Abstract
AIM: To investigate whether therapeutic treatment with melatonin could protect rats against acute pancreatitis and its associated lung injury.
METHODS: Seventy-two male Sprague-Dawley rats were randomly divided into three groups: the sham operation (SO), severe acute pancreatitis (SAP), and melatonin treatment (MT) groups. Acute pancreatitis was induced by infusion of 1 mL/kg of sodium taurocholate (4% solution) into the biliopancreatic duct. Melatonin (50 mg/kg) was administered 30 min before pancreatitis was induced, and the severity of pancreatic and pulmonary injuries was evaluated 1, 4 and 8 h after induction. Serum samples were collected to measure amylase activities, and lung tissues were removed to measure levels of mRNAs encoding interleukin 22 (IL-22) and T helper cell 22 (Th22), as well as levels of IL-22.
RESULTS: At each time point, levels of mRNAs encoding IL-22 and Th22 were significantly higher (P < 0.001) in the MT group than in the SAP group (0.526 ± 0.143 vs 0.156 ± 0.027, respectively, here and throughout, after 1 h; 0.489 ± 0.150 vs 0.113 ± 0.014 after 4 h; 0.524 ± 0.168 vs 0.069 ± 0.013 after 8 h, 0.378 ± 0.134 vs 0.122 ± 0.015 after 1 h; 0.205 ± 0.041 vs 0.076 ± 0.019 after 4 h; 0.302 ± 0.108 vs 0.045 ± 0.013 after 8 h, respectively) and significantly lower (P < 0.001) in the SAP group than in the SO group (0.156 ± 0.027 vs 1.000 ± 0.010 after 1 h; 0.113 ± 0.014 vs 1.041 ± 0.235 after 4 h; 0.069 ± 0.013 vs 1.110 ± 0.213 after 8 h, 0.122 ± 0.015 vs 1.000 ± 0.188 after 1 h; 0.076 ± 0.019 vs 0.899 ± 0.125 after 4 h; 0.045 ± 0.013 vs 0.991 ± 0.222 after 8 h, respectively). The mean pathological scores for pancreatic tissues in the MT group were significantly higher (P < 0.01) than those for samples in the SO group (1.088 ± 0.187 vs 0.488 ± 0.183 after 1 h; 2.450 ± 0.212 vs 0.469 ± 0.242 after 4 h; 4.994 ± 0.184 vs 0.513 ± 0.210 after 8 h), but were significantly lower (P < 0.01) than those for samples in the SAP group at each time point (1.088 ± 0.187 vs 1.969 ± 0.290 after 1 h; 2.450 ± 0.212 vs 3.344 ± 0.386 after 4 h; 4.994 ± 0.184 vs 6.981 ± 0.301 after 8 h). The severity of SAP increased significantly (P < 0.01) over time in the SAP group (1.088 ± 0.187 vs 2.450 ± 0.212 between 1 h and 4 h after inducing pancreatitis; and 2.450 ± 0.212 vs 4.994 ± 0.184 between 4 and 8 h after inducing pancreatitis).
CONCLUSION: Melatonin protects rats against acute pancreatitis-associated lung injury, probably through the upregulation of IL-22 and Th22, which increases the innate immunity of tissue cells and enhances their regeneration.
Keywords: Interleukin 22, Melatonin, Pancreatitis-associated lung injury, Severe acute pancreatitis, T helper 22 cell
INTRODUCTION
Severe acute pancreatitis (SAP) is an inflammatory disease characterized by tissue edema, acinar necrosis, hemorrhage, and fat necrosis in the pancreas. Subsequent entry of proteolytic enzymes and vasoactive mediators into the systemic circulation always leads to multi-organ complications[1]. Pulmonary dysfunction is the most prominent manifestation of extra-abdominal organ dysfunction in pancreatitis, and its severity ranges from mild oxygenation abnormalities to severe acute respiratory distress syndrome (ARDS)[2]. Respiratory failure is associated with approximately 60% of the deaths that occur within the first week of pancreatitis[3,4]. Thus, the success of efforts to identify new therapeutic strategies for SAP will likely depend largely on understanding the molecular pathogenesis of damage to both the pancreas and the lungs.
