Abstract
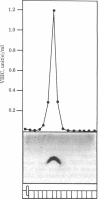
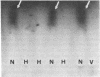
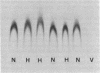
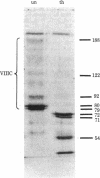
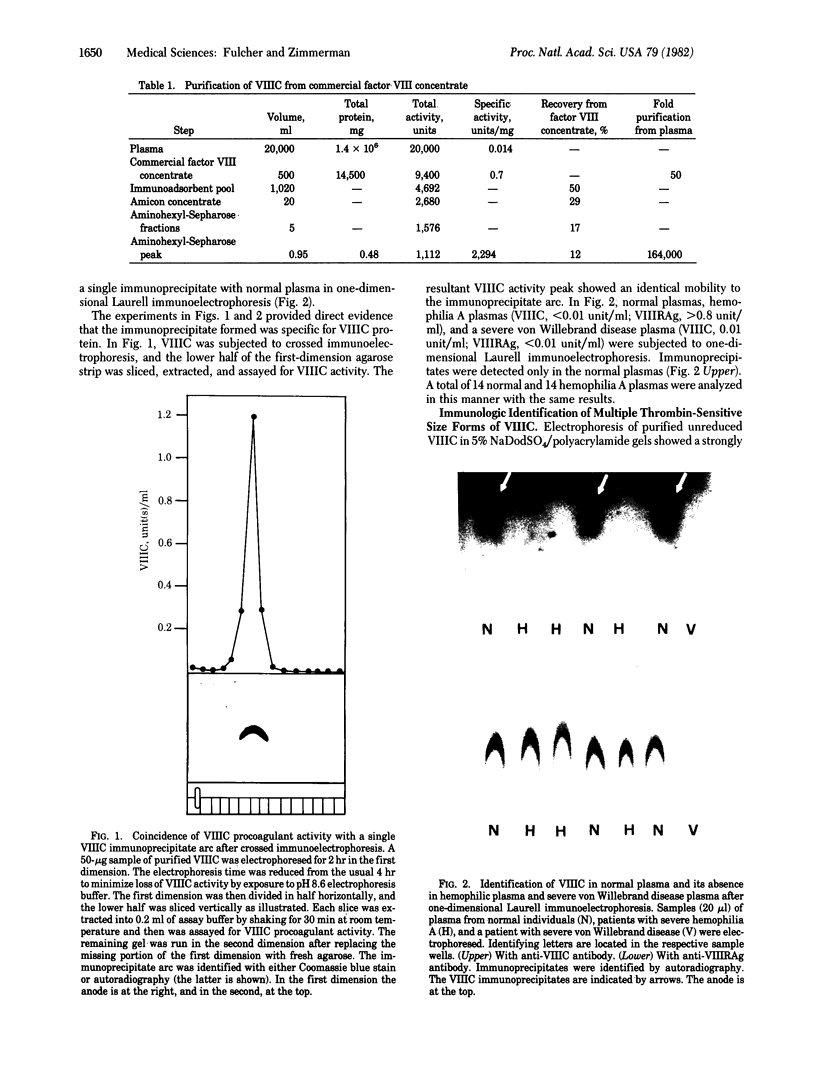
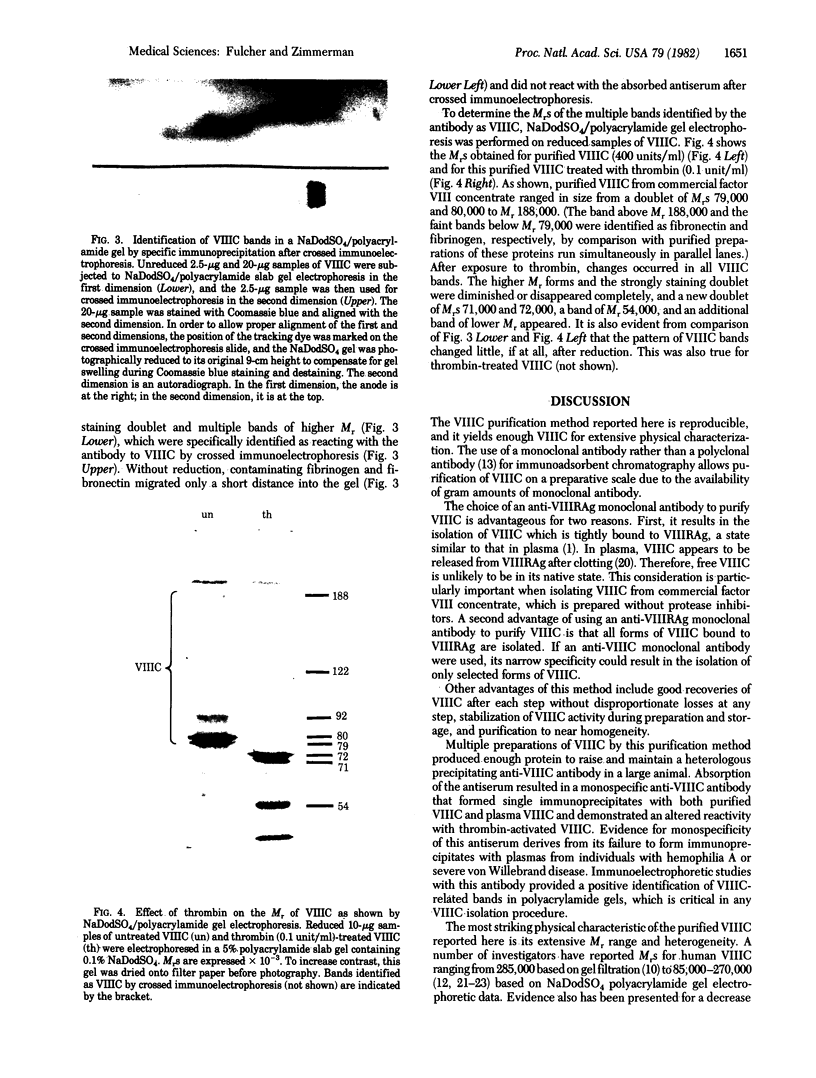
The human factor VIII procoagulant protein (VIIIC) was purified from the VIIIC--factor VIII--related antigen (VIIIRAg) complex in commercial factor VIII concentrate by immunoadsorbent chromatography with a monoclonal anti-VIIIRAg antibody bound to Sepharose. In this complex, VIIIC is noncovalently bound to factor VIII-related antigen. The VIIIC eluted from the complex was free of VIIIRAg as determined by immunoassay and had a specific activity of 2,294 VIIIC units/mg of protein, representing a 164,000-fold purification from plasma. Electrophoresis of this VIIIC preparation in reduced sodium dodecyl sulfate containing 5% polyacrylamide slab gels and subsequent staining with Coomassie blue showed the VIIIC to be a strongly staining doublet of Mrs 79,000 and 80,000 and more faintly staining bands of up to Mr 188,000. Treatment of the VIIIC with catalytic amounts of thrombin resulted in diminution or complete disappearance of all of these bands and appearance of a doublet of Mrs 71,000 and 72,000, a band at Mr 54,000, and material of lower Mr. The purified VIIIC was used to produce a precipitating heterologous anti-VIIIC antibody, which was shown to be monospecific for VIIIC after adsorption with factor VIII-depleted commercial concentrate. Use of this antibody in crossed immunoelectrophoresis positively identified the VIIIC bands in the sodium dodecyl sulfate/polyacrylamide gels. These techniques have allowed the identification of VIIIC protein and description of its extensive Mr heterogeneity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen D. E. The chromatographic separation of factor VIII on aminohexyl sepharose. Br J Haematol. 1979 Dec;43(4):669–674. doi: 10.1111/j.1365-2141.1979.tb03800.x. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Wright P. W., Hart C. E., Woodbury R. G., Hellström K. E., Hellström I. Protein antigens of normal and malignant human cells identified by immunoprecipitation with monoclonal antibodies. J Biol Chem. 1980 Jun 10;255(11):4980–4983. [PubMed] [Google Scholar]
