Abstract
The urinary trypsin inhibitor (UTI) is responsible for most of the antitryptic activity in urine and is excreted in increased amounts in urine under certain pathological conditions such as cancer and bacterial infections. Our aim in this study was to better understand the mechanisms responsible for the increase in UTI excretion on surgical stress and thus to better appreciate the information provided by inflammatory mediators. Thirty-one consecutive patients who underwent radical esophagectomy for esophageal cancer were investigated in this study. We determined serum UTI and polymorphonuclear cell elastase (PMNE), urine UTI and evaluated the effectiveness of preoperative administration of methylprednisolone on the postoperative clinical course and adverse inflammatory reactions. The results revealed that urine UTI and serum PMNE levels in the steroid group were significantly lower than those in the non-steroid group. In addition, UTI levels correlated positively with serum levels of aminotransferases. More importantly, the maximum level of urine UTI in patients without complications was lower than that in patients with complications. These results suggest that urine UTI provides useful information concerning postoperative clinical course, and that preoperative administration of methylprednisolone may contribute to decrease postoperative complications following esophagectomy.
Keywords: urinary trypsin inhibitor, steroid, esophageal cancer
Introduction
Major surgery is associated with a transient severe inflammatory response involving the release of proinflammatory mediators and leads to systemic inflammation (1). Esophagectomy for esophageal carcinoma is one of the most invasive surgical procedures and is associated with a generalized systemic inflammatory response characterized by the activation of proinflammatory cytokines and other chemical mediators (2). After inflammatory stimulus, the pattern of plasma proteins synthesized by the liver changes significantly. In general, the concentration of positive acute phase proteins (APPs), including C-reactive protein (CRP), increases. These proteins are widely used as indicators in rapid diagnosis and the assessment of the response to therapy in inflammatory diseases. In addition, it has been reported that polymorphonuclear cell elastase (PMNE) is released from granulocytes during surgery and that the postoperative serum level of PMNE is a good indicator of surgical stress. More importantly, the monitoring of PMNE was found to be more useful than that of serum CRP for estimation of the inflammatory status and early detection of an acute-phase response (3–5).
The urinary trypsin inhibitor (UTI) is one of the Kunitz-type trypsin inhibitors found in human urine and is synthesized by the inter-α-trypsin inhibitor (ITI) family (6). As previously reviewed by Fries and Blom (7), UTI is responsible for most of the antitryptic activity in urine and is excreted in increased amounts in urine under certain pathological conditions such as cancer and bacterial infections (8–10). Thus, UTI is an important anti-inflammatory substance, and is also considered to be a positive APP (11,12). UTI has previously been suggested as a possible screening test for bacterial and/or viral infections (13). More importantly, the urine concentration of UTI could be useful for predicting the risk of complications and outcome of bone marrow transplantation (14).
We previously reported that preoperative administration of methylprednisolone successfully suppresses the release of certain indicators of response to surgical injury such as IL-6 and IL-8, and plays a significant role in the prevention of systemic inflammatory responses (15). Postoperative complications, so-called stress-induced organ dysfunction states, are thought to be caused by an uncontrolled inflammatory response due to the overproduction of proinflammatory cytokines and chemokines (3,16). However, the mechanisms involved in reduced postoperative complications upon preoperative administration of methylprednisolone have not been clearly elucidated (17–19). Focusing on UTI and PMNE, our aim in the present study was to better understand the relationship between steroid and inflammatory mediators and thus to better appreciate the information provided by preoperative administration of methylprednisolone. We determined serum UTI and PMNE and urine UTI levels using an enzyme-linked immunosorbent assay (ELISA). We also evaluated the preoperative administration of methylprednisolone on the postoperative clinical course and adverse inflammatory reactions.
Patients and methods
Patients and study design
Thirty-one consecutive patients who underwent radical esophagectomy for esophageal cancer at Nippon Medical School Main Hospital between 2002 and 2004 were investigated in this study. The patients did not receive previous intravenous treatment with UTI. Patients who had a preoperative inflammatory reaction within 7 days prior to surgery, preoperative complications such as liver cirrhosis or diabetes mellitus, or tuberculosis lesions were excluded from the study. There were no significant differences in preoperative characteristics between the patients in the steroid and the non-steroid groups (Table I). Therefore, even though the study was not performed in a randomized manner, it is reasonable to state that the data shown here suggest objective results. In the steroid group, 10 mg/kg body weight of methylprednisolone was administered intravenously to each patient just before the start of esophagectomy. Informed consent according to the Declaration of Helsinki was obtained from all patients.