Interleukin 22 (IL-22) is a member of the IL10 family of cytokines. It is an important effector of activated IL-22-producing T helper cells 22 (Th22), Th1 cells, and Th17 cells, as well as cytotoxic T-cell subsets, γδT cells, natural killer (NK), and NKT cells[5-7]. Activated T cells and NK cells are major sources of IL-22, and the highest levels of IL-22 expression have been detected in CD4+ memory cells. In contrast, IL-22 is not produced by either resting or activated monocytes, or B cells[8]. Like all other members of the IL10 family, IL-22 exerts its biological effects via heterodimeric transmembrane receptor complexes that each comprise of a type-1 and a type-2 receptor chain. These receptor chains belong to the cytokine receptor family class 2[5,9].
Since its discovery in 2000, several research groups have studied the unique biological activities of IL-22 and its significance in diseases. IL-22 protects tissues from damage, enhances tissue repair, maintains tissue integrity, increases innate immunity, and promotes anti-microbial defenses[5,10]. Therapy involving IL-22 improves liver regeneration after 70% hepatectomy[11] and also ameliorates high-fat diet-induced liver lipogenesis and hepatic steatosis[12]. Similarly, therapy with IL-22 counteracts the destructive nature of inflammatory bowel disease and ulcerative colitis[13] and prevents lung inflammation and fibrosis[14].
The recently discovered Th22 subset of T cells, which differs from Th17 and other known T-cell subsets based on its unique function[15], produces cytokines such as IL-22, IL26, and IL13. Th22 cells express several fibroblast growth factors, are associated with epidermal repair responses, and synergize with tumor necrosis factor α to induce a characteristic Th22 signature in keratinocytes[15]. Moreover, Th22 cells may regulate epidermal responses in inflammatory skin diseases and could be involved in unknown pathways that control tissue immunity and remodeling[15].
Although most melatonin is produced by the pineal gland, it can also be secreted by extrapineal organs and tissues such as the gastrointestinal tract, the retina and lens, skin, immune and hematopoietic cells, and some reproductive organs[16,17]. Several studies have demonstrated the beneficial effects of melatonin on SAP. As early as 1999, melatonin was reported to attenuate pancreatic edema and lipid peroxidation in caerulein-induced SAP[18]. Subsequent studies confirmed that pretreatment with melatonin significantly decreases all investigated inflammatory parameters associated with SAP[18]. Moreover, melatonin exerts an anti-inflammatory effect by inhibiting nuclear factor kappa B, a transcription factor with a central role in the development of inflammatory diseases. The present study was undertaken to examine the role of IL-22 in the pathogenesis of AP-induced lung damage, as well as the capacity of melatonin to protect against AP-associated lung injury.
MATERIALS AND METHODS
Animals
A total of 72 clean-grade healthy adult male Sprague-Dawley (SD) rats, each weighing 200-250 g, were obtained from the Experimental Animal Center of Wenzhou Medical College, Wenzhou, China. All animals were fed standard rat chow and had unlimited access to water under conditions that provided a constant room temperature of 25 °C and a 12-h/12-h day/night cycle. All animals were acclimated for at least a week before experimentation. Animals were deprived of rat chow for 12 h before experimentation but were allowed unlimited access to water throughout the experimental period. All procedures were performed in accordance with the guidelines for animal experiments of Wenzhou Medical College, Wenzhou, China.
Animal groups and procedures
SD rats were anesthetized by intraperitoneal (i.p.) injection of 10% chloraldurate (2 mL/kg; Solarbio, Beijing, China) and were randomly assigned to a sham operation group (SO group; n = 24), a SAP group (n = 24), or a melatonin treatment group (MT group; n = 24). For each animal in the SAP group, a laparotomy was performed through a midline incision, and 1 mL/kg of 4% sodium taurocholate (Sigma, St. Louis, United States) was retrogradely injected into the biliopancreatic duct through the papilla using a segmental epidural catheter via a microinjection pump at a rate of 0.2 mL/min. A microclip was placed in the hepatic portion of the biliopancreatic duct to avoid reflux before injection of sodium taurocholate. Rats in the SO group were subjected to the same surgical procedure but without infusion of 4% sodium taurocholate. In the MT group, melatonin (50 mg/kg body weight; Sigma) was administered i.p. 30 min before the injection of taurocholate. After each operation, the abdomen of each rat was closed in two layers. All surgical procedures were performed using sterile techniques.
