Abstract
Scrub typhus is an acute febrile disease caused by Orientia tsutsugamushi (O. tsutsugamushi). We report herein the case of a woman who presented with fever and elevated serum levels of liver enzymes and who was definitively diagnosed with scrub typhus by histopathological examination of liver biopsy specimens, serological tests and nested polymerase chain reaction. Immunohistochemical staining using a monoclonal anti-O. tsutsugamushi antibody showed focally scattered positive immunoreactions in the cytoplasm of some hepatocytes. This case suggests that scrub typhus hepatitis causes mild focal inflammation due to direct liver damage without causing piecemeal necrosis or interface hepatitis. Thus, scrub typhus hepatitis differs from acute viral hepatitis secondary to liver damage due to host immune responses, which causes severe lobular disarray with diffuse hepatocytic degeneration, necrosis and apoptosis as well as findings indicative of hepatic cholestasis, such as hepatic bile plugs or brown pigmentation of hepatocytes.
Keywords: Scrub typhus, Immunohistochemistry, Orientia tsutsugamushi
INTRODUCTION
Scrub typhus is an acute febrile disease caused by Orientia tsutsugamushi (O. tsutsugamushi) that occurs when O. tsutsugamushi is transmitted to humans through the bites of chiggers (Leptotrombidium)[1]. Previous reports from Taiwan have revealed that 97% of hospitalized scrub typhus patients have high serum levels of aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase as well as subsequent abnormal liver function[2,3]. However, only a few studies have evaluated the histopathological features of patients with scrub typhus hepatitis[4]. We report herein the case of a woman who presented with fever and elevated serum levels of liver enzymes and who was definitively diagnosed with scrub typhus by a histopathological examination of liver biopsy specimens, serological tests and a nested polymerase chain reaction (PCR).
CASE REPORT
A 63-year-old woman presented at our clinic with a 5-d history of fever. Five days previously, the patient had visited a primary care clinic due to fever, chills, headache, myalgia, anorexia and diarrhea. She was transferred to our clinic because of her unusually high serum levels of liver enzymes and low platelet count. She was a housewife by occupation and had worked in the field every day for 3 wk prior to her illness. She presented with the following clinical parameters: blood pressure, 100/60 mmHg; pulse rate, 84/min; respiratory rate, 20/min; and body temperature, 38.1 °C. Hematochemical tests revealed the following: white blood cell count, 2.97/L (neutrophils 59%, lymphocytes 25%); hemoglobin, 12.4 g/dL; platelets, 117/L; erythrocyte sedimentation rate, 22 mm/h; C-reactive protein, 5.05 mg/dL; aspartate aminotransferase (AST)/alanine aminotransferase (ALT), 426/299 U/L; gamma-glutamyltransferase (γ-GTP)/alkaline phosphatase, 368/299 U/L; total bilirubin, 1.12 mg/dL; and lactate dehydrogenase/creatinine phosphokinase, 172/110 U/L (normal range, 180-460/26-20 U/L). The patient was negative for antibodies against acute hepatitis viruses A, B, D and E and for immunoglobulin M (IgM) against Epstein-Barr virus capsid antigen. PCR for cytomegalovirus and antigenemia tests were also negative. Antibodies involved in connective tissue diseases were absent. Specimens for immunohistochemical staining were obtained by ultrasound-guided needle aspiration to accurately evaluate the increased serum levels of liver enzymes. For immunohistochemical staining, we used a monoclonal anti-O. tsutsugamushi antibody that targets a recombinant 56 kDa protein of a clinical O. tsutsugamushi Boryong strain. Immunolocalization was performed using a polyvalent horseradish peroxidase polymer detection system according to the manufacturer’s protocol (Lab Vision, Fremont, CA, United States)[5].
On the third day of hospitalization, a fever was evident (≥ 38 °C), chills and headache persisted, and maculopapular rashes occurred. Thus, we conducted serological tests for scrub typhus, leptospirosis and hemorrhagic fever with renal syndrome (HFRS) as well as PCR for scrub typhus and leptospirosis. Hematoxylin and eosin staining of the biopsy specimens revealed mild inflammation with a small number of eosinophils and a dominance of lymphocytes in the portal areas. Piecemeal necrosis and interface hepatitis were not evident in the portal tract (Figure 1). Mild degrees of lobular disarray and ballooning degeneration of hepatocytes were observed in addition to the necrosis of isolated hepatocytes. However, there were no clusters of necrotic hepatocytes, bridging necrosis, intracanalicular bile plugs or intrahepatic cholestasis. Immunohistochemical staining using a monoclonal anti-O. tsutsugamushi antibody showed scattered positive immunoreactions in the cytoplasm of some hepatocytes (Figure 1D). Serological tests for O. tsutsugamushi performed on the third day of hospitalization revealed anti-O. tsutsugamushi IgM and immunoglobulin G (IgG) antibody titers of 1:40 and 1:1024, respectively. Nested PCR using the blood buffy coat was positive for the gene encoding the 56 kDa protein of O. tsutsugamushi. Serological tests were negative for HFRS and leptospirosis. Based on these findings, a comprehensive physical examination was conducted, which revealed an eschar on one of her labia minora. The patient reported that she had urinated during field work. Finally, she was definitively diagnosed with scrub typhus. A 5-d course of doxycycline improved the symptoms of fever, chills and headache and her unusually high levels of liver enzymes and low platelet count. She was discharged from the hospital without any complications. At the time of discharge, serological tests showed anti-O. tsutsugamushi IgM and IgG titers of 1:5120 and 1:16 384, respectively.
