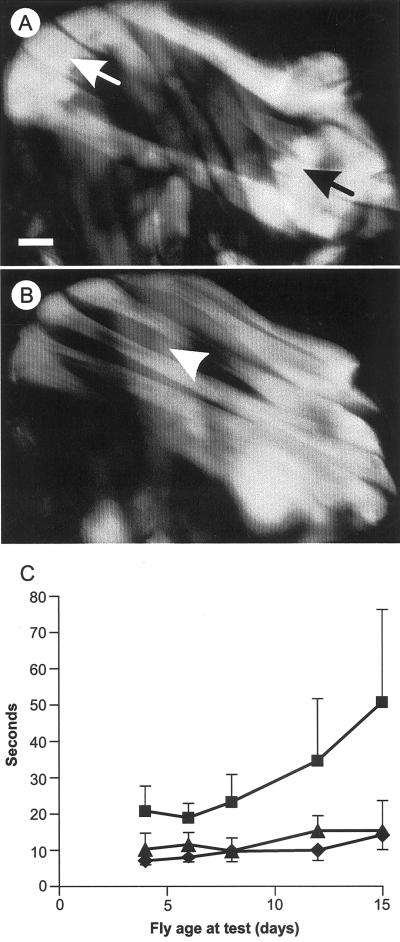
Figure 4.
Suppression of up101 by D53. (A-C) Polarized light microscopy of IFMs. Each thorax is shown with anterior-posterior axis running left to right. Bar = 0.125 mm. (A) In up101/Y birefringence is seen only at the muscle ends. The dorso-longitudinal (DLM) flight muscles are most readily seen, but the opposing dorso-ventral (DVM) muscles are visible. (B) The more complete DLMs typical of partial suppression in up101/Y; D53/+ males. (C) Age effects on walking ability of up101 (▪), up101; D53/+ (▴), and wild-type (♦) flies.
Flies hemi- or homozygous for the hdp3 and hdp5 mutations of wupA also show the wings-up phenotype but in these cases the IFMs fail to develop. These IFM-specific mutations affect alternative transcript splicing of IFM mRNA and produce no TnI protein in the IFMs (Barbas et al., 1993). D53 did not suppress the muscle phenotype of either mutation suggesting that suppression requires the presence of functional TnI protein. The myosin heavy chain “rod” domain mutation Mhc13, which causes a recessive hypercontraction of IFMs (Kronert et al., 1995), is also not suppressed by D53. Thus D53 is not a general suppressor of IFM hypercontraction. Its lack of effect on hdp3 and hdp5 and its more effective suppression of hdp2 than the Tm2 null mutations suggest that it is an allele-specific suppressor.
up101 is a missense mutation of the troponin T gene (Fyrberg et al., 1990). It produces a recessive wings-up phenotype (∼ 90% penetrant) and IFM hypercontraction (100% penetrant) indistinguishable from that of hdp2 (compare Figures 4A and 1C). Homozygous D53 completely suppressed both phenotypes but up101/Y; D53/+ flies showed a range of partially (Figure 4B) or completely suppressed IFM phenotypes. The wing position phenotype was also partially suppressed by D53/+; 39% of the flies had wings in the normal position compared with 10% of control up101/Y flies. No up101; D53/+ or up101; D53/D53 flies could fly.
Adult up101 flies walked more slowly than wild-type (Student's t, P ≪0.001), a difference that increased with age (Figure 4C). This effect was smaller than in hdp2 flies and became extreme only in much older flies (cf. Figure 3A). D53 suppression of up101 was apparently complete in walking muscles because up101/Y; D53/+ flies of all ages walked at wild-type velocities (Figure 4C).
Thus, the suppressing effects of D53 are clearly not gene-specific, but they are weaker in the IFMs with up101 compared with hdp2 (compare Figures 1D and 4B); in walking behavior D53 acts equally well in suppressing both mutations.