Table I.
Comparison of the clinical features between the steroid and the non-steroid group.
| Features | Steroid group (n=19) | Non-steroid group (n=12) | P-value |
|---|---|---|---|
| Male/female | 17/2 | 8/4 | 0.12 |
| Age (year), mean ± SD | 60.9±10.2 | 63.7±9.2 | 0.46 |
| Operative time (min) | 498.6±49.9 | 421.8±80.0 | 0.08 |
| Intraoperative blood loss (ml) | 844.3±364.7 | 891.9±369.0 | 0.56 |
| Tumor stage (I:II:III:IV) | 4:3:5:7 | 5:3:1:3 | 0.40 |
Enzyme immunoassay for PMNE and UTI
Serum and urine samples were obtained and stored at −80°C until being assayed. Serum PMNE was measured the day prior to surgery and 24 h, 3, 5, 7 and 14 days after the start of surgery by enzyme immunoassay (ELISA) using a commercial kit reagent (PMN Elastase; E. Merck, Germany). Plasma and urine UTI were measured using a one-step sandwich enzyme-linked immunosorbent assay (ELISA; Mochida Pharmaceutical Co., Tokyo, Japan). Briefly, purified anti-ulinastatin immunoglobulins (Igs) were labeled with horseradish peroxidase (Toyobo Enzymes, Tokyo, Japan). Each well in the ELISA plates was coated with 100 μl of anti-ulinastatin Igs (5 mg/ml) in carbonate buffer (pH 9.5) at 25°C for 12 h. Coated plates were washed and subsequently blocked with 0.5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) (pH 6.4) for 1 h at room temperature. Then 20 μl of the diluted samples or standards was added, after which 80 ml of peroxidase-conjugated anti-ulinastatin Igs in 10% (v/v) rabbit serum/PBS (pH 6.4) was added. The plate was incubated for 30 min at 37°C and washed three times with 400 μl of physiological saline containing 0.05% Tween-20 (Biosciences Inc., La Jolla, CA, USA). Then 100 μl of freshly prepared tetramethylbenzydine containing hydrogen peroxide (0.02%) was added and incubated for 10 min at room temperature. The reaction was terminated by the addition of 100 μl of 0.5 mol H2SO4. The absorbance was read at 450 nm using a microplate reader (ImmunoMini NJ-2300; Sic, Tokyo, Japan). All samples were assayed in duplicate.
Definition of postoperative complications
The postoperative course of each patient was monitored daily, and the complications were defined as follows. Pulmonary complications were defined by the presence of massive atelectasis, pulmonary edema, or pneumonia. Postoperative hyperbilirubinemia was defined as the peak total bilirubin level >4 mg/dl. Anastomotic leakage was diagnosed by gastrography and clinical features. Liver dysfunction was defined as either the aspartate aminotransferase (AST) or alanine aminotransferase (ALT) value >200 IU/l.
Statistical analysis
The Mann-Whitney’s U test, Chi-square test, and repeated measure ANOVA were used to determine significant differences between the two groups. P-values of <0.05 were considered significant.
Results
Effect of steroid therapy on the changes in acute phase parameters
Postoperative changes in the levels of serum UTI, serum PMNE and urine UTI were compared between both groups. Compared with the non-steroid group, the steroid group had significantly lower levels of urine UTI and serum PMNE (Fig. 1A and C). Regarding the level of serum UTI, no significant difference was noted between the groups (Fig. 1B).
Figure 1.



Changes over postoperative time in (A) urine UTI, (B) serum UTI and (C) PMNE levels in the steroid (n=19) (filled circles) and the non-steroid (n=12) (open circles) groups. Data are expressed as the mean ± SE. *P<0.05 vs. the preoperative values in the same group; †P<0.05 vs. the non-steroid group at the same point. POD, postoperative day.
Correlation between urine UTI and serum levels of aminotransferases
For the evaluation of hepatic injury, the peak levels of AST and ALT in the serum were correlated with that of urine UTI. The peak levels of UTI correlated positively with those of aminotransferases (Fig. 2).
Figure 2:


(A) Correlation between the peak levels of urine UTI and aspartate aminotransferase (AST) in all of the studied patients (n=31; r=0.64, P=0.001). (B) Correlation between the peak levels of urine UTI and alanine aminotransferase (ALT) in all of the studied patients (n=31) (r=0.48, P=0.02).
