Abstract
Degradation of the extracellular matrix and basement membrane is a critical step in tumor progression. Matrix metalloproteinase 2 (MMP-2) and tissue inhibitor of metalloproteinase 2 (TIMP 2) act in a coordinated manner to form an integrated system involved in ovarian cancer (OC) progression. In this study, the authors describe the expression of TIMP-2 detected by immunohistochemistry in 6 OC cell lines and in 43 malignant epithelial ovarian tumors (in tumor and stromal compartments) in sections originating from primary laparotomies. No significant correlations between overall and progression-free survival and TIMP-2 expression in tumor compartment were observed. The analysis demonstrated a significant association between enhanced stromal expression of TIMP-2 and better clinical response to cisplatin- and paclitaxel-based chemotherapy. Increased expression of TIMP-2 in the stromal compartment and simultaneous overexpression in both stromal and tumor compartments strongly correlated with increased survival. No significant correlations were found in vitro between resistance to cisplatin, paclitaxel, or topotecan and the expression of TIMP-2 in the OC cell lines, suggesting stromal influences on tumor chemoresistance in the physiological environment. This study supports the concept of TIMP-2 expression in the stromal compartment of OC as a promising marker of prognosis and response to cisplatin- and paclitaxel-based chemotherapy in OC patients.
Keywords: TIMP-2, prognostic factor, predictive factor, ovarian cancer, immunocytochemistry, immunohistochemistry
Ovarian cancer (OC) is one of the most common gynecologic malignancies, representing a cancer type with a high mortality rate due to its advanced stage at diagnosis (International Federation of Gynecology and Obstetrics [FIGO] III and IV) (Manenti et al. 2003). The clinical symptoms of OC, especially in the early stages, are nonspecific; therefore, approximately 75% of women have an advanced stage of the disease at diagnosis, which is consequently associated with poor outcome. First-line therapy in advanced ovarian cancer commonly consists of surgical resections followed by cisplatin- and taxane-based chemotherapy. Despite a high initial response rate, most patients will relapse, which is strongly related to acquired drug resistance (Surowiak, Materna, Kaplenko, Spaczynski, Dietel, et al. 2006). Knowledge of biological mechanisms mediating chemoresistance may lead to new and more effective therapeutic strategies.
Breakage or degradation of the extracellular matrix (ECM) and basement membrane (BM) is a critical step in tumor progression. Several classes of proteases are involved in tumor invasion by degradation of type IV collagen, which is the main component of the BM. The most important group of proteins is a family of more than 20 extracellular zinc-dependent proteolytic enzymes, that is, the matrix metalloproteinases (MMPs) (Gershtein et al. 2010). The tissue inhibitors of metalloproteinases (TIMPs) are the main physiologic inhibitors of MMPs. TIMPs are secreted proteins that can form complexes with individual MMPs and regulate the activity of specific MMPs. Maintaining the balance between the levels of MMPs and TIMPs is crucial for ECM stability.
TIMP-2 is one of four well-known members of the TIMP family: TIMP-1, TIMP-2, TIMP-3, and TIMP-4. All of these, except for TIMP-3, are secreted in soluble form, whereas TIMP-3 is bound to ECM proteins as an insoluble type (Sternlicht and Werb, 2001). The pleiotropic and multifaceted role of TIMPs in carcinogenesis has been widely discussed in previous studies (Baker et al. 2002; Jiang et al. 2002). The physiological function of TIMP-2 is in the inhibition of MMP-2 activation, but there are data to show that an increasing level of TIMP-2 can promote MMP-2 activity and invasion in some tumors (Lu et al. 2004). Indeed, for over a decade, TIMPs have been described as tumor-promoting factors that are engaged in cellular proliferation (Hayakawa et al. 1992) or as inhibitors of angiogenesis in MMP-independent pathways (Fernandez et al. 2003). Taking into account these paradoxical results, further studies are needed to fully determine the role of TIMPs in human cancer development.
MMP-2/TIMP-2 act in a coordinated manner to form an integrated system involved in OC progression. Data on the function of TIMP-2 in carcinogenesis of the ovary are inconsistent and seem to depend on the detection methods used and the histological type of tumors included in the studies (Davidson et al. 2002; Okamoto et al. 2003; Rauvala et al. 2005; Kim et al. 2006). Davidson et al. (2002) revealed, by using mRNA in situ hybridization, that TIMP-2 expression is a valid marker of poor outcome in advanced-staged OC. However, Okamoto et al. (2003) showed a decreased level of TIMP-2 in ovarian carcinomas, as compared with normal ovarian cells.
This study aimed to assess the prognostic and predictive value of immunohistochemical examination of TIMP-2 expression in patients with ovarian cancer. We examined the relationships between the expression of TIMP-2 in tumor and stromal cells (tumor and stromal compartments) and the clinical data of the studied patients. In addition, TIMP-2 immunoexpression was investigated in six ovarian cancer cell lines (EFO 21, ES-2, Mdah 2774, OAW 42, OVCAR-3, and SKOV-3). Because chemoresistance is a major obstacle in the treatment of ovarian cancer, an analysis of cisplatin, paclitaxel, and topotecan sensitivity in the analyzed cell lines was also carried out.