The rats were killed by exsanguination at defined time points (1, 4 and 8 h; n = 8 for each time point) after the surgical procedure. Postcava puncture was used to obtain 3 mL blood from each animal to assay levels of serum amylase. The concentration of serum amylase (U/L) was assayed using a fully automatic biochemical analyzer (Hitachi, Tokyo, Japan). Lung tissues were harvested immediately before death, and tissue samples were fixed in 4% paraformaldehyde for immunohistochemical analysis. Other tissues were removed and stored in liquid nitrogen for the determination of IL-22 and Th22 mRNA levels using real-time reverse-transcription polymerase chain reaction and IL-22 protein levels using enzyme-linked immunosorbent assay (ELISA).
Histological analysis and pathological scores of pancreatic tissues
Samples of lung and pancreatic tissues were fixed in 40 g/L formaldehyde, embedded with paraffin wax, sectioned (4 μm thick), stained with hematoxylin and eosin (H and E), and observed using light microscopy. Tissue alteration was assessed (20 fields/section) by an experienced histologist who was blinded to the experimental protocol. Ten randomly selected visual fields in each pathological section were observed (H and E staining; ×400) using a high-powered microscope (CX31, Tokyo, Japan) and scored by two experienced pathologists using established standards[19], respectively. The maximum possible score for each visual field was 12, and the pathological score for each section was determined by calculating the mean of the scores from the two pathologists for the 10 visual fields.
Real-time, fluorescence-based quantitative polymerase chain reaction
Total RNA was extracted from lung tissues using TRIzol reagent (Invitrogen, Carlsbad, United States), and cDNA was synthesized using the first strand cDNA synthesis kit (MBI Fermentas, Burlington, Canada). mRNAs encoding IL-22 and Th22 were detected using the PCR mix kit (Daweike, Shanghai, China), and real-time quantitative PCR was performed using the ABI 7500 Sequence Detection System (Applied Biosystems Inc, Carlsbad, United States). For PCR, we used the following primers for IL-22 (forward: 5’-GCCAGCTGCCTGCTTCTCGT-3’, reverse: 5’-CTGGCCTCCTTGGCCAGCAT-3’), Th22 (forward: 5’-GGGCTTCCAGGGTGCTTCGC-3’, reverse: 5’-CCTCAGTTCACCGAGAACCCCA-3’), and β-actin (forward: 5’-GCGTCCACCCGCGAGTACAA-3’, reverse: 5’-CGACGACGAGCGCAGCGATA-3’; Generay, Shanghai, China). The cDNA was denatured at 95 °C for 5 min and amplified over the course of 40 cycles, each involving 95 °C (15 s), 60 °C (45 s), and 72 °C (60 s), with a final extension at 72 °C (5 min). The samples were tested in triplicate, and the expression of IL-22 and Th22 mRNA was calculated using the 2-ΔΔCT method.
Sandwich enzyme-linked immunosorbent assays
Concentrations of IL-22 were measured using commercially available sandwich ELISA kits (all from R and D Systems, Rapidbio, United States). IL-22 levels were assayed using microtiter wells coated with purified rat IL-22 and a solid-phase IL-22-specific antibody. The horseradish peroxidase (HRP)-labeled secondary antibody was detected with the tetramethylbenzidine substrate solution, which creates a blue reaction product. The HRP enzyme-catalyzed reaction was terminated by the addition of a sulfuric acid solution, and the color change was measured spectrophotometrically at a wavelength of 450 nm. The concentration of IL-22 in the samples was then determined by comparing the A450 nm of the samples to a standard curve.
Statistical analysis
Statistical analysis were carried out using the SPSS software (ver. 15.0; SPSS Inc., Chicago, IL, United States). All data are expressed as the mean ± SD. One-way analysis of variance were used to investigate differences among the SO, SAP, and MT groups at each time point, and post hoc comparisons were performed between samples in the SO and SAP groups to check for statistical significance. Differences were considered significant at P < 0.05.