Correlation between urine UTI and postoperative complications
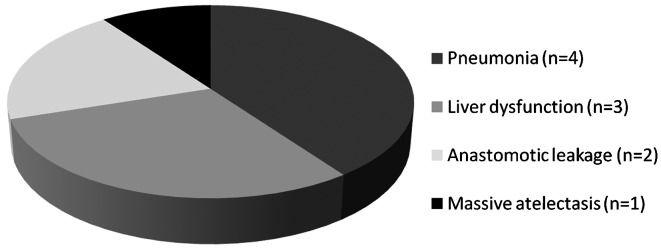
The postoperative complications are shown in Fig. 3. The maximum level of urine UTI in patients without complications was lower than that in patients with complications (Fig. 4).
Figure 3.
The distribution of postoperative complications.
Figure 4.

Peak levels of urine UTI in patients with complications (+) (n=10) and without complications (-) (n=21). The peak level of urine UTI in patients without complication was lower than that in patients with complication.
*P<0.05 in the Student’s t-test.
Discussion
This study was designed to understand the relationship between steroid and inflammatory mediators and to evaluate the effects of preoperative administration of methylprednisolone. Patients with esophageal cancer scheduled for surgery were selected for the following reasons. While a balance of proinflammatory and anti-inflammatory mediators is essential for the appropriate immune response of patients, the postoperative overproduction of proinflammatory mediators may lead to tissue damage via the production of free oxygen species and may also result in systemic inflammatory response syndrome (SIRS) with subsequent multiorgan failure (20). Surgical treatment of esophageal cancer is one of the most stressful surgical procedures, and the frequency of postoperative organ failure remains high (21–23). Esophageal cancer surgery was therefore selected as one of the most appropriate procedures for evaluating the effects of steroids on surgical stress.
UTI is one of the protease inhibitors, and is produced in the liver and kidneys (24,25). It is also one of the acute phase reactants (11,13). The amount of this inhibitor excreted in the urine is considered proportional to the invasiveness of an insult to the host, typically major surgery (14). In the present study, the concentration of serum UTI was significantly lower than that of urine UTI, and no significant difference was noted between the steroid and non-steroid group (Fig. 1). These results are in accord with those from other studies which found that the concentration of serum UTI of patients undergoing partial hepatectomy was only slightly increased and even appeared to be unmodified during the acute phase response (12). Indeed, owing to its small amount, UTI circulating in the blood secondarily diffuses into extracellular spaces and reaches tissues. On the other hand, most of it is quickly excreted in the urine (26). Therefore, the amount of UTI present in urine appears to be a better indicator of UTI released in circulating plasma.
Methylprednisolone has been known to suppress the release of mediators (autocrine and paracrine), such as IL-6, IL-8 and PMNE, following surgical injury, and was found to play a significant role in the prevention of developing the subsequent spread of inflammation. PMNE metabolizes inter-α-inhibitor (IαI) to UTI, which then suppresses the activity of PMNE, thus forming a homeostatic negative feedback loop (27). It has been suggested that when the balance is upset and PMNE activity exceeds that of UTI, inflammation develops (28). As mentioned by Faarvang and Lauritsen (29), increased excretion of urine UTI has been recognized in response to inflammatory conditions. This was confirmed by our results which demonstrated that levels of urine UTI and serum PMNE in the steroid group were significantly decreased compared to those in the non-steroid group (Fig. 1).
During the acute-phase response, a large number of mediators which can cause postoperative complications are generated, and all of these may be markers of inflammation. It has been reported that there is a strong correlation between the concentrations of CRP in whole blood and urine UTI (10,30). More importantly, UTI is a better predictor of the subsequent spread of inflammation compared to CRP (31). In all of our patients, urine UTI levels correlated positively with serum levels of aminotransferases (Fig. 2). This result supports the hypothesis that UTI reflects liver injury as well.
It is of great importance that the possible clinical effects of preoperative steroid administration include the prevention of postoperative complications. Yamashita et al (32) and Shirabe (33) previously reported that the administration of steroids in liver surgery results in decreased values of immunosuppressive acid protein and postoperative positive rate of serum Candida antigen, which is a marker of bacterial translocation. Similar findings were reported for other surgical operations. Shimada et al (34) reported that morbidity rates including hyperbilirubinemia, anastomotic leakage, and liver dysfunction in patients with esophagectomy were significantly lower in a steroid group than in a non-steroid group. In addition, the median hospital stay was reported to be shorter in a steroid group, and adverse effects of steroid use, such as abnormality in glucose tolerance and delay in wound healing, did not occur following liver surgery (35). In the present study, the level of urine UTI in the steroid group was decreased compared to that in the non-steroid group (Fig. 1), and the maximum level of urine UTI in patients without complications was significantly lower than that in patients with complications (Fig. 4). According to these results, the UTI concentration predicts complications following esophagectomy, and the suppression of UTI levels induced by steroid administration may be one of the reasons for the reduction in postoperative complications.