Materials and Methods
Cell Culture
Human carcinoma cells were grown in Leibovitz L-15 medium (Biowhittaker; Walkersville, MD) supplemented with 10% fetal calf serum (FCS; Gibco/BRL, Grand Island, NY), 1 mM L-glutamine, 6.25 mg/1 fetuin, 80 IE/1 insulin, 2.5 mg/ml transferrin, 0.5 g/1 glucose, 1.1 g/1 NaHCO3, 1% minimal essential vitamins, and 20,000 kIE/1 trasylol in a humidified atmosphere of 5% CO2 at 37C, as described previously (Materna et al. 2005, 2006; Kowalski et al. 2005; Surowiak, Materna, Kaplenko, Spaczynski, Dolinska-Krajewska, et al. 2006). The human ovarian carcinoma cell lines, EFO 21, ES-2, Mdah 2774, OAW 42, OVCAR-3, and SKOV-3, were kindly provided by Dr. Carsten Denkert (Institute of Pathology, Charité, Berlin, Germany).
Resistance Tests
The drugs were used in their commercially available form. Each drug was applied to the cells in three concentrations (C1, C2, C3). C1 = 10−1 × C2 and C3 = 10 × C2. Concentration C2 was deduced from levels assessed to be clinically achievable in tumor tissue, as discussed previously (Györffy et al. 2006) (Table 1).
Table 1.
Drugs Used to Establish Cell Line Resistance Patterns and the Clinically Available Drug Concentrations in the Tumors (C2)
| Drug | C2, µg/ml | C2, µM | Supplying Company |
|---|---|---|---|
| Cisplatin | 0.5 | 1.66 × 10−4 | Gry-Pharma |
| Paclitaxel | 0.025 | 0.29 × 10−5 | Bristol |
| Topotecan | 0.01 | 2.18 × 10−3 | GlaxoSmithKline |
In each experiment, 500 cells/microtiter dish were seeded onto 96-well plates. After 2 days, the pre–control cells were fixed and stained using sulforhodamine B (SRB) (Györffy et al. 2006). At the same time, triplicate cultures were prepared with all three of the studied drugs at C1, C2, and C3 concentrations. After 4 days, incubation was terminated by replacing the medium with 10% trichloroacetic acid, followed by incubation at 4C for 1 hr. Subsequently, the plates were washed five times with water and stained by adding 100 µl 0.4% SRB (Sigma; St. Louis, MO) in 1% acetic acid for 10 min at room temperature. Washing the plates five times with 1% acetic acid eliminated unbound dye. After air-drying and resolubilization of the protein-bound dye in 10 mM Tris-HCl (pH 8.0), absorbance was read at 562 nm in an Elisa-Reader (EL 340 Microplate Bio Kinetics Reader; BIO-TEK Instruments, Winooski, VT). The measurements were performed in triplicate in three independent experiments. For calculation of the resistance index (RI) values, the averages of all nine measurements were used.
The RI was estimated by the following formula:
where npre is the medium absorbance value of the precontrol at C2 concentration, npost is the medium absorbance value of control, and n2 is the medium absorbance value of stained cells tested with a chosen concentration of the studied drug. At the C2 concentration of topotecan, we did not have enough resistant and sensitive cell lines to be able to perform a robust statistical calculation; therefore, we used C3. Only the cell lines that fulfilled the following quality criteria of npost > npre and deviation in cell growth within repetitions <15% were included in the evaluation. Cells exhibiting the lowest third RI results were designated as sensitive, the top third as resistant, and the remaining cells as intermediate.
Patients
Forty-three patients who underwent surgery from 1999–2002 due to ovarian carcinoma at the Department of Gynaecology and Obstetrics, University Medical School in Poznan (Poland) were included in the study. The cases were selected based on availability of tissue and were not stratified for known preoperative or pathological prognostic factors. The study was approved by an institutional review board (IRB), and the patients gave their informed consent before being included in the study. Following primary laparotomy (PL), all of the patients were subjected to chemotherapy using cisplatin-based schemes (Table 2). Thirty-six patients from the same group were also subjected to secondary cytoreductions (SCR). In seven cases, no second-look procedure was performed due to advancement of the disease. In six cases, no tumor cells were detected in the material originating from the second-look procedure. The patients were monitored by periodic medical checkups, CA-125 serum levels, and ultrasonographic and radiological examinations. During the follow-up period, 22 patients (51%) had recurrent disease and 13 patients (30%) died of the disease. The mean progression-free survival time was 16.9 months (range, 0–52 months), whereas the mean overall-free survival time was 24.6 months (range, 6–52 months). Only 1 stage I and 1 stage II patients achieved optimal cytoreduction.
Table 2.
