Abstract
Osteoclasts are bone-resorbing multinucleated cells that undergo drastic changes in their polarization due to heavy vesicular trafficking during the resorption cycle. These events require the precise orchestration of membrane traffic in order to maintain the unique characteristics of the different membrane domains in osteoclasts. Rab proteins are small GTPases involved in regulation of most, if not all, steps of vesicle trafficking. The investigators studied RAB genes in human osteoclasts and found that at least 26 RABs were expressed in osteoclasts. Out of these, RAB13 gene expression was highly upregulated during differentiation of human peripheral blood monocytic cells into osteoclasts. To study its possible function in osteoclasts, the investigators performed immunolocalization studies for Rab13 and various known markers of osteoclast vesicular trafficking. Rab13 localized to small vesicular structures at the superior parts of the osteoclast between the trans-Golgi network and basolateral membrane domain. Rab13 localization suggests that it is not involved in endocytosis or transcytosis of bone degradation products. In addition, Rab13 did not associate with early endosomes or recycling endosomes labeled with EEA1 or TRITC-conjugated transferrin, respectively. Its involvement in glucose transporter traffic was excluded as well. It is suggested that Rab13 is associated with a putative secretory function in osteoclasts.
Keywords: osteoclast, bone resorption, Rab, Rab13, vesicle traffic
Bone is a metabolically active organ that is remodeled throughout life by bone-resorbing osteoclasts and bone-forming osteoblasts. Osteoclasts are highly polarized, multinucleated giant cells of hematopoietic origin that contain four functionally distinct membrane domains when activated for bone resorption. An activated osteoclast is attached to the bone surface via the sealing zone, which isolates the microenvironment of the resorption lacuna from the rest of the extracellular space (Lakkakorpi et al. 1989; Vaananen and Horton 1995). The osteoclast ruffled border is the bone-facing, highly convoluted membrane domain responsible for bone degradation. It is formed by the fusion of intracellular acidic vesicles resulting in several late endosomal features (Vaananen et al. 1990). Functionally, this membrane domain is divided into two subdomains: the peripheral vesicle fusion zone and the central degraded matrix uptake zone (Mulari et al. 2003). Osteoclasts secrete acid and proteolytic enzymes, such as cathepsin K, through the fusion zone to dissolve bone matrix in the resorption lacuna. Bone degradation products are endocytosed at the central part of the ruffled border and then transcytosed to the functional secretory domain for release into the non-bone-facing extracellular space (Salo et al. 1996, 1997; Nesbitt and Horton 1997). During this travel across the cell, bone degradation products may be further processed, possibly by tartrate-resistant acid phosphatase (TRACP), a widely used osteoclastic marker that produces reactive oxygen species (Halleen et al. 1999; Vaaraniemi et al. 2004). The changes in osteoclasts following their activation for bone resorption are dramatic, and the vesicular movements involved in the bone degradation process are extensive. Careful regulation of these events is required to create and maintain the distinct membrane domains. Members of the Rab subfamily of small GTPases are most likely involved in this regulation, although they may not be the only players.
Individual Rab proteins are localized to the cytoplasmic surface of distinct compartments in the cell via their C-terminal prenyl groups, although a given Rab protein may appear on multiple types of vesicles (Hutagalung and Novick 2011). Rab proteins provide both temporal and spatial regulation to membrane transport by cycling between their active GTP-bound and inactive GDP-bound state. This cycling, together with their effector proteins, determines the compartmental specificity of organelles in eukaryotic cells (Stenmark 2009). Members of the Rab protein family are known to be involved in all steps of the vesicle life cycle: vesicle budding, tethering, and docking of vesicles to their target compartments. Most recently, Rabs were found to associate with cytoskeletal elements and associated molecular motor proteins (Bielli et al. 2001; Wu et al. 2001; Sun et al. 2005; Grosshans et al. 2006). Although some reports have described the presence of specific Rabs in osteoclasts, no systematic study has been performed (Abu-Amer et al. 1999; Zhao et al. 2001, 2002; Pavlos et al. 2005). Important roles for some Rabs, such as Rab7 and Rab3d, in the formation of the ruffled border in osteoclasts have been proposed (Zhao et al. 2001; Pavlos et al. 2005). The essential role of this protein family in bone resorption has been confirmed further by the use of a specific inhibitor of Rab prenylation, 3-PEHPC (previously, NE10790), that inhibits bone resorption by causing severe morphological changes in osteoclasts (Coxon et al. 2001, 2005). Because the human genome is currently known to contain at least 60 different RAB genes (Seabra et al. 2002), there are most likely more members of this protein family that are important for osteoclastic membrane trafficking. In this study we addressed this question by screening the RAB genes for expression in human bone-resorbing osteoclasts. We then studied the expression levels of three RABs (RAB7, RAB13, and RAB32) during osteoclastogenesis and then chose to focus further studies on Rab13.
Materials and Methods
Osteoclast Culture
Human osteoclasts were differentiated from peripheral blood monocytic cells (PBMCs) as previously described (Husheem et al. 2005). Briefly, peripheral monocytic cells were isolated from buffy coats (obtained from the Finnish Red Cross) or heparinized blood of healthy volunteers by Ficoll-Paque Plus (Amersham Pharmacia Biotech, Uppsala, Sweden) centrifugation. After several washes with phosphate-buffered saline (PBS), 1 × 106 cells were plated on devitalized bovine cortical bone slices and allowed to differentiate in alpha-modified minimum essential medium (αMEM) (Gibco, Invitrogen Corporation, Glasgow, UK) supplemented with 10% heat-inactivated fetal calf serum (iFCS) (Gibco, Invitrogen Corporation), 20 ng/ml Receptor Activator of Nuclear Kappa-B-Ligand (RANK-L, PeproTech Inc, Rocky Hill, NJ), 10 ng/ml Macrophage-Colony Stimulating Factor (M-CSF, R&D Systems, Abingdon, UK), 10–8 M dexamethasone (Sigma-Aldrich, St Louis, MO), 10 ng/ml TNF-α (PeproTech Inc), and penicillin/streptomycin (Gibco, Invitrogen Corporation) for 15 days. An improved osteoclast differentiation assay included the isolation of CD14-positive PBMCs by magnetic separation (Miltenyi Biotech, Bergisch Gladbach, Germany). In this case, only 1 × 105 cells were plated per bone slice, and osteoclasts were differentiated in αMEM supplemented with 10% iFCS, 40 ng/ml RANK-L, 20 ng/ml M-CSF, and penicillin/streptomycin for 10 days. Half of the medium was replaced every 3 or 4 days. The differentiation of PBMC into actively resorbing osteoclasts was monitored by the formation of actin rings (Lakkakorpi and Vaananen 1991) that appeared at day 8 or day 5 of the culture depending on whether osteoclasts were differentiated from the whole PBMC (day 8) or the CD 14-positive cell fractions (day 5).
All animal experiments were carried out in accordance with the guidelines of the Central Animal Laboratory of the University of Turku. Rat osteoclasts were isolated as described previously (Lakkakorpi et al. 1989). Osteoclasts were mechanically removed from the long bones of 1- to 3-day-old rats and cultured on bovine bone slices in aMEM supplemented with 10% iFCS and penicillin/streptomycin for 48 hr.
Total RNA Isolation and Reverse Transcription
Total cellular RNA for PCR was isolated from differentiated human osteoclasts on bovine bone slices with the GenElute mammalian total RNA Miniprep Kit (Sigma-Aldrich) and for real-time PCR experiments with the Total RNA Extraction kit (Qiagen, Hilden, Germany). RNA concentration and quality were measured by UV absorbance. The cDNA was synthesized from purified RNA prior to the PCR reactions using the first strand synthesis reagents from the SMART PCR cDNA Synthesis Kit (BD Biosciences Clontech, San Jose, CA) with oligo-dT primers according to the supplier’s instructions.