A study with a much larger population is required before any firm conclusions can be drawn. Based on findings of the present study, it was suggested that urine UTI provides useful information on postoperative clinical course, and that preoperative administration of methylprednisolone may contribute to decrease postoperative complications following esophagectomy.
Acknowledgments
We thank the hospital committees for endorsing this study.
References
- 1.Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond) 1990;79:161–165. doi: 10.1042/cs0790161. [DOI] [PubMed] [Google Scholar]
- 2.Ono S, Aosasa S, Mochizuki H. Effects of a protease inhibitor on reduction of surgical stress in esophagectomy. Am J Surg. 1999;177:78–82. doi: 10.1016/s0002-9610(98)00300-6. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto K, Arakawa H, Mita S, et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine. 1994;6:181–186. doi: 10.1016/1043-4666(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 4.Kato M, Suzuki H, Murakami M, Akama M, Matsukawa S, Hashimoto Y. Elevated plasma levels of interleukin-6, interleukin-8, and granulocyte colony-stimulating factor during and after major abdominal surgery. J Clin Anesth. 1997;9:293–298. doi: 10.1016/s0952-8180(97)00006-8. [DOI] [PubMed] [Google Scholar]
- 5.Sato N, Murakami K, Ishida K, Ikeda K, Saito K. Pulmonary hypertension and polymorphonuclear leukocyte elastase after esophageal cancer operations. Ann Thorac Surg. 1991;51:754–758. doi: 10.1016/0003-4975(91)90118-a. [DOI] [PubMed] [Google Scholar]
- 6.Salier JP, Rouet P, Raguenez G, Daveau M. The inter-alpha-inhibitor family: from structure to regulation. Biochem J. 1996;315:1–9. doi: 10.1042/bj3150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fries E, Blom AM. Bikunin - not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32:125–137. doi: 10.1016/s1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 8.Mizon C, Balduyck M, Bonneterre JP, Mizon J. Urinary trypsin inhibitory capacity determination: study in patients with disseminated cancers. Bull Cancer. 1983;70:266–270. [PubMed] [Google Scholar]
- 9.Chawla RK, Rausch DJ, Miller FW, Vogler WR, Lawson DH. Abnormal profile of serum proteinase inhibitors in cancer patients. Cancer Res. 1984;44:2718–2723. [PubMed] [Google Scholar]
- 10.Gosset D, Mizon C, Savinel P, et al. Clinical value of the determination of urinary antitrypsin activity. Presse Med. 1988;17:329–332. (In French) [PubMed] [Google Scholar]
- 11.Kuwajima S, Matsui T, Kitahashi S, et al. Automated measurement of trypsin inhibitor in urine with a centrifugal analyzer: comparison with other acute phase reactants. Clin Biochem. 1990;23:167–171. doi: 10.1016/0009-9120(90)80031-d. [DOI] [PubMed] [Google Scholar]
- 12.Noie T, Sugawara Y, Harihara Y, et al. Kinetics of urinary trypsin inhibitor in patients undergoing partial hepatectomy. Scand J Gastroenterol. 2001;36:410–416. doi: 10.1080/003655201300051270. [DOI] [PubMed] [Google Scholar]
- 13.Piette AM, Saba J, Bernard N, et al. Urinary trypsin inhibitory activity for the diagnosis of bacterial infection: a prospective study in 690 patients. Eur J Med. 1992;1:273–276. [PubMed] [Google Scholar]
- 14.Yamada S, Takatsuka H, Takemoto Y, et al. Urinary trypsin inhibitor concentration can predict the immunological insult of chemotherapy and complications after bone marrow transplantation. Bone Marrow Transplant. 2001;27:195–199. doi: 10.1038/sj.bmt.1702755. [DOI] [PubMed] [Google Scholar]
- 15.Takeda S, Takeda S, Kim C, et al. Preoperative administration of methylprednisolone attenuates cytokine-induced respiratory failure after esophageal resection. J Nippon Med Sch. 2003;70:16–20. doi: 10.1272/jnms.70.16. [DOI] [PubMed] [Google Scholar]