DISCUSSION
The D53 suppressor is a missense mutation, S185F, of the Tm2 gene. It suppresses all the effects of hdp2 on muscle function, consistent with D53 being a mutation in constitutively expressed exon 2, and Tm2 expression occurring in the IFMs, TDT, leg muscles and supercontractile larval muscles (Basi et al., 1984; Basi and Storti, 1986; Karlik and Fyrberg, 1986).
The dominant suppression of hdp2 flightlessness in D53/+ heterozygotes, but not D53 homozygotes, deteriorates with age. Clearly a mixture of mutant and wild-type Tm2 proteins suppresses in young flies but incompletely so as progressive degeneration occurs, unusually, from the myofibril centers outwards. A similar disruption pattern occurs in the Drosophila Act88FG245D mutant allele (Sakai et al., 1990) and the intragenic D3 suppressor of hdp2 (Prado et al., 1995). These observations suggest that the myofibrillar center and periphery either have different protein constitutions or experience different physiological conditions. Myofibrillogenesis begins at the center (Reedy and Beall, 1993) and different isoforms could be assembled in the myofibril center compared with the periphery but there is no evidence for this. Calcium diffusion into and out from the myofibril upon activation and relaxation will produce temporal differences of Ca2+ concentration across the myofibril. If hdp2 and D53 affect the response to Ca2+ a trans-myofibrillar calcium gradient could generate damaging forces.
Tm2 null mutant heterozygotes produce myofibrils with a normal central lattice but considerable peripheral disruption (Kreuz et al., 1996). Why do they partially suppress hdp2? Fiber destruction in the hdp2 hypercontraction likely involves actomyosin generated forces. Kreuz et al. (1996) showed that Tm2C10/+ fibers produced significantly less work than wild-type. This would explain the partial suppression by extreme Tm2 alleles and why flight is not restored. A similar argument may explain the effects of the myosin heavy chain gene suppressors of hdp2 (Kronert et al., 1995) though other explanations including direct interactions between the myosin head and the Tn-Tm complex cannot be excluded.
Structural Implications
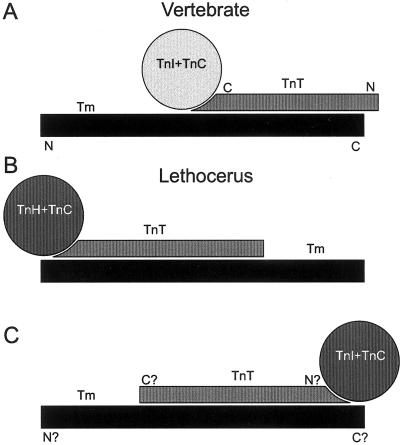
The vertebrate inhibitory, actin-binding TnI and the calcium-binding TnC form a globular domain (Herzberg and James, 1985) which sits on TnT, an elongated molecule (see Figure 6A). TnT is the major structural link between TnI + TnC and Tm binding (see Farah and Reinach, 1995) and extends along the carboxy-terminal third of the Tm coiled-coil to overlap with the N-terminus of the adjacent Tm dimer. Two regions of Tm bind TnT one of which, the Ca2+-sensitive binding region, includes residues 170–190.
Figure 6.
._Cartoon of tropomyosin-troponin complex arrangements redrawn from Wendt et al., (1997) to illustrate that an inversion of their diagram for Lethocerus would lead to a similar orientation between the Tm and TnT in vertebrate and invertebrate troponin-tropomyosin- complexes. (A) vertebrate, (B) Lethocerus redrawn from Wendt et al. (1997 and (C) proposed reorientation. The N- and C- termini labeling of Tm and TnT in (C) is based solely on the implied structural homology to the vertebrate structure.
Residue 185, phenylalanine in D53, is indicated in the alignment (Figure 5) of Tm sequences. The Drosophila Tm2 IFM isoform (TPM0_DROME) has considerable sequence homology with other Tm sequences in this region. TPM0_DROME and TPM1_DROME (the standard muscle Tm encoded by the Tm1 gene) show many residue differences. Vertebrate Tm sequences are more similar to each other than to invertebrate sequences and residue type conservation occurs at many positions (Figure 5). In part, this reflects the α-helical coiled-coil heptad repeat requirement for hydrophobic residues at most ‘a’ and ‘d’ positions. Residue 185 is predicted to be a ‘c’ residue, implying that the phenylalanine side chain of D53 will point away from the dimer axis and not affect coiled-coil stability. More probably it affects interactions between Tm and TnT or F-actin.