Patient and Tumor Characteristics
| Characteristics | No. (%)a |
|---|---|
| All patients | 43 (100) |
| Age, y (mean 51.0)b | |
| ≤50 | 20 (47) |
| 50–60 | 16 (37) |
| >60 | 7 (16) |
| Gradeb | |
| 1 | 7 (16) |
| 2 | 18 (42) |
| 3 | 18 (42) |
| FIGOb | |
| I | 1 (2) |
| II | 1 (2) |
| III | 41 (95) |
| Histologyb | |
| Serous | 37 (86) |
| Endometrioid | 3 (7) |
| Other | 3 (7) |
| Clinical responsec | |
| Complete response | 16 (37) |
| Stable disease | 5 (12) |
| Progressive disease | 22 (51) |
| Chemotherapy (in total) | |
| Cisplatin/paclitaxel | 31 (72) |
| Cisplatin/cyclophosphamide/adriblastin | 6 (14) |
| Cisplatin/cyclophosphamide/paclitaxel | 3 (7) |
| Cisplatin/cyclophosphamide/paclitaxel/adriblastin | 2 (5) |
| Carboplatin/paclitaxel | 1 (2) |
FIGO, International Federation of Gynecology and Obstetrics.
Differences in the sum to 100% in the groups are due to rounding.
Data given for the first operation/diagnosis implemented.
According to RECIST (Response Evaluation Criteria in Solid Tumors) (Therasse et al. 2000).
Tissue samples were fixed in 10% buffered formalin and embedded in paraffin. In each case, hematoxylin and eosin-stained preparations were subjected to histopathological evaluation by two pathologists. The stage of the tumors was assessed according to the International Federation of Gynaecology and Obstetrics (Sobin et al. 1997). The tumors were graded according to the Silverberg grading system (Shimizu et al. 1998).
Immunohistochemistry
An immunohistochemical examination was performed retrospectively on tissue samples taken from primary laparotomies. Formalin-fixed, paraffin-embedded tissue was freshly cut (4 µm). The sections were mounted on Superfrost slides (Menzel Gläser; Göttingen, Germany), dewaxed with xylene, and gradually hydrated. The activity of endogenous peroxidase was blocked by 5-min exposure to 3% H2O2. All of the studied sections were boiled for 15 min at 250W in the Antigen Retrieval Solution (DakoCytomation; Copenhagen, Denmark). Then, immunohistochemical reactions were performed using a mouse monoclonal antibody directed against TIMP-2 (MAB971, clone 89025, R&D Systems, Minneapolis, MN; dilution 1:100 in Antibody Diluent, Background Reducing [DakoCytomation, Carpinteria, CA], 1 hr at 20C). Each reaction was accompanied by a negative control using Primary Mouse Negative Control (DakoCytomation). Subsequent incubations involved biotinylated antibodies (15 min, room temperature) and streptavidin-biotinylated peroxidase complex (15 min, room temperature; LSAB+, horseradish peroxidase [HRP], DakoCytomation). DAB (DakoCytomation) was used as a chromogen (7 min, room temperature). All of the sections were counterstained with Meyer’s hematoxylin. In this study, Ki67 (Surowiak, Materna, Kaplenko, Spaczynski, Dietel, et al. 2006) and p53 (Materna et al. 2007) expression data, which had been investigated previously in the same group of patients, were reused.
Immunocytochemistry
Immunostaining of TIMP-2 was performed using all of the studied cell lines. Cells were grown on microscope slides and fixed in an ice-cold methanol-acetone mixture (1:1) for 10 min. After rehydration, an immunostaining reaction was performed in triplicate as described above.
Evaluation of Immunohistochemical Reaction in Postoperative Cancer Specimens and Immunocytochemical Reaction in Cancer Cell Lines
In this study, we separately evaluated the expression (immunoreactive score [IRS]) in tumor cells (tumor compartment, TIMPc) and in stromal fibroblasts (stromal compartment, TIMPs) of OC specimens originating from laparotomies. For the evaluation of the TIMP-2 expression in the stromal compartment, we used only a percentage of the positive cells (0–4) (Table 3). Intensity of the immunohistochemical reactions in the tumor compartment and immunocytochemical reactions in cell lines was appraised using the semi-quantitative IRS scale, in which intensity of the reaction and percentage of positive cells were scored (Table 3). The final result represented a product of scores given for individual traits and ranged between 0 and 12 (Remmele and Stegner 1987). Intensity of immunohistochemical reactions was evaluated independently by two pathologists. In cases of divergences, the evaluation was repeated using a double-headed microscope.
Table 3.
Procedure for Evaluating TIMP-2 Expression in Cancer Cells Using the IRS
| Percentage of Positive Cells | Points | Intensity of reaction | Points |
|---|---|---|---|
| No positive cells | 0 | No reaction | 0 |
| <10% | 1 | Weak color reaction | 1 |
| 10–50% | 2 | Moderate intensity | 2 |
| 51–80% | 3 | Intense reaction | 3 |
| >80% | 4 |
IRS, immunoreactive score according to Remmele and Stegner (1987); TIMP-2, tissue inhibitor of metalloproteinase 2.