- Cockburn C. G., de Beaufre-Apps R. J., Wilson J., Hardisty R. M. Parallel destruction of factor VIII procoagulant activity and an 85,000 dalton protein in highly purified factor VIII/VWF. Thromb Res. 1981 Feb 1;21(3):295–309. doi: 10.1016/0049-3848(81)90167-5. [DOI] [PubMed] [Google Scholar]
- Davies B. L., Furlong R. A., Peake I. R. Studies on the relationship between factor VIII related antigen (VIIIRAg) and factor VIII clotting antigen (VIIICAg) by immunoelectrophoresis and autoradiography using 125I anti VIIICAg. Thromb Res. 1981 Apr 1;22(1-2):87–96. doi: 10.1016/0049-3848(81)90311-x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Harris R. B., Johnson A. J., Hodgins L. T. Partial purification of biologically active, low molecular weight, human antihemophilic factor free of Von Willebrand factor. II. Further purification with thiol-disulfide interchange chromatography and additional evidence for disulfide bonds susceptible to limited reduction. Biochim Biophys Acta. 1981 May 29;668(3):471–480. doi: 10.1016/0005-2795(81)90181-1. [DOI] [PubMed] [Google Scholar]
- Harris R. B., Newman J., Johnson A. J. Partial purification of biologically active, low molecular weight, human antihemophilic factor free of Von Willebrand factor. I. Partial characterization and evidence for disulfide bond(s) susceptible to limited reduction. Biochim Biophys Acta. 1981 May 29;668(3):456–470. doi: 10.1016/0005-2795(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W. The factor VIII complex: structure and function. Blood. 1981 Jul;58(1):1–13. [PubMed] [Google Scholar]
- Hoyer L. W., Trabold N. C. The effect of thrombin on human factor VIII. Cleavage of the factor VIII procoagulant protein during activation. J Lab Clin Med. 1981 Jan;97(1):50–64. [PubMed] [Google Scholar]