Figure 5.
CLUSTALX multiple sequence alignment of muscle tropomyosin sequences corresponding to the TnT-2 binding site to show (a) position of S185F substitution in the protein sequence of the Drosophila Tm2 product expressed in the IFMs (TPM0_DROME); (b) consensus sequence (4 = KR, 6 = LIVM); (c) U = residues unique to the Tm2 product; (d) Heptad repeat ‘abcdefg ’ prediction generated with MacStripe (Dr. A. E. Knight, http://motility.york.ac.uk:85/) using the algorithm of Lupas et al. (1991). SwissProt Sequences: TPM0_DROME, Drosophila Tm2 gene product (P09491); TPM1 DROME, Drosophila Tm1 gene product (P06754); TPM1_HUMAN (P09493) and TPM3_HUMAN (P06753), human skeletal muscle α-chains; TPMA_BRARE (P13104), TPMA_COTJA (P18442), and TPMA_RABIT (P46902), zebrafish, quail, and rabbit skeletal/cardiac muscle α-chains; TPMA_RANTE (P13105), TPMA_RAT (P04692), TPMA_XENLA (Q01173), European common frog, rat, and South African claw toed frog skeletal muscle α-chains; TPMB_CHICK (P19352), TPMB_HUMAN (P07951), and TPMB_RABIT (P02560), chicken, human, and rabbit skeletal muscle β-chains; TPMC PIG (P42639), pig cardiac muscle α-chain; TPMM LOCMI (P31816) and TPMM_TRICO (P12324), locust and nematode muscle tropomyosins. Dots indicate residues identical to those in the TPM0 DROME sequence.
Our interpretations assume that current models for the vertebrate complex are relevant. This seems likely as Drosophila thin filament proteins show substantial sequence homology to their vertebrate counterparts although TnI (Barbas et al., 1991; Beall and Fyrberg, 1991), TnT (Fyrberg et al., 1990), and two IFM-specific Tm isoforms (Karlik et al., 1984; Hanke and Storti, 1988) have N- or C-terminal polypeptide additions. Drosophila and Lethocerus (waterbug) IFMs have a very similar physiology (Peckham et al., 1990) but in Lethocerus IFMs TnI is not detectable and is replaced by a unique 80 kDa molecule called TnH (Wendt et al., 1997). EM studies of Lethocerus Tn-Tm complexes (Wendt et al., 1997) suggest that the globular portion of the Tn complex lies not along the Tm as in vertebrates (Figure 6A) but at the Tm dimer overlap region (Figure 6B). While sequence homology strongly implies structural homology of the Drosophila Tn-Tm complex to that of vertebrates, common physiology argues for a structural similarity to Lethocerus. However, the orientation of the Lethocerus Tn complex with respect to the Tm N- and C-termini could not be determined from electron micrographs (Wendt et al., 1997). When their figure (Figure 6B) is inverted (Figure 6C) it shows that the Tm, TnT and F-actin in both insects and vertebrates could maintain the same relative position though the remainder of the troponin complex is located differently.
Functional Implications
Current models (Geeves and Lehrer, 1998; Squire and Morris, 1998) suggest that in the absence of Ca2+, TnI binds actin maintaining the ‘blocked’ filament state (Geeves and Lehrer, 1998) with Tm occluding much of the myosin binding site on actin. Ca2+ binding to TnC changes TnC/TnI conformation, releasing the TnI inhibitory domain from actin, producing the ‘closed’ state, and Tm with its attached Tn complex, can now move across the F-actin surface. This exposes the complete myosin binding surface on actin (‘open’ state), activating the actomyosin cross-bridge cycle.