In addition, cases with high intensity of immunohistochemical reaction both in the tumor cells (IRS 6–12) and in the stromal fibroblasts (2–4), defined as TIMP-2c-s = 1, and with low intensity or without reaction, defined as TIMP-2c-s = 0 (TIMP-2c, IRS 0–4; TIMP-2s, IRS 0–1), were extracted for statistical analysis.
Statistical Analysis
Statistica 98 PL software (StatSoft, Krakow, Poland) was used for a statistical analysis of the results. The tests used included the ANOVA Kruskal-Wallis rank test, Spearman’s rank correlation, Kaplan-Meier statistics, and log-rank tests. In the univariate analysis, we did not find any significant relationships (p>0.05) between the studied clinicopathological parameters (age, histology, grade, CA-125 at primary laparotomy level) and overall and progression-free survival time; therefore, we did not perform a multivariate analysis. Because 95% of the studied patients were in stage FIGO III, we did not investigate the relationships between stage and survival data.
We also performed Kaplan-Meier statistics and log-rank tests on a subgroup of 35 FIGO III patients receiving postsurgical platinum- and paclitaxel-containing combination therapy.
Results
Cisplatin, Paclitaxel, and Topotecan Resistance of Cell Lines
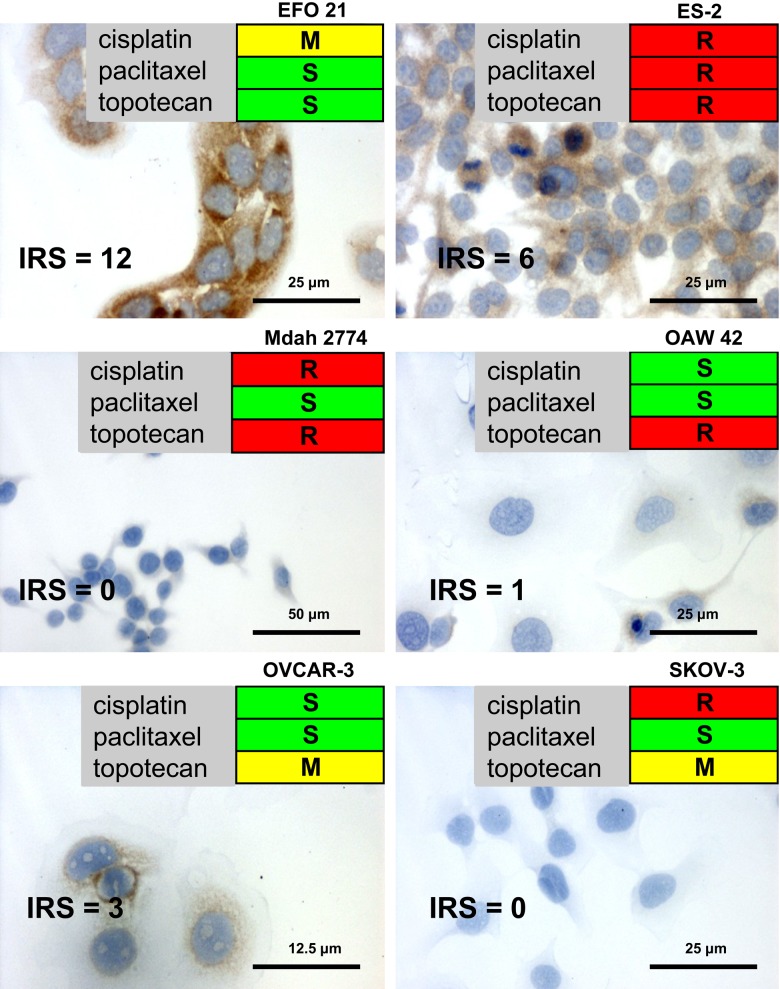
The resistance of various human ovarian carcinoma cell lines against treatment with cisplatin, paclitaxel, and topotecan was determined by an assessment of the RI, as described in Materials and Methods. An ovarian cancer cell line that was completely resistant to cisplatin, paclitaxel, and topotecan was ES-2. In turn, the EFO 21 and OVCAR-3 cell lines were characterized by a relatively good response to the applied dose of anticancer agents. Mdah 2774 was resistant to cisplatin and topotecan, but the analysis revealed complete sensitivity to paclitaxel. Furthermore, OAW-42 was only resistant to topotecan, and SKOV-3 was characterized by sensitivity only to paclitaxel. Figure 1 presents all the results of chemoresistance analysis in the studied cell lines.
Figure 1.
Immunocytochemical expression of tissue inhibitor of metalloproteinase 2 (TIMP-2) in ovarian carcinoma cell lines (×200; hematoxylin) with response to anticancer agents. IRS, immunoreactive score. S (green) = sensitive; M (yellow) = moderate; R (red) = resistant.