Primer Design and Polymerase Chain Reactions
Primer pairs were designed for Rab proteins listed by M.C. Seabra in 2002 (Seabra et al. 2002). Rabs that are involved in the regulation of basic functions of the cells (e.g., ER to Golgi trafficking) and thus considered ubiquitously expressed (e.g., Rab1, 2, and 6 isoforms) were omitted from the screen. Some Rabs already described in rat osteoclasts were also analyzed in human osteoclasts (e.g., Rab7 and 9), whereas others were excluded (Rab1b, 4b, 5c, 11b, 35, and 6); (Zhao et al. 2002; Taylor et al. 2007). The selected 43 RAB mRNA sequences for primer design were found in two public, international databases: NCBI GenBank and Ensembl. Each primer pair was designed to be specific for one RAB sequence and to span at least two exons, thus avoiding possible genomic DNA amplification. Primer sequences and expected PCR product molecular sizes are available as supplemental material (Suppl. Table S1). Oligonucleotides were purchased from Sigma-Genosys Ltd (Cambridgeshire, UK) and TAG Copenhagen A/S (Copenhagen, Denmark). PCR reactions were optimized for each primer pair. The 25-µl reactions consisted of 0.2 µM dNTP mix, 0.2 µM primers, approximately 1 ng of human osteoclast cDNA, and 0.5 units of Advantage 2 PCR enzyme (Clontech Laboratories Inc, Mountain View, CA). PCR reactions were incubated in a thermal cycler (Eppendorf Mastercycler Gradient, Hamburg, Germany) with 33 cycles of denaturation at 94C for 30 sec, hybridization for 30 sec, and elongation at 70C for 45 sec. PCR products were separated by electrophoresis on a 1.2% (w/v) agarose gel and DNA bands visualized by staining with ethidium bromide. All PCR products of expected size were confirmed by sequencing at the sequencing laboratories of the Turku University Genetics Institute or the Center for Biotechnology (Turku, Finland).
Real-Time PCR
Total RNA was isolated from human osteoclasts differentiated from CD14-positive PBMCs at four time points, days 0, 3, 6, and 10, and reverse transcribed as described above. Real-time PCR reactions were performed using the DNA Engine Opticon System (MJ Research, San Francisco, CA) with the Quantitect SYBR Green PCR Kit (Qiagen, Hilden, Germany) in the presence of 0.25 µM primer and approximately 1 ng of one of the human osteoclast cDNA templates. After enzyme activation at 95C for 15 min, the PCR reactions were cycled 40 times at 94C for 30 sec followed by 30 sec of annealing and elongation at 72C for 45 sec. The fluorescence signal was read at the end of each elongation step. The specificity of each PCR product was ascertained by performing melting curves after cycling and agarose gel electrophoresis (not shown). For relative quantitation, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene, and the relative expressions were calculated by comparing the Ct value of the gene of interest to the Ct value of the reference gene within a sample. Day 0 values were chosen to represent 1-fold expression of each gene, and all other samples were compared with this using the relative real-time quantitative PCR analysis method 2-ΔΔCt (Livak and Schmittgen 2001). Greater than 2-fold upregulation of gene expression was considered significant. Data presented are one example of three independent experiments.
Immunofluorescence
Double- and triple-staining experiments were performed to study the intracellular localization of Rab13 in rat and human osteoclasts. Polyclonal rabbit anti-Rab13 antibody was a generous gift from Dr Simone Di Giovanni (Di Giovanni et al. 2005). Monoclonal mouse anti-cathepsin K antibody was from Fuji-Chemical Industries (Japan). Tartrate-resistant acid phosphatase was visualized with monoclonal anti-TRACP antibody DB-13Z1 (Kaija et al. 1999). Glucose transporters 1 and 6 were visualized with monoclonal antibodies from Abcam (Cambridge, UK) and Novus Biologicals (Cambridge, UK), respectively. Mouse monoclonal anti-TGN38 and anti-EEA1 antibodies were obtained from BD Transduction Laboratories (Lexington, KY). Tetramethylrhodamine-conjugated transferrin, Alexa Fluor 488-conjugated phalloidin, and TO-PRO-3 were purchased from Molecular Probes (Eugene, OR). The secondary antibodies used were FITC- and TRITC-conjugated donkey anti-mouse and anti-rabbit immunoglobulins from Jackson ImmunoResearch Laboratories (West Grove, PA). The 5-(6)-carboxyfluorescein succimidyl ester used to fluorescently label surface of bovine bone slices was purchased from Molecular Probes.
Cultured rat and human osteoclasts were rinsed once with PBS and permeabilized with 0.05% saponin in 80 mM K-Pipes, pH 6.8, 5 mM EGTA, and 1 mM MgCl2 prior to fixation with 3% paraformaldehyde in PBS for 20 min. Free aldehyde groups were quenched with 50 mM NH4Cl in PBS for 10 min. All antibody incubations and washing steps were performed in PBS containing 0.2% BSA and 0.05% saponin. Nonspecific binding was blocked with 2% BSA before incubation with primary antibodies. Primary antibody binding was visualized using fluorescent dye-conjugated secondary antibodies and labeled cells observed with a Leica fluorescence microscope and a Leica TCS-SP confocal laser scanning microscope equipped with an Argon-Krypton laser (Leica Microsystems, Mannheim, Germany). Confocal images were acquired by scanning all channels separately to avoid bleed-through. More than 20 osteoclasts were analyzed in at least two separate experiments for each marker.
Immunohistochemistry
Rat long bones were removed from rats aged 1–3 days and immersion fixed in 4% paraformaldehyde overnight. After formic acid decalcification, the bones were processed for immunohistochemistry, embedded in paraffin, and cut into 5-µm sections with a microtome. Immunohistochemical stainings were performed according to the following protocol: after rehydration the slides were incubated in hot 1.5 mM EDTA (pH 9.0) for 5 min and cooled on ice for 15 min. After washing with 0.9% NaCl, the slides were dipped in ice cold acetone:acetic acid (1:1) for 1 min and washed with PBS. Endogenous peroxidase activity was blocked by 3% H2O2 in methanol treatment, and nonspecific antibody reactions were blocked with SuperBlock buffer (Pierce, Rockford, IL). Endogenous biotin-binding sites were blocked using the Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA). Following overnight incubation with primary antibodies, slides were incubated with streptavidin-conjugated, species-specific secondary antibodies (Vector Laboratories) and detected with ABC and DAB reactions (Standard ABC Elite and Peroxidase Substrate Kits, Vector Laboratories). Mayer hematoxylin was used as a counterstain.
Western Blot
Rat osteoclasts were purified from newborn rat long bones by magnetic separation as previously described (Zhao et al. 2002). Briefly, mouse anti-rat b3-integrin monoclonal antibody (a gift from Dr. M.A. Horton, University College London, UK) was adsorbed to sheep anti-mouse IgG magnetic Dynabeads (Invitrogen Dynal, Oslo, Norway) overnight at 4C. Bone marrow cells were mechanically removed from long bones of rat pups into αMEM and incubated with labeled beads for 20 min. After extensive washing, isolated osteoclasts and bone marrow cells deprived of osteoclasts (non-osteoclasts) were lysed in RIPA buffer. For each sample, equal amounts of total protein (15 micrograms) of samples were loaded on a 12% SDS-polyacrylamide gel and transferred to PVDF membrane (BioRad, Hercules, CA). Following the transfer, membranes were blocked in 5% skim milk, incubated with polyclonal rabbit anti-Rab13 antibody, HRP-conjugated swine anti-rabbit immunoglobulin (DAKO, Glostrup, Denmark), and developed using an enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Buckinghamshire, UK).