Figure 1.
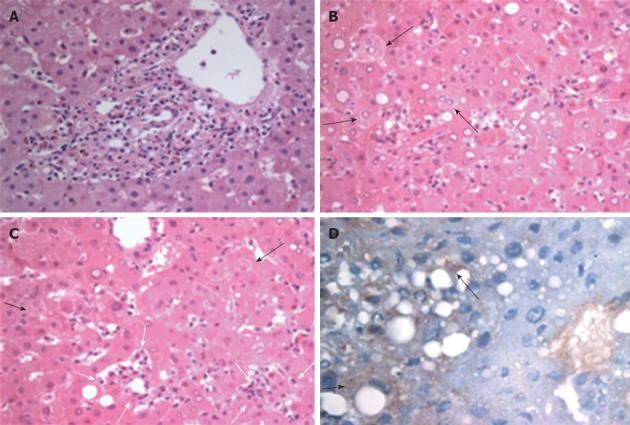
Histopathological analysis of a liver specimen from a patient with scrub typhus. A: Portal inflammation showing lymphocytes and some eosinophils devoid of distinct interface hepatitis; B, C: The degeneration of individual hepatocytes (black arrows), mild ballooning changes with lobular disarray and small clusters of mononuclear cell infiltration (white arrows) identified in the lobules. Neither cholestasis nor hepatocyte apoptosis are evident (hematoxylin and eosin stain, ×400 magnification); D: Immunohistochemical staining for Orientia tsutsugamushi demonstrating scattered positive immunoreactions (arrows) in the cytoplasm of the hepatocytes (labeled streptavidin-biotin method, counterstained by hematoxylin, ×400 magnification).
DISCUSSION
Fever, headache and skin rash are potential indicators of rickettsial diseases[6]. In the present case, the patient presented with fever, diarrhea and elevated serum levels of liver enzymes without a typical skin rash or eschar suggestive of scrub typhus. We initially considered infections caused by hepatitis virus, other infectious diseases such as tuberculosis and autoimmune hepatitis. Serological tests, serum protein electrophoresis and the use of antinuclear antibodies all yielded negative results, but immunohistochemical staining of a biopsy specimen revealed hepatocytes positive for O. tsutsugamushi. Because a maculopapular skin rash developed during hospitalization and because positive immunohistochemical staining was confirmed, a thorough history and a comprehensive physical examination were conducted, which confirmed an eschar on a labium minus and a history of urination during field work. These results reflect the importance of a thorough physical examination that includes the genitalia[7].
Although many studies have reported increases in liver enzymes in patients with scrub typhus[2], there have been only a few reports on the histopathological features of this disease[4]. In this case, the primary lesion was portal triad inflammation, which is consistent with the results of a previous study indicating that Rickettsia and Orientia species mainly cause such inflammation due to tropism for the vascular endothelium[8]. Rickettsioses cause focal lesions in various organs but do not cause extensive hepatic lesions that are sufficient to cause organ failure[9]. In addition, although Rocky Mountain Spotted Fever causes the focal death of some hepatocytes and increases in the serum levels of liver enzymes due to hepatic infection, hepatic failure does not occur[8]. In our case, immunohistochemical staining showed scattered focal positive immunoreactions. O. tsutsugamushi hepatitis is speculated to cause primarily intrahepatic sinusoidal endothelial vasculitis and to increase the serum levels of AST, ALT and γ-GTP due to direct cytopathic liver damage[10]. In contrast, host immune responses have been shown to cause viral hepatitis-induced liver damage[11,12]. The hepatitis virus multiplies within the cytoplasm of hepatocytes, causing non-cytopathic inflammation and hepatocellular damage as well as the destruction of infected hepatocytes through human leukocyte antigen-restricted, hepatitis A virus-specific CD8+ T lymphocytes and natural killer cells. Because hepatocyte degeneration, necrosis and apoptosis are more severe in acute viral hepatitis, it is conceivable that the serum levels of AST and ALT would be significantly higher in acute viral hepatitis than in scrub typhus hepatitis. Unlike patients with scrub typhus hepatitis, patients with viral hepatitis exhibit hepatic cholestasis, including intracanalicular bile plugs or the brown pigmentation of hepatocytes; this hepatic cholestasis can markedly increase the serum levels of γ-GTP and alkaline phosphatase[13,14]. Further studies are needed to confirm the relationship between the laboratory and histopathological findings.
Although granuloma formation in the liver is a characteristic finding in patients with scrub typhus, our patient did not have granulomatous hepatitis. It is thought that granulomatous hepatitis can be observed in some patients with scrub typhus.