RESULTS
Histopathological examination of lung tissues and pathological scores of pancreatic tissues after SAP induction
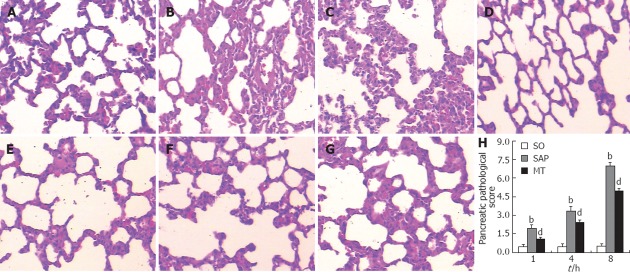
The appearance of lung tissue samples collected 1 h (Figure 1A), 4 h (Figure 1B), and 8 h (Figure 1C) after the induction of SAP was much more severely affected than the appearance of tissue from animals in the SO group (Figure 1D), which tended to remain morphologically normal even 8 h after surgery. Microscopic examination of lung tissue from animals in the SAP group provided extremely clear evidence of edema, hemorrhage complicated by microthrombosis, cell degeneration, and inflammation (Figure 1A-C). The extent of damage observed in the lungs of SAP animals was largely alleviated in lung tissues of animals in the MT group (Figure 1E-G).
Figure 1.
Histology of pulmonary tissues and pathological scores of pancreatic tissues from rats in the sham operation, severe acute pancreatitis, and melatonin treatment groups. A-C: Histological changes of pulmonary tissues in the SAP group (A) 1 h, (B) 4 h, and (C) 8 h after experimental induction of SAP; D: Histological changes of pulmonary tissues in the SO group 8 h after mock induction of SAP; E-G: Histological changes of pulmonary tissues in the MT group (E) 1 h, (F) 4 h, and (G) 8 h after experimental induction of SAP (hematoxylin and eosin stain; ×200). H: Pathological scores of pancreatic tissues (n = 8). bP < 0.01 vs the MT group; dP < 0.01 vs the SO and MT groups. SO: Sham operation; SAP: Severe acute pancreatitis; MT: Melatonin treatment.
Pancreatic tissue samples were also collected and analyzed from each group of animals. At each time point, the mean pathological score for pancreatic tissues in the MT group was significantly higher (P < 0.01) than for samples in the SO group, but significantly lower (P < 0.01) than for samples in the SAP group at each time point. The severity of SAP increased significantly over time in the SAP group (P < 0.01, Figure 1H).
Serum amylase levels after SAP induction
Levels of serum amylase at each time point were markedly elevated in the SAP group relative to the SO (P < 0.01) and MT (P < 0.05) groups (Figure 2). In addition, the levels of amylase in the MT group increased compared with those in the SO group. In the SAP group, amylase levels increased over time, indicating the development of SAP. There was no significant difference between the levels of serum amylase at the three time points in the SO group (Figure 2).
Figure 2.
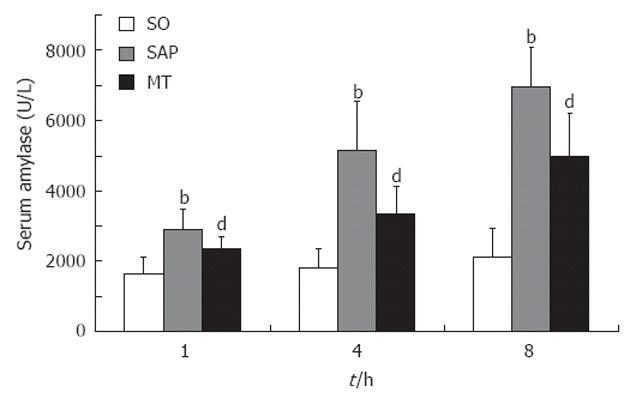
Changes in serum amylase levels from rats in the sham operation, severe acute pancreatitis, and melatonin treatment groups over time. Serum amylase levels differed significantly (P < 0.01) between each pair of time points in the SAP group, but not at different levels in the SO group (n = 8 rats per group). bP < 0.01 vs the SO group; dP < 0.05 vs the SO and SAP groups. SO: Sham operation; SAP: Severe acute pancreatitis; MT: Melatonin treatment.