Results
RAB Gene Expression in Human Osteoclasts
The vesicular trafficking pathways in osteoclasts during bone resorption and cell polarization have recently been characterized (Nesbitt and Horton 1997; Salo et al. 1997; Palokangas et al. 1997; Mulari et al. 2003), but the regulation of these events remains mostly unknown. Earlier we used degenerate primers to identify RAB genes expressed in rat osteoclasts and found seven Rab family members (Zhao et al. 2002). To gain further insight into the regulation of vesicular trafficking events in osteoclasts, we decided to screen the RAB genes for expression in resorbing human osteoclasts differentiated on bovine bone slices. At the time of cell lysis for RNA isolation, osteoclast cultures contained multinuclear resorbing osteoclasts that were demonstrated by phalloidin staining of the actin rings, indicators of active bone resorption (Lakkakorpi and Vaananen 1991) (not shown). Using specific primer pairs for the selected 43 human RAB genes, we observed the expression of 26 of the RABs in resorbing human osteoclasts differentiated from PBMCs (Fig. 1, Table 1) by PCR. The expression of several members of the Rab protein family in osteoclasts reflects the high volume of membrane traffic present during osteoclast polarization and bone resorption as well as the precise orchestration of these events.
Figure 1.
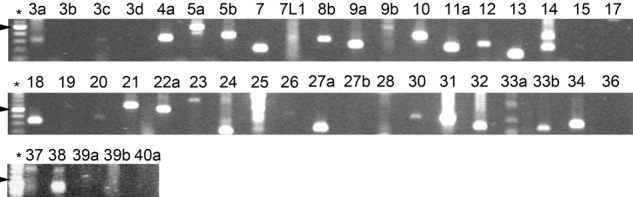
Expression of RAB genes in human osteoclasts. Osteoclasts were differentiated from PBMCs for 15 days, total RNA was isolated, and expression of RAB genes was detected with gene-specific primer pairs by PCR. Agarose gel electrophoresis of RAB PCR products, where a number designates the RAB number and an asterisk represents the 100-bp DNA ladder. Arrowheads indicate the 500-bp band. Expected PCR product sizes are listed in a supplementary table.
Table 1.
Expression of RAB Genes in Human Osteoclasts
| Present | Not Detected | ||
|---|---|---|---|
| Rab3c | Rab20 | Rab3a | Rab37 |
| Rab4a | Rab21 | Rab3b | Rab39a |
| Rab5a | Rab22a | Rab3d | Rab39b |
| Rab5b | Rab23 | Rab7L1(29) | Rab40 |
| Rab7 | Rab24 | Rab9b | |
| Rab8b | Rab27a | Rab15 | |
| Rab9a | Rab30 | Rab17 | |
| Rab10 | Rab31 | Rab19 | |
| Rab11a | Rab32 | Rab25 | |
| Rab12 | Rab33a | Rab26 | |
| Rab13 | Rab33b | Rab27b | |
| Rab14 | Rab34 | Rab28 | |
| Rab18 | Rab38 | Rab36 | |
RAB13 Expression Is Induced during Osteoclast Differentiation
Because of their expression in osteoclasts and the described functions in other cell types, RAB7, 13, and 32 were selected for expression analysis by real-time PCR to estimate their functional importance in osteoclast biology. Rab7 is a late-endosome- and lysosome-associated Rab protein that is directed almost exclusively to the ruffled border in resorbing osteoclasts and is essential for the ruffled border formation and bone resorption (Palokangas et al. 1997; Zhao et al. 2001). When we compared RAB7 expression during osteoclast differentiation and activation for bone resorption, we observed only slight changes (Fig. 2). Rab32 is a recently described protein involved in mitochondrial dynamics and melanosome transport (Alto et al. 2002; Wasmeier et al. 2006). As mitochondria are abundant in bone-resorbing osteoclasts, we followed RAB32 expression during osteoclastogenesis. It was upregulated approximately 2-fold, but the expression pattern of RAB32 fluctuated during the osteoclast differentiation unlike that of RAB7 (Fig. 2). Rab13 associates with tight junctions in polarized epithelial cells (Zahraoui et al. 1994), and as it was expressed in osteoclasts, we speculated it has a related function in osteoclasts regulating the formation of the sealing zone, the tight attachment of the resorbing cell to the bone surface. Interestingly, the expression of RAB13 dramatically increased by up to 20-fold during osteoclast differentiation. The signal for this gene was almost non-detectable in monocytes at day 0, but it increased by day 3 (Fig. 2). Bone-resorbing multinucleated osteoclasts begin to appear and resorb bone at day 5 in this culture system. These expression patterns were supported by our Affymetrix microarray data that followed universal gene expression levels during human osteoclast differentiation (H. Vuorikoski et al. manuscript in preparation).
Figure 2.
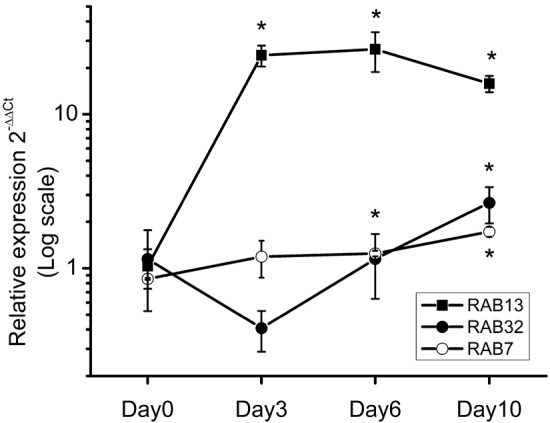
Expression of RAB7, RAB13, and RAB32 during osteoclast differentiation of CD14-positive PBMCs followed by real-time PCR. Expression levels of each gene in CD14-positive monocytes (day 0) are set to one and then other time points are compared with that, as indicated in the Materials and Methods. Changes greater than 2-fold are considered significant (*). Rab13 expression increased during osteoclastogenesis.
Rab13 and Bone Resorption Markers in Osteoclasts
To gain further insight into the possible role of Rab13 in osteoclasts, we studied its subcellular distribution by immunofluorescence. We detected endogenous Rab13 in osteoclasts of rat and human origin using purified rabbit polyclonal anti-Rab13 antibody kindly provided by Dr. S. Di Giovanni and Dr. Alan Faden (Di Giovanni et al. 2005). This antibody detects one major band of approximately 25 kDA in several rat tissues and recognizes bacterially expressed glutathione S-transferase (GST)-tagged Rab13 but not Rab7, 11, or 32 (Suppl. Fig. S1). We observed labeling of cell–cell contacts as well as numerous small vesicles in the cytoplasm at perinuclear areas and cell periphery in HeLa cells (Suppl. Fig. S3). These observations are in agreement with the previous reports of Rab13 localization (Zahraoui et al. 1994; Marzesco et al. 2002). In osteoclasts cultured on bone, Rab13 antibody detected numerous small vesicles throughout the cytoplasm, whereas no labeling without the primary antibody could be observed (Suppl. Fig. S2). Because Rab13 is known to associate with tight junctions in polarized epithelial cells (Marzesco et al. 2002), we first tested our initial hypothesis of Rab13 regulating the formation of the tight attachment of osteoclasts to the bone surface and its location at the sealing zone. This area is characterized by the formation of thick actin bundles in a ring-like structure and can be visualized by phalloidin staining (Lakkakorpi and Vaananen 1991). Osteoclasts from rat bone marrow were cultured on bovine bone slices and stained for Rab13 after 48 hr of culture. Rab13 antibody identified a finely granulated vesicle population at the upper perinuclear and peripheral parts of osteoclasts (Fig. 3A–D), but these Rab13-positive vesicles did not associate with the sealing zone area. Similar results were obtained from human osteoclasts differentiated from PBMCs (data not shown). This result suggests that Rab13 is not involved in the formation of the tight attachment of osteoclasts to the bone surface and instead may have some other function.