TnI sequence alignments (not shown) show that Drosophila TnI residue A116, (A116V in hdp2) coincides with conserved residue A25 of vertebrate skeletal muscle isoforms. A25 contributes to a contact with the ‘E’ α-helix of TnC (Vassylyev et al., 1998). The hdp2 A116V substitution increases residue size which must affect TnC-TnI binding by changing TnI α-helix and TnC ‘E’ α-helix interactions. These could result in hdp2 either lowering the threshold for calcium activation or affecting the ability of the Tn-Tm complex to return to the relaxed state. We cannot yet distinguish which explanation is correct for hdp2 but the latter one was previously proposed for a hypercontracting TnT mutant in Caenorhabditis elegans (McArdle et al., 1998) and would readily explain hypercontraction of hdp2 IFMs. The less extreme effects on nonflight muscles argue strongly that hdp2 can produce near-normal thin filament regulation.
The S185F substitution introduces a more bulky, hydrophobic residue. Its heptad ‘c’ position means that the phenylalanine ring will point outwards from the Tm axis. It could suppress hdp2 either by altering Tn-Tm complex movement across the F-actin surface or by changing the Tn complex orientation on Tm through the Ca2+ sensitive TnT-Tm binding site. The former suggestion is supported by observations (Bing et al., 1998) that the Act88F gene E93K mutation reduces Tm movement across the F-actin surface in vitro and suppresses hdp2 IFM hypercontraction in vivo (Nongthomba and Sparrow, unpublished). In the latter suggestion S185F would reverse the conformational effects of hdp2 on the TnI/TnC interaction by altering structural relationships between Tm and the Tn complex. Reversal of the TnI/TnC conformational effects of hdp2 could require very specific changes such as found with the intragenic D3 suppressor, a TnI mutation (L188F) which suppresses hdp2 (Prado et al., 1995) perhaps by changes in actin binding (L188 is close to an actin -binding domain). However, alterations in Tm mobility could affect any Tn mutant which causes slight regulatory changes. The partial suppression of up101 by D53 might thus seem to favor this latter explanation, except for the observation that up101, as a TnT mutation, is also located in the Ca2+-sensitive TnT-Tm interaction site.
Clinical Implications
Human hypertrophic cardiomyopathies (HCM) include two dominant mutations, D175N and E180G, in highly conserved residues (Figure 5) of the TPM1 gene (Thierfelder et al., 1994). As with D53, these mutations map within the tropomyosin region which interacts with TnT in a Ca2+-sensitive manner. In vitro motility experiments using reconstituted cardiac thin filaments showed that these HCM mutations affect the Ca2+ sensitivity of the fraction of filaments moving compared with wild-type, although in the absence of structural information on the Ca2+-sensitive Tm binding site for TnT, it is not clear how these mutations could cause this (Bing et al., 2000). There is evidence that HCM TnT mutations, I79N and R92Q, located within the same TnT-Tm interaction (residues 70–180) may affect Ca2+ sensitivity in tension versus force measurements (see Tobacman et al., 1999 for discussion). Further biochemical studies of all these human and Drosophila Tm and TnT mutants are required to ascertain the role of this Ca2+-sensitive contact between Tm and TnT in thin filament regulation.
Typically HCM symptoms do not manifest before adolescence, but penetrance and expressivity of HCM mutations are highly variable (Coonar and McKenna, 1997). This suggests that HCM mutations have relatively mild effects, but the functional deficits can induce cardiac changes, such as hypertrophy, leading to premature death. The variable expression of HCM mutations must be partly due to genetic background. Modifier genes may reduce or enhance HCM severity by affecting either the contractile machinery or the response to cardiac dysfunction. D53 is similar to HCM mutations in that reduced IFM function is detectable only in older organisms. The D53 suppression of the hdp2 and up101 progressive myopathies suggests that Drosophila muscles may provide a useful genetic model with which to study HCM genes and their genetic modifiers.