Expression of IL-22 and Th22 mRNAs after SAP induction
Levels of the IL-22 and Th22 mRNAs in lung tissues were significantly decreased within 1 h after the induction of SAP (P < 0.01; Figure 3A and B), and levels of both mRNAs remained low during the remaining time points. Importantly, this decrease in both mRNAs in the SAP group relative to the SO group was partly rescued in the MT group. Differences between levels in the SAP group and the MT group were significant (P < 0.01), and expression differed significantly between the MT group and the SO group (P < 0.01, Figure 3A and B).
Figure 3.
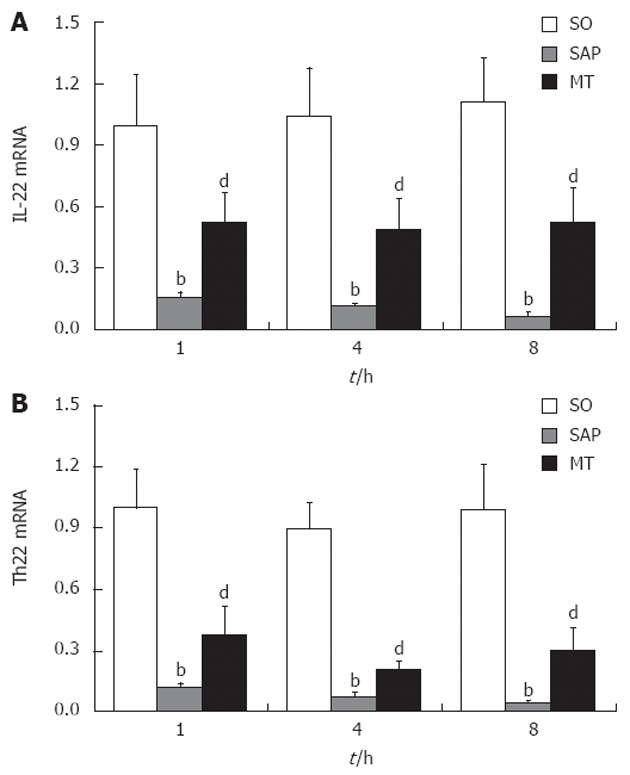
Levels of mRNAs encoding interleukin 22 and T helper cell 22 in lung tissue samples from rats in the sham operation, severe acute pancreatitis, and melatonin treatment groups. A: Expression of IL-22 mRNA 1, 4 and 8 h after induction of SAP; B: Expression of Th22 mRNA 1, 4 and 8 h after induction of SAP. Levels of mRNAs encoding IL-22 and Th22 decreased with continued progression of SAP (P < 0.01). bP < 0.01 vs the SO group; dP < 0.01 vs the SO and SAP groups (n = 8 rats per group). SO: Sham operation; SAP: Severe acute pancreatitis; IL: Interleukin; Th: T helper cell; MT: Melatonin treatment.
Levels of IL-22 in lung tissue after SAP induction
The level of IL-22 in lung tissue in the MT group was significantly lower than that in the SO group and significantly higher than that in the SAP group at each time point (P < 0.01; Figure 4). The level of IL-22 in the lung tissue samples decreased significantly over time in the SAP group (P < 0.01), although IL-22 levels did not differ significantly between each pair of time points in the SO group.
Figure 4.
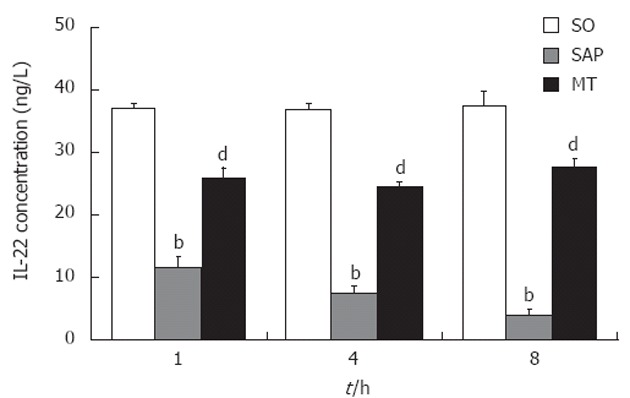
Levels of interleukin 22 in lung tissue samples from rats in the sham operation, severe acute pancreatitis, and melatonin treatment groups. Levels of IL-22 at 1, 4 and 8 h after induction of SAP. Levels of IL-22 decreased with continued progression of SAP (P < 0.01). bP < 0.01 vs the SO group; dP < 0.01 vs the SO and SAP groups (n = 8 rats per group). SO: Sham operation; SAP: Severe acute pancreatitis; IL: Interleukin; Th: T helper cell; MT: Melatonin treatment.