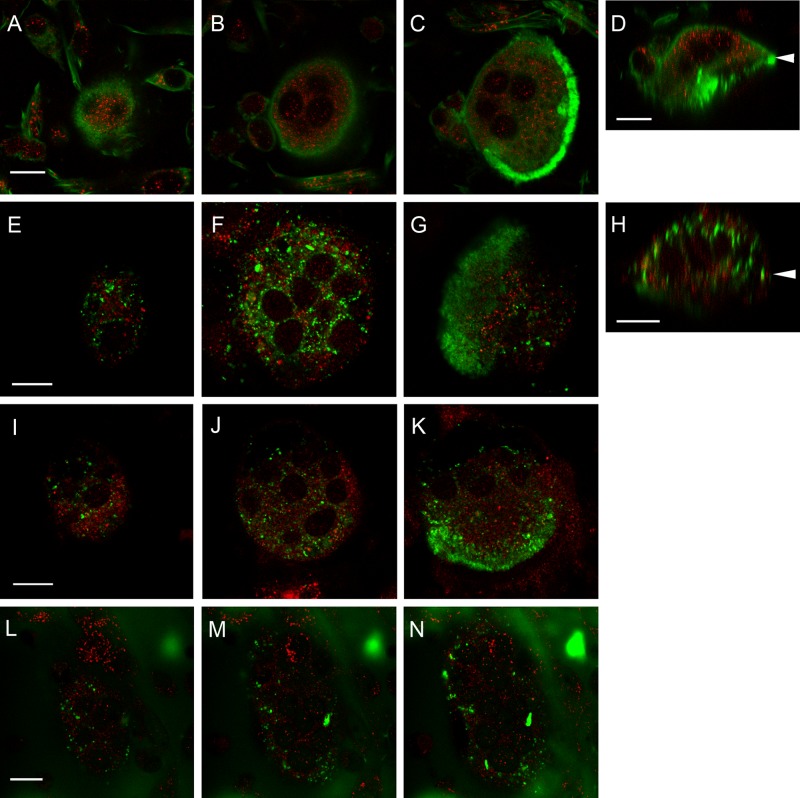
Figure 3.
Rab13 does not colocalize with the osteoclastic markers, F-actin (A–D), TRACP (E–H), cathepsin K (I–K), or FITC-labeled bone (L–N). Rat osteoclasts cultured on bovine bone slices were fixed and immunolabeled for Rab13 (red) and the indicated markers for bone resorption (green). Pictures represent single-optical confocal microscopic sections from the superior part of the cell (A, E, I, L), the nuclear level (B, F, J, M), and the ruffled border area (C, G, K, N). Bone surface is marked by white arrowheads in vertical sections (D and H). Scale bars = 10µm.
To evaluate whether Rab13 is involved in resorption in osteoclasts, we double-stained rat bone marrow osteoclasts with different resorption-specific markers. TRACP has previously been shown to localize mostly to intracellular vesicles and partly associate in the large transcytotic vesicles that transport bone degradation particles from the ruffled border to the functional secretory domain (Halleen et al. 1999; Vaaraniemi et al. 2004); additionally, some studies detected TRACP in the ruffled border and the resorption lacuna (Reinholt et al. 1990; Fukushima et al. 1991). In rat osteoclasts, both TRACP and Rab13 localized to perinuclear vesicles in the upper parts of the cells, but the two markers were completely distinct and no colocalization was observed. TRACP-containing vesicles were usually larger than the Rab13-positive vesicles (Fig. 3E–H). In some cells, we detected TRACP at the ruffled border area, whereas very little Rab13 was present in the lower part of the cell (Fig. 3G).
During bone resorption, osteoclasts secrete cathepsin K, the most abundant cysteine protease in osteoclasts capable of organic matrix degradation, in the resorption lacuna. Strong cathepsin K staining was observed at the ruffled border (Fig. 3K), where it is secreted via the biosynthetic route (Baron et al. 1988). It was also present in vesicles at the medial and upper parts of the cells that most likely represent the portion of the secreted cathepsin K that is endocytosed from the resorption lacuna together with the bone degradation products (Vaaraniemi et al. 2004) (Fig. 3I–K). Rab13 was absent from these cathepsin K-positive vesicles, and we did not detect colocalization of Rab13 and cathepsin K in bone-resorbing osteoclasts.
Finally, to determine whether Rab13 was involved in the uptake and transcytosis of the degraded bone particles through the cell to the functional secretory domain for release to the extracellular space, we cultured rat osteoclasts on FITC-labeled bone slices. These osteoclasts were highly active, and several large transcytotic vesicles were observed being transported from the ruffled border to the functional secretory domain (Fig. 3L–N). Rab13 did not colocalize with these vesicles at any level of the cell, indicating that Rab13 is not involved in the initial uptake of bone degradation products from the resorption lacuna or in the further processing of them during transcytosis prior to their release at the functional secretory domain.
Rab13 and Endosomal Markers in Osteoclasts
Localization of Rab13 in endosomal vesicles of resorbing osteoclasts was studied by double-staining experiments with transferrin and early endosomal antigen (EEA1). Transferrin is a commonly used marker for the endosomal recycling pathway, and the route of endocytosed iron-loaded transferrin in bone-resorbing osteoclasts has been well described (Palokangas et al. 1997; Mulari et al. 2003). It is endocytosed on the basolateral, non-bone-facing plasma membrane domain and reaches the perinuclear recycling compartment following 10 min of incubation. After 30 min of incubation, intensive accumulation of transferrin is seen in the peripheral subdomain of the ruffled border. This accumulation most likely resembles the fraction of transferrin that is directed to the late endosome in other cell types. After longer incubation times, transferrin can also be detected at the functional secretory domain area. To observe the entire transferrin route in osteoclasts, we incubated human osteoclasts differentiated from PBMCs in the presence of TRITC-labeled human transferrin for 60 min and stained them with Rab13 antibody. At this time point, transferrin was detected in early endosomes close to the basolateral plasma membrane, the circumnuclear recycling compartment, at the ruffled border and the functional secretory domain. Rab13 antibody labeled perinuclear vesicles but did not colocalize with the transferrin-containing vesicles (Fig. 4A–D).
Figure 4.