ACKNOWLEDGMENTS
We acknowledge contributions from Daniel Johnson, Steph Lawlor, Sarah Withers, Nick Brown, Claire Bryan, Philip Ash, Paul Rowan, Ky Hutchinson, Chris Robinson, and Colin Civil in undergraduate projects. We thank the BBSRC, the EU (TMR Network grant ERB-CHRXCT920249), the British Council/Acciones Integradas Program from the Autonomous Community of Madrid (CAM 08.5/0043/1998), and the Spanish Ministry of Culture (PM96–06) for their support.
REFERENCES
- Al Khayat HA, Yagi N, Squire JM. Structural changes in actin-tropomyosin during muscle regulation: computer modelling of low-angle X-ray diffraction data. J Mol Biol. 1995;252:611–632. doi: 10.1006/jmbi.1995.0524. [DOI] [PubMed] [Google Scholar]
- Barbas JA, Galceran J, Krah-Jentgens I, de la Pompa JL, Canal I, Pongs O, Ferrus A. Troponin I is encoded in the haplolethal region of the Shaker gene complex of Drosophila. Genes Dev. 1991;5:132–140. doi: 10.1101/gad.5.1.132. [DOI] [PubMed] [Google Scholar]
- Barbas JA, Galceran J, Torroja L, Prado A, Ferrus A. Abnormal muscle development in the heldup3 mutant of Drosophila melanogaster is caused by a splicing defect affecting selected troponin I isoforms. Mol Cell Biol. 1993;13:1433–1439. doi: 10.1128/mcb.13.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi GS, Boardman M, Storti RV. Alternative splicing of a Drosophila tropomyosin gene generates muscle tropomyosin isoforms with different carboxy-terminal ends. Mol Cell Biol. 1984;4:2828–2836. doi: 10.1128/mcb.4.12.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi GS, Storti RV. Structure and DNA sequence of the tropomyosin I gene from Drosophila melanogaster. J Biol Chem. 1986;261:817–827. [PubMed] [Google Scholar]
- Bautch VL, Storti RV, Mischke D, Pardue ML. Organization and expression of Drosophila tropomyosin genes. J Mol Biol. 1982;162:231–250. doi: 10.1016/0022-2836(82)90524-1. [DOI] [PubMed] [Google Scholar]
- Beall CJ, Fyrberg EA. Muscle abnormalities in Drosophila melanogaster heldup mutants are caused by missing or aberrant troponin-I isoforms. J Cell Biol. 1991;114:941–951. doi: 10.1083/jcb.114.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein SI, O'Donnell PT, Cripps RM. Molecular genetic analysis of muscle development, structure and function in Drosophila. Int Rev Cytol. 1993;143:63–152. doi: 10.1016/s0074-7696(08)61874-4. [DOI] [PubMed] [Google Scholar]
- Bing W, Razzaq A, Sparrow J, Marston S. Tropomyosin and troponin regulation of wild type and E93K mutant actin filaments from Drosophila flight muscle: charge reversal on actin changes actin-tropomyosin from ON to OF.F. state. J Biol Chem. 1998;273:15016–15021. doi: 10.1074/jbc.273.24.15016. [DOI] [PubMed] [Google Scholar]
- Bing W, Knott A, Redwood C, Esposito G, Pircell I, Watkins H, Marston S. Effect of hypertrophic cardiomyopathy mutations in human cardiac muscle α-tropomyosin (Asp175Asn and Glu180Gly) on the regulatory properties of human cardiac troponin determined by in vitro motility assay. J Mol Cell Cardiol. 2000;32:1489–1498. doi: 10.1006/jmcc.2000.1182. [DOI] [PubMed] [Google Scholar]
- Cabral-Lilly D, Tobacman LS, Mehegan JP, Cohen C. Molecular polarity in tropomyosin-troponin T co-crystals. Biophys J. 1997;73:1763–70. doi: 10.1016/S0006-3495(97)78206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonar AS, McKenna WJ. Molecular genetics of familial cardiomyopathies. Adv Genet. 1997;35:285–324. doi: 10.1016/s0065-2660(08)60453-8. [DOI] [PubMed] [Google Scholar]