DISCUSSION
Patients with SAP and the associated multiple-organ failure in which it frequently results[20], require admission to a high-dependency or intensive-care unit. Despite recent progress in revealing the mechanisms that underlie SAP, which include inflammation, cell injury, and cell death, the exact pathogenesis of SAP is not fully understood. The uncontrolled generation of reactive oxygen species (ROS) during SAP causes oxidative damage, both in the pancreas and distant organs. The lung is especially vulnerable to damage by ROS because it possesses the largest endothelial surface area of any organ in the body[21].
IL-22 is a member of the IL-10 cytokine family, which additionally comprises IL-10, IL-19, IL-20, IL-24, IL-26, IL-28α, IL-28β and IL-29[22-24]. IL-22 is a key player in antimicrobial defenses, tissue regeneration, and protection against damage that may serve either protective or highly pathogenetic roles in chronic inflammatory disorders, depending on the nature of the affected tissue and the local cytokine milieu[5]. IL-22 targets cells from the skin, liver, and kidney and from organs of the respiratory and gastrointestinal systems. The effects of IL-22 in AP are mediated by direct and/or indirect inhibition of the activities and/or production of cycloxygenase-2, prostaglandin E2, IL-1β, and monocyte chemoattractant protein-1, all of which contribute to the progression of SAP[25]. In fact, IL-22 is a potential therapeutic target for the treatment of chronic inflammatory diseases, and treatment with recombinant cytokine or delivery of IL-22 by gene therapy may alleviate tissue destruction during inflammatory responses[26]. Experimental delivery of IL-22 has been efficacious in treating autoimmune disorders such as experimental autoimmune myocarditis in rats[27,28]. Western blotting for IL-22 in bronchoalveolar lavage fluid revealed lower levels of IL-22 in patients with ARDS[29]. This is the first study to suggest the protective actions of IL-22 in a rat model of pancreatitis-associated lung injury.
In this study, we measured the levels of mRNAs encoding IL-22 and Th22 in rat pulmonary tissues to explore the mechanism of acute pancreatitis-associated lung injury. We also examined the effects of SAP, with and without prior treatment with melatonin, on IL-22 protein levels. The reduced levels of IL-22 and Th22 mRNAs that accompanied taurocholate-induction of SAP suggest that downregulation of mRNAs encoding IL-22 and Th22 contribute to the pathogenesis of pancreatitis-associated lung injury. However, future studies are needed to determine more precisely the underlying mechanisms by which downregulation of IL-22 and Th22 contributes to acute pancreatitis-associated lung injury.
Melatonin is a hormone that can cross all body barriers as well as the placenta[30,31]. It is not stored and is rapidly released into the general circulation immediately after its synthesis. Melatonin is useful not only for the acute treatment of ischemic stroke and neurodegenerative disease[32] but also as a prophylactic treatment against stroke[33]. Melatonin is a more highly efficient ROS scavenger than other ROS scavengers and general antioxidants such as glutathione and ascorbic acid, which protect DNA, lipids, and proteins from oxidative damage[34,35]. The previous results have indicated that melatonin prevents or attenuates the severity of experimental SAP[18], but few published studies have examined the relevance between the effect of melatonin and IL-22 or Th22. The main finding of this study was the demonstration that the ameliorative effect of melatonin on pancreatitis-associated lung injury is correlated with an elevation in the levels of IL-22 and Th22, and IL-22 has been demonstrated to protect tissues against caerulein-induced SAP by their anti-inflammatory effects[25].
The levels of mRNAs encoding IL-22 and Th22 were lower in rats with SAP and gradually decreased with the progression of SAP. The levels of IL-22 and Th22 mRNAs in pulmonary tissues from the MT group were, however, partially restored to the levels seen in the SO group, and histopathological examination indicated that the damage to pulmonary tissues from the MT group was reduced relative to that of the SAP group. This suggests that the alleviation of acute pancreatitis-associated lung injury by melatonin is mediated, at least in part, by upregulation of IL-22 and Th22 activities.