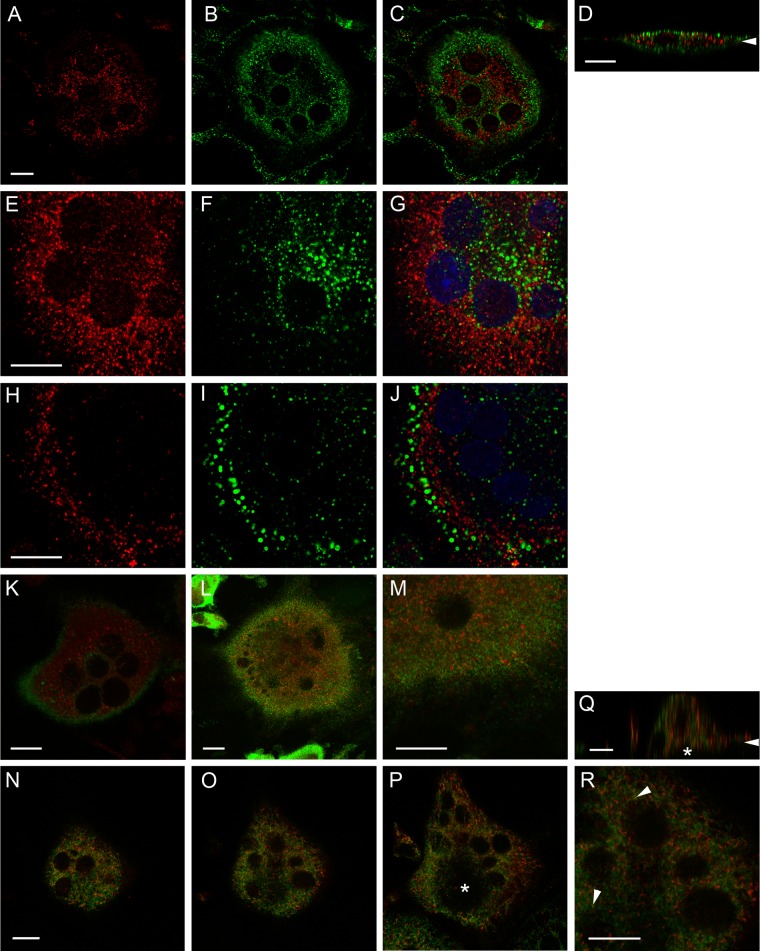
Rab13 is not localized to endosomes or recycling endosomes nor is it involved in glucose transporter traffic in osteoclasts. Rab13 (red) and the indicated markers (green) were immunolabeled in human osteoclasts on bovine bone slices. Osteoclasts were incubated with TRITC-labeled transferrin (pseudocolored green) for 60 min to visualize recycling endosomes prior to fixation (A–D). Early endosomes were marked by immunolabeling with anti-EEA1 antibody, and nuclei were visualized by TO-PRO-3 staining (blue) (E–J). The distribution of early endosomes was perinuclear in non-resorbing (E–G) and more peripheral in resorbing (H–J) osteoclasts. The resorptive status of the observed osteoclasts was estimated by cell morphology; active cells are thick and round whereas inactive osteoclasts are planar. Rab13 does not associate with the glucose transporters Glut1 (K) or Glut6 (L-M) in bone-resorbing osteoclasts. Rab13 colocalization with TGN38, a marker for TGN (N-R), is weak (R, arrowhead). An asterisk marks the ruffled border (P and Q). Images represent single-optical confocal microscopic sections at the nuclear level. The bone surface is marked by arrowheads in vertical sections (D and Q). Scale bars = 10µm.
Finally, to determine whether Rab13 could regulate the early phases of endocytosis, we labeled human osteoclasts with EEA1 antibody, a marker for early endosomes and a well-known Rab5 effector protein. In agreement with our previous studies (Zhao et al. 2002), EEA1 was detected in cytoplasmic vesicular structures throughout the cell. Interestingly, a strong difference in the distribution of EEA1-containing vesicles was observed between resorbing and non-resorbing osteoclasts. In inactive osteoclasts, EEA1-positive vesicles were mostly located in the central parts of the cell between the nuclei (Fig. 4E–G), whereas in active osteoclasts, early endosomes were located more peripherally (Fig. 4H-J). This change in the distribution of early endosomes to the cell periphery may reflect the early phases of endocytic delivery of basolateral membranes to the ruffled border in bone-resorbing osteoclasts (Palokangas et al. 1997). In resorbing osteoclasts, Rab13-positive vesicles were also located more peripherally than those in inactive osteoclasts, but the change was not as dramatic as was observed with EEA1. Rab13 staining did not colocalize with EEA1 staining (Fig. 4E-J). Thus, Rab13 is most likely not involved in the early phases of endocytosis in bone-resorbing osteoclasts.
Rab13 Is Not Associated with Glut1 or Glut6 Glucose Transporters in Osteoclasts
Rab13 was recently reported to regulate the translocation of the insulin-sensitive glucose transporter, Glut4 (Sun et al. 2010). Because osteoclast formation and bone resorption are energy-intensive processes and the principal energy source for this process is glucose (Williams et al. 1997), we investigated whether Rab13 is involved in glucose transport in osteoclasts. We did not detect expression of Glut4 in osteoclasts (data not shown), but osteoclasts and osteoclast-like cells have been shown to express Glut1 (Kim et al. 2007; Knowles and Athanasou 2008). In our experiments, Glut1 was mainly located on the plasma membrane in bone-resorbing osteoclasts, but some Glut1-positive vesicular structures were observed. However, these vesicular structures were distinct from the Rab13 vesicles (Fig. 4K). We also determined whether Rab13 colocalized with Glut6, originally found in leucocytes (Doege et al. 2000). Glut6 was located in small intracellular vesicles throughout the cell in bone-resorbing osteoclasts, but these vesicles did not colocalize with the Rab13 vesicles (Fig. 4L–M).
Rab13 Colocalization with TGN38 Is Light in Osteoclasts
Rab13 localization to the biosynthetic route was studied by its colocalization with TGN38, an integral membrane protein that predominantly locates in the trans-Golgi network (TGN) but is also cycled to the plasma membrane in some cell types but not in rat osteoclasts (Palokangas et al. 1997). TGN38 was located in perinuclear structures in resorbing human osteoclasts, as previously reported. Although we observed a similar distribution of these markers in bone-resorbing cells (Fig. 4N–Q), higher magnifications revealed only weak colocalization in a few vesicles per cell (Fig. 4R). The ruffled border was devoid of both Rab13 and TGN38 (Fig. 4P–Q, asterisk).
Inhibition of Rab13 Expression Does Not Affect Osteoclast Differentiation
The role of Rab13 on osteoclastogenesis was evaluated by inhibiting its expression by electroporation of the osteoclast precursors with specific siRNA molecules (Taylor et al. 2007). With these methods, we observed a minor trend toward a reduced number of resorbing cells in Rab13 siRNA-treated cells compared with the negative control siRNA-treated cells, but the difference did not reach statistical significance (Suppl. Fig. S4C). However, we also observed a decrease in the viability of Rab13 siRNA-transfected cells, which at least in part may explain the difference in the numbers of actin rings in these cells (Suppl. Fig. S4B). These experiments may suggest, however, that the impairment of Rab13 expression has only a minimal—if any—effect on the differentiation and resorption activity of osteoclasts.
Rab13 Expression in Osteoclasts In Vivo
Sections of long bones from newborn rats were immunostained to confirm the presence of Rab13 in osteoclasts in vivo. Sequential sections were stained for Rab13 and cathepsin K to identify osteoclasts (Fig. 5B–D). Controls were mock labeled without primary antibody (Fig. 5A). Multinuclear osteoclasts were observed on bone trabeculae (Fig. 5A–D) with strong cathepsin K staining through the cell including the ruffled border (Fig. 5B, arrowhead). Osteoclasts were Rab13 positive, but the staining was not restricted to osteoclasts; a fraction of the mononuclear cells of the bone marrow, as well as the cells lining the bone trabeculae, also expressed Rab13 (data not shown). Rab13 staining was located in the medial and superior parts of the osteoclasts, whereas the ruffled border facing the bone was almost devoid of Rab13 (Fig. 5C, D, arrowhead).