In summary, this case has 2 clinical implications. First, our patient was diagnosed early with scrub typhus by immunohistochemistry using a monoclonal antibody for O. tsutsugamushi. The immunohistochemical analysis showed scattered focal positive immunoreactions in the cytoplasm of some hepatocytes. Second, our report supports previous findings demonstrating that scrub typhus hepatitis causes mild portal inflammation and lobular activities without causing significant interface hepatitis or intralobular hepatocyte death due to focal direct liver damage. In contrast, acute viral hepatitis secondary to host immune response-induced liver damage causes severe lobular disarray with diffuse hepatocyte degeneration, necrosis and apoptosis as well as findings of hepatic cholestasis, including intracanalicular bile plugs and the brown pigmentation of hepatocytes.
Footnotes
Supported by The Korea Science and Engineering Foundation Grant Funded by the Korean Government through the Research Center for Resistant Cells, No. R13-2003-009
Peer reviewer: Jian-Zhong Zhang, Professor, Department of Pathology and Laboratory Medicine, Beijing 306 Hospital, 9 North Anxiang Road, PO Box 9720, Beijing 100101, China
S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Lee SH, Kim DM, Cho YS, Yoon SH, Shim SK. Usefulness of eschar PCR for diagnosis of scrub typhus. J Clin Microbiol. 2006;44:1169–1171. doi: 10.1128/JCM.44.3.1169-1171.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu ML, Liu JW, Wu KL, Lu SN, Chiou SS, Kuo CH, Chuah SK, Wang JH, Hu TH, Chiu KW, et al. Short report: Abnormal liver function in scrub typhus. Am J Trop Med Hyg. 2005;73:667–668. [PubMed] [Google Scholar]
- 3.Basnyat B, Belbase RH, Zimmerman MD, Woods CW, Reller LB, Murdoch DR. Clinical features of scrub typhus. Clin Infect Dis. 2006;42:1505–1506. doi: 10.1086/503680. [DOI] [PubMed] [Google Scholar]
- 4.Silpapojakul K, Mitarnun W, Ovartlarnporn B, Chamroonkul N, Khow-Ean U. Liver involvement in murine typhus. QJM. 1996;89:623–629. doi: 10.1093/qjmed/89.8.623. [DOI] [PubMed] [Google Scholar]
- 5.DeRoche T, Walker E, Magi-Galluzzi C, Zhou M. Pathologic characteristics of solitary small renal masses: can they be predicted by preoperative clinical parameters? Am J Clin Pathol. 2008;130:560–564. doi: 10.1309/YR7P42XUVQHPHDWL. [DOI] [PubMed] [Google Scholar]
- 6.Dumler JS, Taylor JP, Walker DH. Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA. 1991;266:1365–1370. [PubMed] [Google Scholar]
- 7.Pau WS, Tan KK. Importance of a thorough examination. Pediatr Infect Dis J. 2008;27:569–570. doi: 10.1097/INF.0b013e318168db08. [DOI] [PubMed] [Google Scholar]
- 8.Adams JS, Walker DH. The liver in Rocky Mountain spotted fever. Am J Clin Pathol. 1981;75:156–161. doi: 10.1093/ajcp/75.2.156. [DOI] [PubMed] [Google Scholar]
- 9.Randall MB, Walker DH. Rocky Mountain spotted fever. Gastrointestinal and pancreatic lesions and rickettsial infection. Arch Pathol Lab Med. 1984;108:963–967. [PubMed] [Google Scholar]
- 10.Watanabe H, Saito T, Misawa K, Suzuki A, Sanjo M, Okumoto K, Hattori E, Adachi T, Takeda T, Ito JI, et al. Direct cytopathic liver injury and acute respiratory distress syndrome associated with gilliam-type tsutsugamushi disease. J Gastroenterol Hepatol. 2005;20:969–971. doi: 10.1111/j.1440-1746.2005.03802.x. [DOI] [PubMed] [Google Scholar]
- 11.Fleischer B, Fleischer S, Maier K, Wiedmann KH, Sacher M, Thaler H, Vallbracht A. Clonal analysis of infiltrating T lymphocytes in liver tissue in viral hepatitis A. Immunology. 1990;69:14–19. [PMC free article] [PubMed] [Google Scholar]
- 12.Baba M, Hasegawa H, Nakayabu M, Fukai K, Suzuki S. Cytolytic activity of natural killer cells and lymphokine activated killer cells against hepatitis A virus infected fibroblasts. J Clin Lab Immunol. 1993;40:47–60. [PubMed] [Google Scholar]
- 13.Walker D, Raoult D, Dumler JS, Marries T. Rickettsial diseases. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, et al., editors. Harrison’s principles of internal medicine. 15th ed. Tokyo: McGraw-Hill; 2001. pp. 1065–1073. [Google Scholar]
- 14.Chien RN, Liu NJ, Lin PY, Liaw YF. Granulomatous hepatitis associated with scrub typhus. J Gastroenterol Hepatol. 1995;10:484–487. doi: 10.1111/j.1440-1746.1995.tb01605.x. [DOI] [PubMed] [Google Scholar]