In summary, this study indicates, for the first time, that the downregulation of pulmonary IL-22 and Th22 may be associated with the pathogenesis of acute pancreatitis-associated lung injury. Melatonin, functioning as a protectant and antioxidant, may reduce lung injury by increasing IL-22 and Th22 activity.
ACKNOWLEDGMENTS
We thank Ji-Wei Xu and Qi-Jian Zhu, who are experienced pathologists in the First Affiliated Hospital, Wenzhou Medical College, Wenzhou, China, for their valuable assistance.
COMMENTS
Background
Severe acute pancreatitis (SAP) is a potentially serious disease for which there are limited specific therapies. Pulmonary dysfunction is the most prominent manifestation of extra-abdominal organ dysfunction in pancreatitis. Previous studies assessed the therapeutic efficacy of interleukin 22 (IL-22) and melatonin for the treatment of SAP in a rat model of the disease, although the exact mechanism(s) by which melatonin mediates its effects remains obscure.
Research frontiers
Previous research indicated that therapy involving recombinant IL-22 markedly attenuates the severity of SAP in rats. The study has found that melatonin, which can reduce pancreatic damage in rats with SAP, has a protective effect on the lungs of rats with SAP. This protection is associated with increases in the levels of mRNAs that encode IL-22 and T helper cell 22 (Th22) and increased levels of IL-22 protein. Further research is needed to explore the mechanism by which melatonin upregulates IL-22 and Th22 and how IL-22 and Th22 mediate their protective effects on the lungs of animals with SAP.
Innovations and breakthroughs
The mechanism(s) responsible for the previously proposed anti-inflammatory effects of IL-22 and melatonin during SAP remains to be elucidated. The authors studied the effect of melatonin on levels of mRNAs encoding IL-22 and Th22 and the level of IL-22 in lung tissues from rats with SAP. The results indicated that the downregulation of pulmonary mRNAs encoding IL-22 and Th22 in animals with SAP may be associated with the pathogenesis of acute pancreatitis-associated lung injury, and melatonin is potentially capable of reducing lung injury by increasing IL-22 and Th22 activities. This paper provides a plausible explanation of the mechanism underlying the therapeutic effects of melatonin in the treatment of SAP.
Applications
Given the established therapeutic efficacy of melatonin for treating SAP, elucidation of the underlying mechanism involved is the primary obstacle to translation of this approach to clinical practice. The results suggest that the therapeutic effects of melatonin in reducing acute pancreatitis-associated lung injury can potentially be attributed to increased IL-22 and Th22 activities.
Terminology
SAP is principally caused by autodigestion of the pancreas, and pulmonary dysfunction is the most pertinent manifestation of extra-abdominal organ dysfunction in patients with pancreatitis. IL-22 is a member of the IL10 family of cytokines and represents an important effector molecule of activated IL-22-expressing Th22 cells, Th1, and Th17 cells. Melatonin, which is secreted by the pineal gland, prevents or attenuates the severity of experimental SAP.
Peer review
The authors of this study focus on the value of melatonin in alleviating the severity of acute pancreatitis-associated lung injury and the possible molecular mechanism that underlies this effect. The results suggest that the down-regulation of pulmonary IL-22 and Th22 may be associated with the pathogenesis of acute pancreatitis-associated lung injury and that melatonin is potentially capable of reducing lung injury by increasing IL-22 and Th22 activity.