Figure 5.
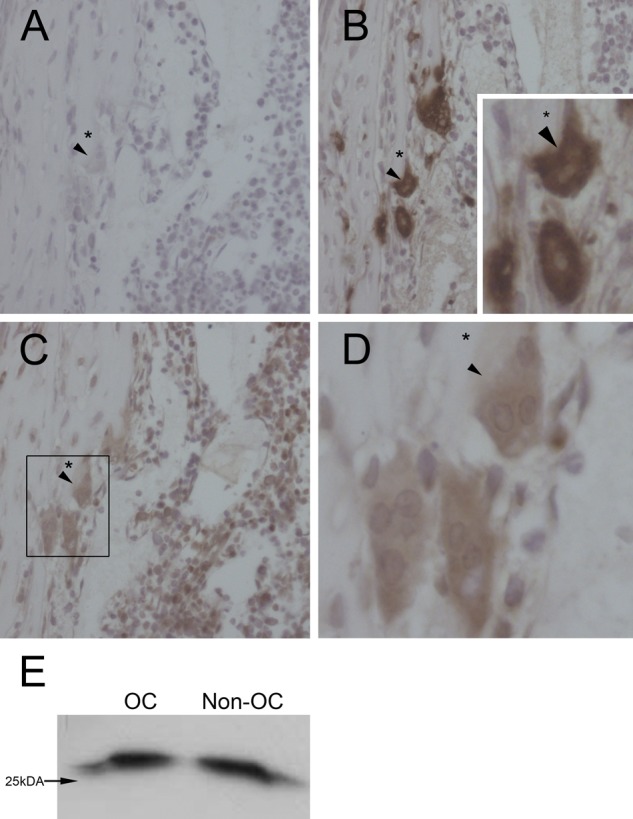
Rab13 is expressed in rat long bones in vivo. Rat long bones were processed for immunohistochemistry as described in the Materials and Methods. Sequential slices were incubated without primary antibody (A), with anti-cathepsin K antibody to indicate osteoclasts (B), or with anti-Rab13 antibody (C, D). Rab13 staining was restricted to the superior part of osteoclasts, excluding the ruffled border, whereas strong labeling of cathepsin K was detected also at the lower parts of osteoclasts (arrowheads). Asterisks mark the bone matrix under the osteoclasts. Some of the mononuclear cells in the bone marrow were also Rab13-positive. Rat osteoclasts were purified from bone marrow of newborn rat long bones by magnetic separation using the vitronectin receptor antibody, lysed, and immunoblotted with anti-Rab13 antibody. Rab13 expression was detected in both fractions, osteoclasts (OC) and non-osteoclasts (Non-OC) (E). Equal amounts of total protein were loaded per lane (15µg).
Osteoclasts were affinity-purified from the bone marrow of newborn rat long bones, lysed, and processed for immunoblotting. Consistent with the results from immunohistochemistry, Rab13 was detected in purified osteoclasts (OC) as well as in the remaining cell population of the bone marrow (non-OC) (Fig. 5E). Therefore, we concluded that Rab13 is also present in osteoclasts in vivo.
Discussion
Bone resorption by osteoclasts is a complex process requiring strong cellular polarization and extensive vesicular trafficking. Rab GTPases serve as molecular switches that provide specificity to vesicular trafficking and determine the identity and characteristics of the cellular organelles. Expression of some Rab proteins (Rab1B, Rab4B, Rab5C, Rab9, Rab35, Rab6, Rab7, and Rab3 isoforms) (Abu-Amer et al. 1999; Zhao et al. 2001, 2002; Pavlos et al. 2005; Taylor et al. 2007) has been described in osteoclasts, but only the functions of Rab7 and Rab3d have been described in greater detail (Zhao et al. 2001; Pavlos et al. 2005).
To gain further insight into the regulation of osteoclast vesicle trafficking, we determined which RAB genes are expressed in bone-resorbing human osteoclasts differentiated in cell culture from human monocytic cells. We found that 26 RAB genes are expressed in osteoclasts. Osteoclasts expressed several exocytic Rabs (Wixler et al. 2011), RAB8b, RAB10, RAB12, RAB13 and RAB18, indicating high levels of secretion, but we were not able to detect expression of the previously described RAB3 isoforms in human osteoclasts. This might be due to our inability to design efficient primer pairs, despite several attempts. Interestingly, we also observed the expression of RAB27a, RAB32, and RAB38 in human osteoclasts. These Rabs have previously been shown to regulate the specialized lysosomal secretion of melanin in melanocytes (Bahadoran et al. 2001; Wasmeier et al. 2006),
The expression of Rab7 is essential for the formation of the ruffled border and bone resorption (Zhao et al. 2001). The observed minor change in the expression of RAB7 during osteoclast differentiation may indicate that Rab7 is retargeted to the ruffled border from the late endosomal compartment as monocytic cells start to differentiate to osteoclasts, whereas the mRNA expression levels do not vary during this process. The expression of RAB32, a mitochondria- and melanosome-associated protein (Alto et al. 2002; Wasmeier et al. 2006), was also induced during osteoclastogenesis, which is in agreement with the abundance of mitochondria in osteoclasts. Studies on Rab32 at the protein level are needed, however, to reveal its role in osteoclast function.
RAB13 expression increased during osteoclast differentiation in culture, and its expression pattern was similar to the described expression pattern of TRACP (Alatalo et al. 2000). Our microarray experiments seem to indicate that RAB13 expression is not restricted to osteoclasts but is present in all macrophage-related cells, although the expression levels are somewhat higher in osteoclasts (H. Vuorikoski et al., manuscript in preparation). Rab13 was originally described as localizing to tight junctions in polarized epithelial cells and to vesicular structures in non-polarized cells (Zahraoui et al. 1994). Overexpression of the constitutively active, GTP-bound form of Rab13 has been shown to inhibit the assembly of tight junctions (Marzesco et al. 2002). Rab13 affects the transport of Claudin-1 to the cell surface in non-polarized BHK cells and regulates the endocytic recycling of occludin in both fibroblastic BHK and epithelial MTD-1A cells (Marzesco et al. 2002; Yamamoto et al. 2003; Morimoto et al. 2005). Rab13 has also been shown to interact directly with the catalytic subunit of PKA, inhibit its activity, and prevent phosphorylation of vasodilator-stimulated phosphoprotein, a key actin remodeling protein located in tight junctions in MDCK kidney cells (Kohler et al. 2004). Emerging data indicate more versatile functions for Rab13 in several other cell types. In regenerating neurons, Rab13 is localized to growth cones, and its downregulation was shown to inhibit neurite extension in PC-12 neuronal-like cells (Di Giovanni et al. 2005). Most recently, Rab13 was shown to be activated by insulin and regulate the traffic of the glucose transporter Glut4 to the cell surface in rat L6 muscle cells (Sun et al. 2010).
Our results show that Rab13 is expressed in human osteoclasts in vitro and in rat osteoclasts in vivo. In resorbing osteoclasts, Rab13 localized to a distinct vesicle population in the vicinity of the nuclei in medial sections of the cell, but it did not colocalize with the sealing zone or the commonly used bone resorption markers, TRACP, cathepsin K, and FITC-labeled bone. The fact that the ruffled border had only slight Rab13 staining suggests that Rab13 is not involved in the resorption process itself but may have some other function in osteoclasts.