TIMP-2 Immunostaining in Cell Lines
Strong cytoplasmic TIMP-2 expression (IRS 12) was shown in EFO 21 cells (Fig. 1). Moderately enhanced cytoplasmic immunoreactivity was also observed in the ES-2 and OVCAR-3 cell lines (IRS 6 and 3, respectively). The other cell lines were TIMP-2 negative (Fig. 1). No significant correlations were found between cisplatin, paclitaxel, and topotecan resistance and expression of TIMP-2 among the cell lines.
TIMP-2 Expression and Clinicopathological Parameters
In postoperative specimens of ovarian cancer, a cytoplasmic reaction with variable intensity in individual cases (Fig. 2) was observed. Furthermore, we noted strong TIMP-2 immunoreactivity in the stromal compartment of ovarian tumors (Fig. 3). The mean ± SD IRS score of TIMP-2 expression in the tumor compartment was 4.69 ± 3.35 (minimum, 0; maximum, 12), and the mean ± SD reactivity of TIMP-2 in the stromal compartment was 1.02 ± 1.14 (minimum, 0; maximum, 4). We observed seven cases defined as TIMP-2c-s = 1 with high intensity of an immunohistochemical reaction, both in the tumor cells (IRS 6–12) and in the stromal fibroblasts (2–4). There were 36 specimens defined as TIMP-2c-s = 0 with simultaneous low TIMP-2 expression intensity or without reaction (TIMP-2c, IRS 0–4; TIMP-2s, IRS 0–1) in both stromal and tumor compartments.
Figure 2.
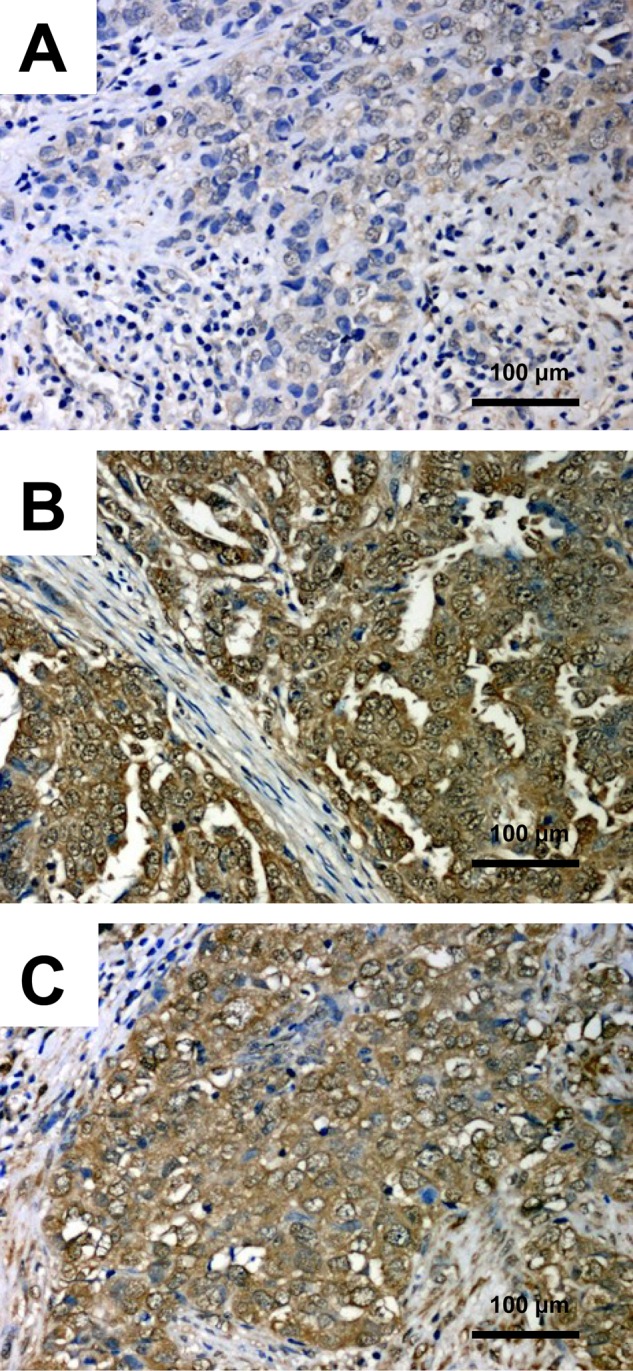
Immunohistochemical expression of tissue inhibitor of metalloproteinase 2 (TIMP-2) in ovarian cancer tissue. (A) IRS 2, ×200; (B) IRS 12, ×200; (C) IRS 8, ×200; hematoxylin. IRS, immunoreactive score.
Figure 3.