Footnotes
Supported by Wenzhou Municipal Science and Technology Commission major projects funds, No. 20090006; The scientific committee of Wenzhou and the Department of Gastroenterology and Hepatology, First Affiliated Hospital, Wenzhou Medical College, Wenzhou, China
Peer reviewers: Maha Maher Shehata, Professor, Internal Medicine, Mansoura University, Medical Specialized Hospital, 35516 Mansoura, Egypt; Hongjoo Kim, Professor, Sungkyunkwan University Kangbuk Samsung Hospital, 108, Pyung-Dong, Jongro-Ku, Seoul 110-746, South Korea
S- Editor Gou SX L- Editor Webster JR E- Editor Yan JL
References
- 1.Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- 2.Shields CJ, Winter DC, Redmond HP. Lung injury in acute pancreatitis: mechanisms, prevention, and therapy. Curr Opin Crit Care. 2002;8:158–163. doi: 10.1097/00075198-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Renner IG, Savage WT, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30:1005–1018. doi: 10.1007/BF01308298. [DOI] [PubMed] [Google Scholar]
- 4.Steer ML. Relationship between pancreatitis and lung diseases. Respir Physiol. 2001;128:13–16. doi: 10.1016/s0034-5687(01)00259-6. [DOI] [PubMed] [Google Scholar]
- 5.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 6.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 7.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 8.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 9.Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17:367–380. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 11.Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74–G80. doi: 10.1152/ajpgi.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, Ruan X, Huang H, Wang Z, Huang Z, et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53:339–347. doi: 10.1016/j.jhep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, Zwissler B, Muhl H. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:369–376. doi: 10.1165/rcmb.2009-0440OC. [DOI] [PubMed] [Google Scholar]
- 15.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 17.Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Qi W, Tan DX, Reiter RJ, Kim SJ, Manchester LC, Cabrera J, Sainz RM, Mayo JC. Melatonin reduces lipid peroxidation and tissue edema in cerulein-induced acute pancreatitis in rats. Dig Dis Sci. 1999;44:2257–2262. doi: 10.1023/a:1026656720868. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakafika A, Papadopoulos V, Mimidis K, Mikhailidis DP. Coagulation, platelets, and acute pancreatitis. Pancreas. 2007;34:15–20. doi: 10.1097/01.mpa.0000240617.66215.d2. [DOI] [PubMed] [Google Scholar]
- 21.Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol. 1999;277:L1067–L1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- 22.Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, Vazquez N, Pestka S, Donnelly RP, Kotenko SV. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Shemi AG, Basalamah MA, Kensara OA, Ashshi AM. Interleukin-22 therapy attenuates the development of acute pancreatitis in rats. Clinical Medicine and Research. 2011;3:82–88. [Google Scholar]
- 26.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang H, Hanawa H, Liu H, Yoshida T, Hayashi M, Watanabe R, Abe S, Toba K, Yoshida K, Elnaggar R, et al. Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J Immunol. 2006;177:3635–3643. doi: 10.4049/jimmunol.177.6.3635. [DOI] [PubMed] [Google Scholar]
- 28.Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1:43–49. [PubMed] [Google Scholar]
- 29.Whittington HA, Armstrong L, Uppington KM, Millar AB. Interleukin-22: a potential immunomodulatory molecule in the lung. Am J Respir Cell Mol Biol. 2004;31:220–226. doi: 10.1165/rcmb.2003-0285OC. [DOI] [PubMed] [Google Scholar]
- 30.Bülbüller N, Doğru O, Umaç H, Gürsu F, Akpolat N. [The effects of melatonin and pentoxiphylline on L-arginine induced acute pancreatitis] Ulus Travma Acil Cerrahi Derg. 2005;11:108–114. [PubMed] [Google Scholar]
- 31.Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Cześnikiewicz-Guzik M, Kwiecień S, Brzozowski T, Bubenik GA, Pawlik WW. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT) J Physiol Pharmacol. 2007;58:381–405. [PubMed] [Google Scholar]
- 32.Reiter RJ. Oxidative damage in the central nervous system: protection by melatonin. Prog Neurobiol. 1998;56:359–384. doi: 10.1016/s0301-0082(98)00052-5. [DOI] [PubMed] [Google Scholar]
- 33.Kilic E, Kilic U, Reiter RJ, Bassetti CL, Hermann DM. Prophylactic use of melatonin protects against focal cerebral ischemia in mice: role of endothelin converting enzyme-1. J Pineal Res. 2004;37:247–251. doi: 10.1111/j.1600-079X.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- 34.Vural H, Sabuncu T, Arslan SO, Aksoy N. Melatonin inhibits lipid peroxidation and stimulates the antioxidant status of diabetic rats. J Pineal Res. 2001;31:193–198. doi: 10.1034/j.1600-079x.2001.310301.x. [DOI] [PubMed] [Google Scholar]
- 35.Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]