Bone resorption by osteoclasts is an energy-intensive process, indicated by the abundance of mitochondria in these cells. The principal energy source for bone degradation is glucose, and osteoclasts cultured on bone transport glucose at almost twice the rate of those cultured on other substrates (Williams et al. 1997). In light of the recent finding of the role of Rab13 in regulating the insulin-sensitive transport of Glut4 to the plasma membrane in muscle cells, we speculated that it might have an analogous function in osteoclasts, but neither we nor others (Kim et al. 2007) detected Glut4 mRNA or protein expression in osteoclasts (data not shown). However, osteoclasts and osteoclast precursors have been described as expressing Glut1 (Kim et al. 2007; Knowles and Athanasou 2008). Rab13 did not colocalize with Glut1 or Glut6, the two glucose transporters tested in our study; thus, it may not be involved in glucose transport in bone-resorbing osteoclasts.
The secretory pathways described in osteoclasts thus far have mostly been related to bone resorption. Vesicular traffic is directed to the ruffled border, where proteolytic enzymes and acid are secreted to resorption lacuna, or to the functional secretory domain, where TRACP and bone degradation products are released (Salo et al. 1997; Mulari et al. 2003). l-Glutamate secretion by osteoclasts was described recently, but this occurs with the release of bone degradation products at the functional secretory domain (Morimoto et al. 2006). Rab13 does not seem to participate in these events. The localization of Rab13 and the fact that it did not colocalize with the endosomes suggest that Rab13 is involved in the specialized trafficking of a novel vesicle type, possibly secretory, between the TGN and basolateral membrane in osteoclasts. In transfected MDCK cells, Rab13 partially colocalizes with transferrin receptor and regulates the biosynthetic transport from the TGN to the plasma membrane via the recycling endosome (Nokes et al. 2008). However, the Rab13-mediated recycling of occludin to the cell surface in MTD-1A and BHK cells is independent of the transferrin receptor-labeled recycling endosomes (Morimoto et al. 2005). In osteoclasts, the putative Rab13-regulated secretory route must be distinct from the recycling endosomes, as we did not observe any colocalization of Rab13 with transferrin-labeled recycling compartments. The observed weak localization of Rab13 to TGN38-labeled compartments may arise from fast sorting of the secretory cargo at the TGN to its destination in bone-resorbing osteoclasts, thus making Rab13 localization at the TGN very transient. Clearly, osteoclasts and epithelial cells are essentially different, as demonstrated by the unique organization of the plasma membrane domains in osteoclasts. The designated function of a Rab in a cell type is highly dependent on the presence of a specific array of Rab effector proteins that confer specificity to the Rab protein. Therefore, we may find a completely novel function for Rab13 in hematopoietic cells once its effectors are identified.
Inhibition of Rab13 expression by electroporation of specific siRNA molecules into the precursor cells did not produce significant differences in osteoclast differentiation. This may indicate that Rab13 is not directly linked to the differentiation of osteoclasts, but it does not exclude a possible functional importance of Rab13, for instance, for cell–cell communication or some other cellular function needed for proper osteoclast function in vivo. Alternatively, a compensating mechanism may exist.
In conclusion, the regulators of the vesicular trafficking pathways in bone-resorbing osteoclasts are mostly unknown. We addressed this question by identifying several RABs that are expressed in osteoclasts, but studies at the protein level are needed to reveal the function of individual Rab proteins during bone resorption. RAB13 expression increased during osteoclastogenesis, leading us to investigate its role in bone resorption further. We have excluded its presence in either the endocytotic or transcytotic pathways in bone resorption and its involvement in glucose transport. The distribution of Rab13 in bone-resorbing osteoclasts suggests its association with vesicles transporting cargo between the TGN and the basolateral membrane, possibly for specialized secretion or membrane protein delivery. We suggest that Rab13 is located in a specific vesicular compartment that remains to be identified in osteoclasts.
Supplementary Material
Acknowledgments
We are grateful to Teuvo Hentunen for his critical reading of the manuscript.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Turku University Doctoral Programme of Biomedical Sciences and grants from the Academy of Finland and Sigrid Juselius Foundation.
Supplementary material for this article is available on the Journal of Histochemistry and Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
Literature Cited
- Abu-Amer Y, Teitelbaum SL, Chappel JC, Schlesinger P, Ross FP. 1999. Expression and regulation of RAB3 proteins in osteoclasts and their precursors. J Bone Miner Res. 14:1855–1860 [DOI] [PubMed] [Google Scholar]
- Alatalo SL, Halleen JM, Hentunen TA, Monkkonen J, Vaananen HK. 2000. Rapid screening method for osteoclast differentiation in vitro that measures tartrate-resistant acid phosphatase 5b activity secreted into the culture medium. Clin Chem. 46: 1751–1754 [PubMed] [Google Scholar]
- Alto NM, Soderling J, Scott JD. 2002. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J Cell Biol. 158:659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadoran P, Aberdam E, Mantoux F, Busca R, Bille K, Yalman N, de Saint-Basile G, Casaroli-Marano R, Ortonne JP, Ballotti R. 2001. Rab27a: A key to melanosome transport in human melanocytes. J Cell Biol. 152:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Neff L, Brown W, Courtoy PJ, Louvard D, Farquhar MG. 1988. Polarized secretion of lysosomal enzymes: co- distribution of cation-independent mannose-6-phosphate receptors and lysosomal enzymes along the osteoclast exocytic pathway. J Cell Biol. 106:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielli A, Thornqvist PO, Hendrick AG, Finn R, Fitzgerald K, McCaffrey MW. 2001. The small GTPase Rab4A interacts with the central region of cytoplasmic dynein light intermediate chain-1. Biochem Biophys Res Commun. 281:1141–1153 [DOI] [PubMed] [Google Scholar]
- Coxon FP, Ebetino FH, Mules EH, Seabra MC, McKenna CE, Rogers MJ. 2005. Phosphonocarboxylate inhibitors of Rab geranylgeranyl transferase disrupt the prenylation and membrane localization of Rab proteins in osteoclasts in vitro and in vivo. Bone. 37:349–358 [DOI] [PubMed] [Google Scholar]
- Coxon FP, Helfrich MH, Larijani B, Muzylak M, Dunford JE, Marshall D, McKinnon AD, Nesbitt SA, Horton MA, Seabra MC, Ebetino FH, Rogers MJ. 2001. Identification of a novel phosphonocarboxylate inhibitor of Rab geranylgeranyl transferase that specifically prevents Rab prenylation in osteoclasts and macrophages. J Biol Chem. 276:48213–48222 [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, De Biase A, Yakovlev A, Finn T, Beers J, Hoffman EP, Faden AI. 2005. In vivo and in vitro characterization of novel neuronal plasticity factors identified following spinal cord injury. J Biol Chem. 280:2084–2091 [DOI] [PubMed] [Google Scholar]
- Doege H, Bocianski A, Joost HG, Schürmann A. 2000. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem J. 350(Pt 3):771–776 [PMC free article] [PubMed] [Google Scholar]