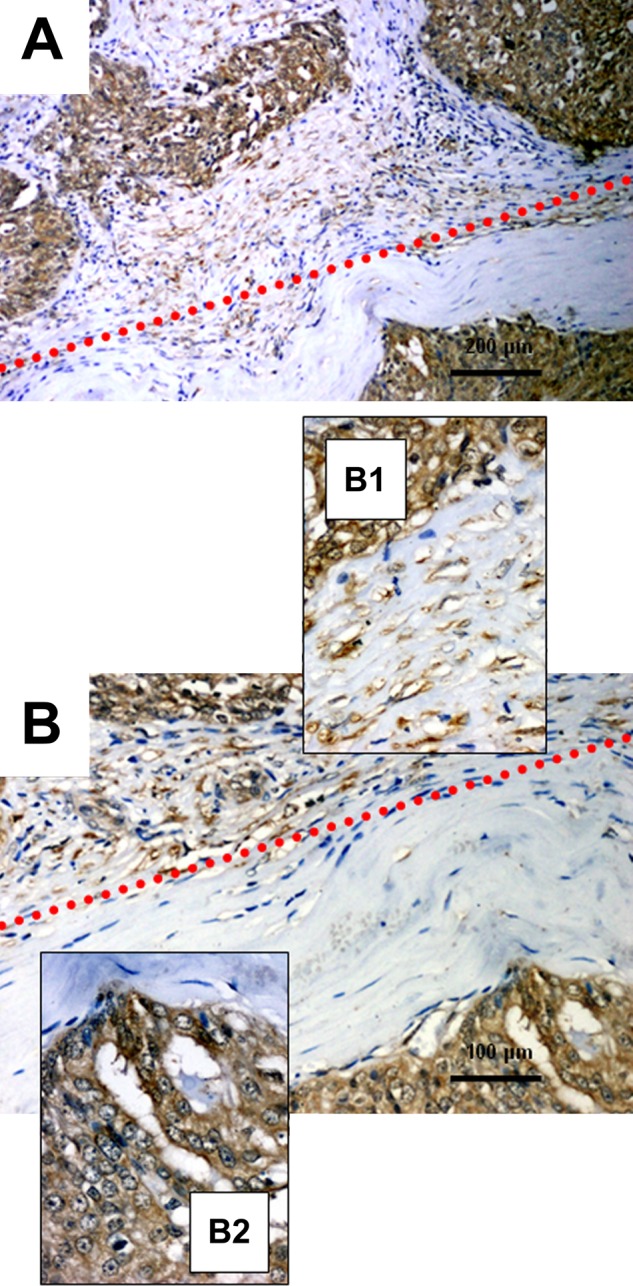
Immunohistochemical patterns of tissue inhibitor of metalloproteinase 2 (TIMP-2) expression in the tumor and stromal compartments of ovarian cancer: Both types of expression (cancer and stromal) are above the red dots; only the cancer cells are below. (A) Ovarian cancer tissue, ×100, hematoxylin; (B) ovarian cancer tissue, ×200, hematoxylin; (B1) ovarian cancer tissue with stromal details, ×400, hematoxylin; (B2) ovarian cancer tissue with tumor compartment details, ×400, hematoxylin.
At the first stage of statistical analysis of the relationship between TIMP-2 expression and the clinicopathological parameters of patients (age, CA-125 serum levels at primary laparotomy, histological tumor type, grade and clinical response to chemotherapy), Spearman’s rank correlation and the ANOVA Kruskal-Wallis rank test were used. In addition, correlations between TIMP-2 reactivity in the tumor and stromal compartments and immunoreactivity of Ki-67 and p53 were examined (Table 4).
Table 4.
Correlation between TIMP-2 Expression and Various Clinicopathological and Immunohistochemical Parameters
| Characteristics | TIMP-2c p Value | TIMP-2s p Value | TIMP-2c-s p Value |
|---|---|---|---|
| Age | 0.9803a | 0.9391a | 0.3649b |
| CA-125 serum levels at primary laparotomies | 0.2571a | 0.7603a | 0.7236b |
| Histologic type | 0.9609b | 0.4347b | 0.8572b |
| Grade | 0.2006b | 0.4882b | 0.5757b |
| Clinical response | 0.3257b | 0.0497b | 0.0824b |
| Ki67 | 0.0506a | 0.1947a | 0.1226b |
| p53 | 0.5644a | 0.8929a | 0.9867b |
TIPM-2, tissue inhibitor of metalloproteinase 2; TIPM-2c, TIMP-2 expression in tumor compartment (cancer cells); TIMP-2s, TIMP-2 expression in the stromal compartment; TIMP2c-s, TIMP-2 coexpression in tumor and stromal compartments.
Spearman’s rank correlation.
ANOVA Kruskal-Wallis rank test.
In cases that responded well (complete response) to cisplatin- and paclitaxel-based chemotherapy, the immunoreactivity of TIMP-2 expression in the stromal compartment was stronger (p=0.0497) than in patients with progressive disease (Table 4). Interestingly, we observed a tendency (p=0.0506) for simultaneous Ki67 overexpression and the enhanced reactivity of TIMP-2 in the tumor compartment (Table 4). Except the above-mentioned two relationships, the statistical analysis did not reveal any significant correlations between TIMP-2 expression (in both localizations of expression: in stromal and cancer cells) and other analyzed clinical parameters (Table 4).