- Deak II. Mutations in Drosophila melanogaster that affect flight muscles. J Embryol Exp Morphol. 1977;40:35–63. [PubMed] [Google Scholar]
- Deak II, Bellamy PR, Bienz M, Dubuis Y, Fenner E, Gollin M, Rahmi A, Ramp T, Reinhardt CA, Cotton B. Mutations affecting the indirect flight muscles of Drosophila melanogaster. J Embryol Exp Morphol. 1982;69:61–81. [PubMed] [Google Scholar]
- Drummond DR, Hennessey ES, Sparrow JC. Characterization of missense mutations in the Act88F gene of Drosophila melanogaster. Mol Gen Genet. 1991;226:70–80. doi: 10.1007/BF00273589. [DOI] [PubMed] [Google Scholar]
- Erdelyi M, Michon AM, Guichet A, Glotzer JB, Ephrussi A. Requirement for Drosophila cytoplasmic tropomyosin in oskar mRNA localization. Nature. 1995;377:524–527. doi: 10.1038/377524a0. [DOI] [PubMed] [Google Scholar]
- Farah CS, Miyamoto CA, Ramos CHI, Da Silva ACR, Quaggio RB, Fujimori K, Smillie LB, Reinach FC. Structural and regulatory functions of the NH2- and COOH-terminal regions of skeletal muscle troponin I. J Biol Chem. 1994;269:5230–5240. [PubMed] [Google Scholar]
- Fyrberg EA, Fyrberg CC, Beall C, Saville DL. Drosophila melanogaster troponin-T mutations engender three distinct syndromes of myofibrillar abnormalities. J Mol Biol. 1990;216:657–675. doi: 10.1016/0022-2836(90)90390-8. [DOI] [PubMed] [Google Scholar]
- Geeves MA, Lehrer S. The muscle thin filament as a classical cooperative/allosteric regulatory system. J Mol Biol. 1998;277:1081–1089. doi: 10.1006/jmbi.1998.1654. [DOI] [PubMed] [Google Scholar]
- Hanke PD, Storti RV. Nucleotide sequence of a cDNA clone encoding a Drosophila muscle tropomyosin II isoform. Gene. 1986;45:211–214. doi: 10.1016/0378-1119(86)90256-8. [DOI] [PubMed] [Google Scholar]
- Hanke PD, Storti RV. The Drosophila melanogaster tropomyosin II gene produces multiple proteins by use of alternative tissue-specific promoters and alternative splicing. Mol Cell Biol. 1988;8:3591–3602. doi: 10.1128/mcb.8.9.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg O, James MNG. Structure of the calcium regulatory muscle protein troponin-C at 2.8 Å resolution. Nature. 1985;313:653–659. doi: 10.1038/313653a0. [DOI] [PubMed] [Google Scholar]
- Hurd DD, Saxton WM. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H, Takuwa K, Sakube Y. Mutations and expressions of the tropomyosin gene and the troponin-C gene of Caenorhabditis elegans. Cell Struct Funct. 1997;22:213–218. doi: 10.1247/csf.22.213. [DOI] [PubMed] [Google Scholar]
- Karlik CC, Fyrberg EA. Two Drosophila melanogaster tropomyosin genes: structural and functional aspects. Mol Cell Biol. 1986;6:1965–1973. doi: 10.1128/mcb.6.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlik CC, Mahaffey JW, Coutu MD, Fyrberg EA. Organization of contractile protein genes within the 88F subdivision of the Drosophila melanogaster third chromosome. Cell. 1984;37:469–481. doi: 10.1016/0092-8674(84)90377-5. [DOI] [PubMed] [Google Scholar]