- 16.Sato N, Koeda K, Ikeda K, et al. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg. 2002;236:184–190. doi: 10.1097/00000658-200208000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 18.Tonnesen E, Wanscher M, Hohndorf K, et al. Effect of methylprednisolone on the cytokine response in patients undergoing lung surgery. Acta Anaesthesiol Scand. 1993;37:410–414. doi: 10.1111/j.1399-6576.1993.tb03738.x. [DOI] [PubMed] [Google Scholar]
- 19.Miyashita M. Controversy of corticosteroids in septic shock. J Nippon Med Sch. 2010;77:67–70. doi: 10.1272/jnms.77.67. [DOI] [PubMed] [Google Scholar]
- 20.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 21.Swisher SG, Hunt KK, Holmes EC, Zinner MJ, McFadden DW. Changes in the surgical management of esophageal cancer from 1970 to 1993. Am J Surg. 1995;169:609–614. doi: 10.1016/s0002-9610(99)80231-1. [DOI] [PubMed] [Google Scholar]
- 22.Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology. 1991;48:411–420. doi: 10.1159/000226971. [DOI] [PubMed] [Google Scholar]
- 23.Fok M, Law SY, Wong J. Operable esophageal carcinoma: current results from Hong Kong. World J Surg. 1994;18:355–360. doi: 10.1007/BF00316814. [DOI] [PubMed] [Google Scholar]
- 24.Kaumeyer JF, Polazzi JO, Kotick MP. The mRNA for a proteinase inhibitor related to the HI-30 domain of inter-alpha-trypsin inhibitor also encodes alpha-1-microglobulin (protein HC) Nucleic Acids Res. 1986;14:7839–7850. doi: 10.1093/nar/14.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiki M, Sumi H, Maruyama M, Yoshida E, Mihara H. Clearance and distribution of acid-stable trypsin inhibitor (ASTI) Enzyme. 1989;42:31–38. doi: 10.1159/000469004. [DOI] [PubMed] [Google Scholar]
- 26.Mizon C, Piva F, Queyrel V, Balduyck M, Hachulla E, Mizon J. Urinary bikunin determination provides insight into proteinase/proteinase inhibitor imbalance in patients with inflammatory diseases. Clin Chem Lab Med. 2002;40:579–586. doi: 10.1515/CCLM.2002.100. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi H. Endogenous anti-inflammatory substances, inter-alpha-inhibitor and bikunin. Biol Chem. 2006;387:1545–1549. doi: 10.1515/BC.2006.192. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa M, Nishibe S, Mori T, Neumann S. Effect of human urinary trypsin inhibitor on granulocyte elastase activity. Res Commun Chem Pathol Pharmacol. 1987;55:271–274. [PubMed] [Google Scholar]
- 29.Faarvang HJ, Lauritsen OS. Circadian variations in sensitivity to glucocorticoid evaluated by urinary trypsin inhibitor excretion. Proc Soc Exp Biol Med. 1965;120:338–340. doi: 10.3181/00379727-120-30530. [DOI] [PubMed] [Google Scholar]
- 30.Ueki M, Yokono S, Taie S, Nogaya J, Komatsu H, Ogli K. Changes of urinary ulinastatin and serum CRP after elective surgery for gastric cancer. Masui. 1996;45:933–936. (In Japanese) [PubMed] [Google Scholar]
- 31.Pugia MJ, Lott JA. Pathophysiology and diagnostic value of urinary trypsin inhibitors. Clin Chem Lab Med. 2005;43:1–16. doi: 10.1515/CCLM.2005.001. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita Y, Shimada M, Hamatsu T, et al. Effects of preoperative steroid administration on surgical stress in hepatic resection: prospective randomized trial. Arch Surg. 2001;136:328–333. doi: 10.1001/archsurg.136.3.328. [DOI] [PubMed] [Google Scholar]
- 33.Shirabe K, Takenaka K, Yamatomto K, et al. Impaired systemic immunity and frequent infection in patients with Candida antigen after hepatectomy. Hepatogastroenterology. 1997;44:199–204. [PubMed] [Google Scholar]
- 34.Shimada H, Ochiai T, Okazumi S, et al. Clinical benefits of steroid therapy on surgical stress in patients with esophageal cancer. Surgery. 2000;128:791–798. doi: 10.1067/msy.2000.108614. [DOI] [PubMed] [Google Scholar]
- 35.Aldrighetti L, Pulitano C, Arru M, et al. Impact of preoperative steroid administration on ischemia-reperfusion injury and systemic responses in liver surgery: a prospective randomized study. Liver Transpl. 2006;12:941–949. doi: 10.1002/lt.20745. [DOI] [PubMed] [Google Scholar]