Using the Kaplan-Meier analysis, overall and progression-free survival were compared in patients with low (0–4 IRS) and high (6–12 IRS) expression of TIMP-2 in cancer tissue (Figs. 4A, 5A) and in stromal cells (Figs. 4B, 5B). In addition, we examined the influence on survival for patients with coexisting expression in the tumor and stromal compartments (Figs. 4C, 5C). Patients with enhanced immunoreactivity of TIMP-2 in the stromal compartment had a significantly increased overall survival (p=0.00008; Fig. 4B) and progression-free survival time (p=0.001; Fig. 5B). No significant correlations between overall survival and progression-free survival and TIMP-2 in the tumor compartment were detected. Interestingly, in cases of simultaneous high expression of TIMP-2 in tumor and stromal compartments, we observed that this type of reactivity is strongly correlated with a good clinical outcome (p=0.00056 for overall survival time; Fig. 4C).
Figure 4.
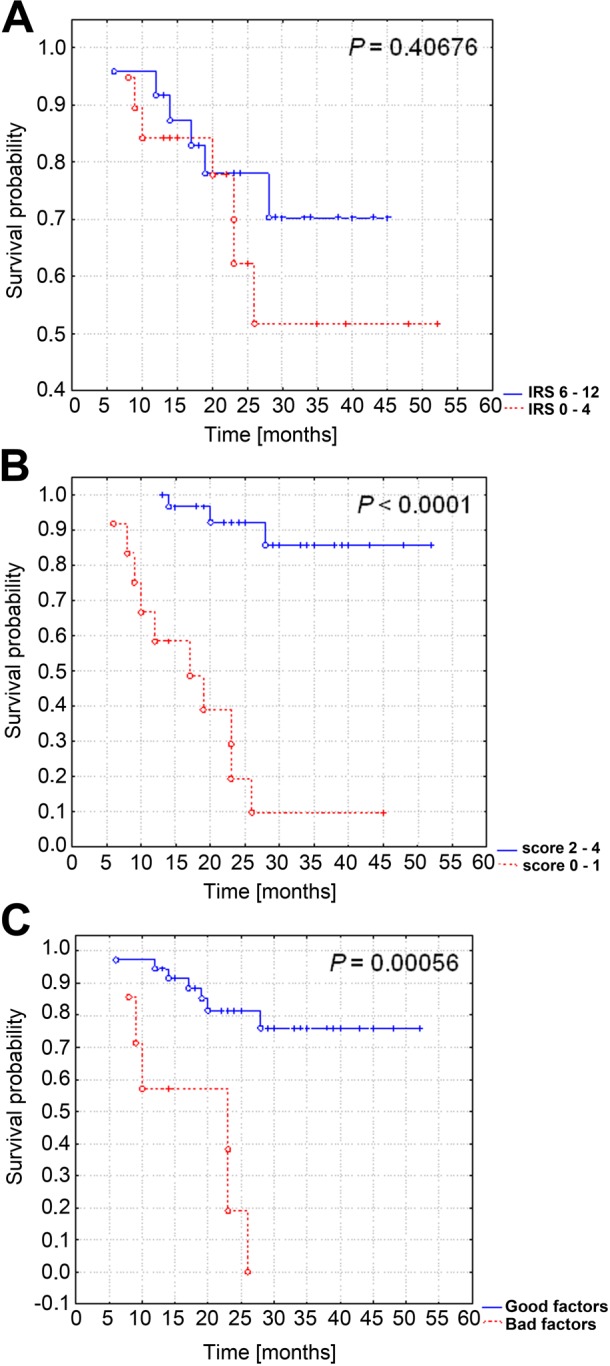
Kaplan-Meier curves for overall survival and tissue inhibitor of metalloproteinase 2 (TIMP-2) expression in 43 patients with ovarian cancer. No significant difference in overall survival time and TIMP-2 expression in cancer cells (A). Patients with enhanced immunoreactivity of TIMP-2 in the stromal compartment (B) and simultaneous overexpression in the tumor and stromal compartments (C) had an increased overall survival time. IRS, immunoreactive score.
Figure 5.