- Kreuz AJ, Simcox A, Maughan D. Alterations in flight muscle ultrastructure and function in Drosophila tropomyosin mutants. J Cell Biol. 1996;135:673–687. doi: 10.1083/jcb.135.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronert WA, O'Donnell PT, Fieck A, Lawn A, Vigoreaux JO, Sparrow JC, Bernstein SI. Defects in the Drosophila myosin rod permit sarcomere assembly but cause flight muscle degeneration. J Mol Biol. 1995;249:111–125. doi: 10.1006/jmbi.1995.0283. [DOI] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- McArdle K, Allen TStC, Bucher EA. Ca2+-dependent muscle dysfunction caused by mutation of the Caenorhabditis elegans troponin T1 gene. J Cell Biol. 1998;143:1201–1213. doi: 10.1083/jcb.143.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongthomba U, Ramachandra NB. A direct screen identifies new flight muscle mutants on the Drosophila second chromosome. Genetics. 1999;153:261–274. doi: 10.1093/genetics/153.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham M, Molloy JE, Sparrow JC, White DCS. Physiological properties of the dorsal longitudinal flight muscle and the tergal depressor of the trochanter muscle of Drosophila melanogaster. J Muscle Res Cell Motil. 1990;11:203–215. doi: 10.1007/BF01843574. [DOI] [PubMed] [Google Scholar]
- Prado A, Canal I, Barbas JA, Molloy J, Ferrus A. Functional recovery of troponin-I in a Drosophila heldup mutant after a second site mutation. Mol Biol Cell. 1995;6:1433–1441. doi: 10.1091/mbc.6.11.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood CS, Moolman-Snook JC, Watkins H. Properties of mutant contractile proteins that cause hypertrophic cardiomyopathy. Cardiovasc Res. 1999;44:20–36. doi: 10.1016/s0008-6363(99)00213-8. [DOI] [PubMed] [Google Scholar]
- Reedy MC, Beall C. Ultrastructure of developing flight muscle in Drosophila. Dev Biol. 1993;160:443–465. doi: 10.1006/dbio.1993.1320. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Okamoto H, Mogami K, Yamada T, Hotta Y. Actin with tumor-related mutation is antimorphic in Drosophila muscle: two distinct modes of myofibrillar disruption by antimorphic actins. J Biochem (Tokyo) 1990;107:499–505. doi: 10.1093/oxfordjournals.jbchem.a123074. [DOI] [PubMed] [Google Scholar]
- Schmitz S, Clayton J, Nongthomba U, Veigel C, Geeves M, Sparrow J. Drosophila ACT88F indirect flight muscle-specific actin is not N-terminally acetylated: a mutation in N-terminal processing affects actin function. J Mol Biol. 2000;295:201–210. doi: 10.1006/jmbi.1999.3407. [DOI] [PubMed] [Google Scholar]
- Squire JM, Morris EP. A new look at thin filament regulation in vertebrate skeletal muscle. FASEB J. 1998;12:761–771. doi: 10.1096/fasebj.12.10.761. [DOI] [PubMed] [Google Scholar]
- Tobacman LS, Lin D, Butters C, Landis C, Back N, Pavlov D, Homsher E. Functional consequences of troponin T mutations found in hypertrophic cardiomyopathy. J Biol Chem. 1999;274:28363–28370. doi: 10.1074/jbc.274.40.28363. [DOI] [PubMed] [Google Scholar]
- Thierfelder L, Watkins H, MacCrae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy; a disease of the sarcomeres. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Vassylyev DG, Takeda S, Wakatsuki S, Maeda K, Maeda Y. Crystal structure of troponin C in complex with troponin I fragment at 2.3-Å resolution. Proc Natl Acad Sci USA. 1998;95:4847–4852. doi: 10.1073/pnas.95.9.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert PJ, Craig R, Lehman W. Steric model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- Wendt T, Guénebaut V, Leonard KR. Structure of the Lethocerus troponin-tropomyosin complex as determined by electron microscopy. J Struct Biol. 1997;118:1–8. doi: 10.1006/jsbi.1996.3834. [DOI] [PubMed] [Google Scholar]
- White SP, Cohen C, Phillips GN. Structure of co-crystals of tropomyosin and troponin. Nature. 1987;325:826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]