- Fukushima O, Bekker PJ, Gay CV. 1991. Ultrastructural localization of tartrate-resistant acid phosphatase (purple acid phosphatase) activity in chicken cartilage and bone. Am J Anat. 191:228–236 [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. 2006. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 103:11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleen JM, Raisanen S, Salo JJ, Reddy SV, Roodman GD, Hentunen TA, Lehenkari PP, Kaija H, Vihko P, Vaananen HK. 1999. Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. J Biol Chem. 274:22907–22910 [DOI] [PubMed] [Google Scholar]
- Husheem M, Nyman JK, Vaaraniemi J, Vaananen HK, Hentunen TA. 2005. Characterization of circulating human osteoclast progenitors: development of in vitro resorption assay. Calcif Tissue Int. 76:222–230 [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. 2011. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 91:119–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaija H, Jia J, Lindqvist Y, Andersson G, Vihko P. 1999. Tartrate-resistant bone acid phosphatase: large-scale production and purification of the recombinant enzyme, characterization, and crystallization. J Bone Miner Res. 14:424–430 [DOI] [PubMed] [Google Scholar]
- Kim J-M, Jeong D, Kang HK, Jung SY, Kang SS, Min B-M. 2007. Osteoclast precursors display dynamic metabolic shifts toward accelerated glucose metabolism at an early stage of RANKL-stimulated osteoclast differentiation. Cell Physiol Biochem. 20:935–946 [DOI] [PubMed] [Google Scholar]
- Knowles HJ, Athanasou NA. 2008. Hypoxia-inducible factor is expressed in giant cell tumour of bone and mediates paracrine effects of hypoxia on monocyte-osteoclast differentiation via induction of VEGF. J Pathol. 215: 56–66 [DOI] [PubMed] [Google Scholar]
- Kohler K, Louvard D, Zahraoui A. 2004. Rab13 regulates PKA signaling during tight junction assembly. J Cell Biol. 165: 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkakorpi P, Tuukkanen J, Hentunen T, Jarvelin K, Vaananen K. 1989. Organization of osteoclast microfilaments during the attachment to bone surface in vitro. J Bone Miner Res. 4:817–825 [DOI] [PubMed] [Google Scholar]
- Lakkakorpi PT, Vaananen HK. 1991. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J Bone Miner Res. 6:817–826 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408 [DOI] [PubMed] [Google Scholar]
- Marzesco AM, Dunia I, Pandjaitan R, Recouvreur M, Dauzonne D, Benedetti EL, Louvard D, Zahraoui A. 2002. The small GTPase Rab13 regulates assembly of functional tight junctions in epithelial cells. Mol Biol Cell. 13:1819–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R, Uehara S, Yatsushiro S, Juge N, Hua Z, Senoh S, Echigo N, Hayashi M, Mizoguchi T, Ninomiya T, Udagawa N, Omote H, Yamamoto A, Edwards RH, Moriyama Y. 2006. Secretion of L-glutamate from osteoclasts through transcytosis. EMBO J. 25:4175–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. 2005. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem. 280:2220–2228 [DOI] [PubMed] [Google Scholar]
- Mulari MT, Zhao H, Lakkakorpi PT, Vaananen HK. 2003. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic. 4:113–125 [DOI] [PubMed] [Google Scholar]
- Nesbitt SA, Horton M. 1997. Traffiking of matrix collagens through bone-resorbing osteoclasts. Science. 276:266–269 [DOI] [PubMed] [Google Scholar]
- Nokes RL, Fields IC, Collins RN, Fölsch H. 2008. Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells. J Cell Biol. 182:845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palokangas H, Mulari M, Vaananen HK. 1997. Endocytic pathway from the basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. J Cell Sci. 110(Pt 15): 1767–1780 [DOI] [PubMed] [Google Scholar]
- Pavlos NJ, Xu J, Riedel D, Yeoh JS, Teitelbaum SL, Papadimitriou JM, Jahn R, Ross FP, Zheng MH. 2005. Rab3D regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol Cell Biol. 25:5253–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholt FP, Widholm SM, Ek-Rylander B, Andersson G. 1990. Ultrastructural localization of a tartrate-resistant acid ATPase in bone. J Bone Miner Res. 5:1055–1061 [DOI] [PubMed] [Google Scholar]
- Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. 1997. Removal of osteoclast bone resorption products by transcytosis. Science. 276:270–273 [DOI] [PubMed] [Google Scholar]
- Salo J, Metsikko K, Palokangas H, Lehenkari P, Vaananen HK. 1996. Bone-resorbing osteoclasts reveal a dynamic division of basal plasma membrane into two different domains. J Cell Sci. 109(Pt 2): 301–307 [DOI] [PubMed] [Google Scholar]
- Seabra MC, Mules EH, Hume AN. 2002. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 8:23–30 [DOI] [PubMed] [Google Scholar]
- Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 10:513–525 [DOI] [PubMed] [Google Scholar]
- Sun Y, Bilan PJ, Liu Z, Klip A. 2010. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc Natl Acad Sci U S A. 107:19909–19914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Buki KG, Ettala O, Vaaraniemi JP, Vaananen HK. 2005. Possible role of direct Rac1-Rab7 interaction in ruffled border formation of osteoclasts. J Biol Chem. 280:32356–32361 [DOI] [PubMed] [Google Scholar]
- Taylor A, Rogers MJ, Tosh D, Coxon FP. 2007. A novel method for efficient generation of transfected human osteoclasts. Calcif Tissue Int. 80:132–136 [DOI] [PubMed] [Google Scholar]
- Vaananen HK, Horton M. 1995. The osteoclast clear zone is a specialized cell-extracellular matrix adhesion structure. J Cell Sci. 108(Pt 8):2729–2732 [DOI] [PubMed] [Google Scholar]
- Vaananen HK, Karhukorpi EK, Sundquist K, Wallmark B, Roininen I, Hentunen T, Tuukkanen J, Lakkakorpi P. 1990. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell Biol. 111:1305–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaraniemi J, Halleen JM, Kaarlonen K, Ylipahkala H, Alatalo SL, Andersson G, Kaija H, Vihko P, Vaananen HK. 2004. Intracellular machinery for matrix degradation in bone-resorbing osteoclasts. J Bone Miner Res. 19:1432–1440 [DOI] [PubMed] [Google Scholar]
- Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. 2006. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 175:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Blair HC, McDonald JM, McKenna MA, Jordan SE, Williford J, Hardy RW. 1997. Regulation of osteoclastic bone resorption by glucose. Biochem Biophys Res Commun. 235:646–651 [DOI] [PubMed] [Google Scholar]
- Wixler V, Wixler L, Altenfeld A, Ludwig S, Goody RS, Itzen A. 2011. Identification and characterisation of novel Mss4-binding Rab GTPases. Biol Chem. 392:239–248 [DOI] [PubMed] [Google Scholar]
- Wu X, Rao K, Bowers MB, Copeland NG, Jenkins NA, Hammer JA. 2001. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J Cell Sci. 114:1091–1100 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nishimura N, Morimoto S, Kitamura H, Manabe S, Kanayama HO, Kagawa S, Sasaki T. 2003. Distinct roles of Rab3B and Rab13 in the polarized transport of apical, basolateral, and tight junctional membrane proteins to the plasma membrane. Biochem Biophys Res Commun. 308:270–275 [DOI] [PubMed] [Google Scholar]
- Zahraoui A, Joberty G, Arpin M, Fontaine JJ, Hellio R, Tavitian A, Louvard D. 1994. A small rab GTPase is distributed in cytoplasmic vesicles in non polarized cells but colocalizes with the tight junction marker ZO-1 in polarized epithelial cells. J Cell Biol. 124:101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ettala O, Vaananen HK. 2002. Intracellular membrane trafficking pathways in bone-resorbing osteoclasts revealed by cloning and subcellular localization studies of small GTP-binding rab proteins. Biochem Biophys Res Commun. 293:1060–1065 [DOI] [PubMed] [Google Scholar]
- Zhao H, Laitala-Leinonen T, Parikka V, Vaananen HK. 2001. Downregulation of small GTPase Rab7 impairs osteoclast polarization and bone resorption. J Biol Chem. 276:39295–39302 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.