- Kang E. P., Cruickshank W. H., Rock G. Visualization of low molecular weight factor VIII purified in the presence of benzamidine. Thromb Res. 1980 Feb 1;17(3-4):337–346. doi: 10.1016/0049-3848(80)90068-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letter: A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975 Dec 15;34(3):869–872. [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. Radioiodination of proteins by the use of the chloramine-T method. Methods Enzymol. 1980;70(A):210–213. doi: 10.1016/s0076-6879(80)70050-2. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Jaffe E. A., Miller C., Brown W. T. Structural analysis of factor VIII antigen in von Willebrand disease. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6832–6836. doi: 10.1073/pnas.77.11.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPAPORT S. I., SCHIFFMAN S., PATCH M. J., AMES S. B. The importance of activation of antihemophilic globulin and proaccelerin by traces of thrombin in the generation of intrinsic prothrombinase activity. Blood. 1963 Feb;21:221–236. [PubMed] [Google Scholar]
- Shapiro S. S. Antibodies to blood coagulation factors. Clin Haematol. 1979 Feb;8(1):207–214. [PubMed] [Google Scholar]
- Smith J. A., Hurrell J. G., Leach S. J. Elimination of nonspecific adsorption of serum proteins by Sepharose-bound antigens. Anal Biochem. 1978 Jul 1;87(2):299–305. doi: 10.1016/0003-2697(78)90679-6. [DOI] [PubMed] [Google Scholar]
- Tuddenham E. G., Trabold N. C., Collins J. A., Hoyer L. W. The properties of factor VIII coagulant activity prepared by immunoadsorbent chromatography. J Lab Clin Med. 1979 Jan;93(1):40–53. [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Weinstein M., Chute L., Deykin D. Analysis of factor VIII coagulant antigen in normal, thrombin-treated, and hemophilic plasma. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5137–5141. doi: 10.1073/pnas.78.8.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]