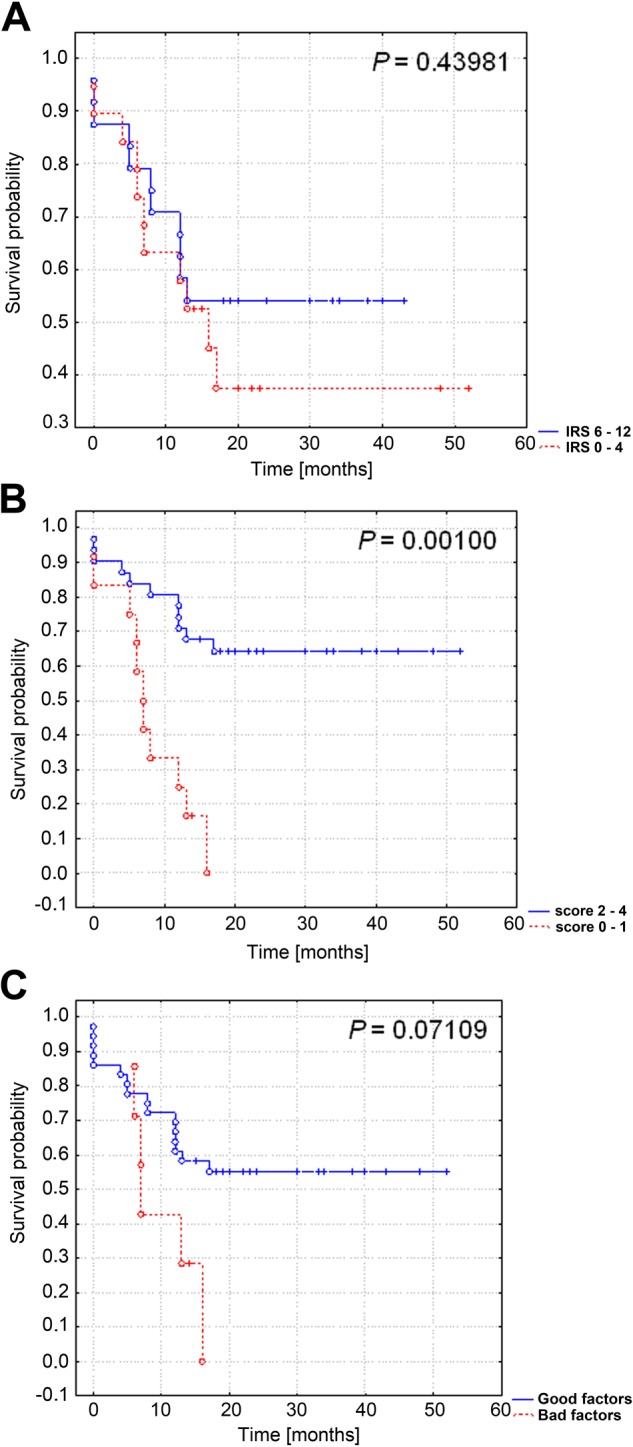
Kaplan-Meier curves for progression-free survival and tissue inhibitor of metalloproteinase 2 (TIMP-2) expression in 43 patients with ovarian cancer. No significant differences in progression-free survival and TIMP-2 expression in the tumor compartment (A) and in cases with simultaneous tumor and stromal overexpression of TIMP-2 (C). Patients with enhanced immunoreactivity of TIMP-2 in the stromal compartment had an increased progression-free survival time (B). IRS, immunoreactive score.
TIMP-2 Expression and Results of Chemotherapy
The relationships between overall and progression-free survival and expression of TIMP-2 in the subgroup of FIGO stage III patients treated with platinum-based drugs and paclitaxel were evaluated. Overexpression of TIMP-2 in the stromal compartment in this subgroup was associated (p=0.0321) with longer progression-free survival (Table 5). In cases with tumor and stromal TIMP-2 coexpression, increased overall (p=0.001) and progression-free survival (p=0.0034) were observed (Table 5).
Table 5.
Relationships between Overall Survival Time and Progression-Free Survival and Expression of TIMP-2 in the Subgroup of FIGO Stage III Patients Treated with Platinum-Based Drugs and Paclitaxel (n=35)
| TIPM-2c | TIMP-2s | TIMP2c-s | |
|---|---|---|---|
| Overall survival, p value | 0.4231 | 0.0743 | 0.0010 |
| Progression-free survival, p value | 0.9465 | 0.0321 | 0.0034 |
FIGO, International Federation of Gynecology and Obstetrics; TIMP-2, tissue inhibitor of metalloproteinase 2; TIPM-2c, TIMP-2 expression in tumor compartment (cancer cells); TIMP-2s, TIMP-2 expression in the stromal compartment; TIMP2c-s, TIMP-2 coexpression in tumor and stromal compartments.
Discussion
Matrix metalloproteinases and their inhibitors (TIMPs) play a crucial regulatory role in the homeostasis of the extracellular matrix. TIMP-2 has additional functions, unrelated to its anti-MMP activity, that are associated with cell proliferation and survival (Bourboulia et al. 2011). In addition to the key role of TIMP-2 in the process of inhibiting the activity of metalloproteinases, especially of MMP-2, it is also involved in activation of this enzyme along with membrane type 1 metalloproteinase (MT1-MMP). The main mechanism of this process is based on the fact that MT1-MMP is a specific receptor for TIMP-2. The MT1-MMP/TIMP-2 complex has been shown to function as a cytobiochemical active base for proMMP-2, which is finally activated by another MT1-MMP (Butler et al. 1998; Kinoshita et al. 1998).
In this study, we detected by immunohistochemistry the expression of TIMP-2 in malignant epithelial ovarian tumors (sections originating from primary laparotomies). In addition, we conducted an immunocytochemical analysis of TIMP-2 reactivity in six ovarian carcinoma cell lines and determined their resistance to cisplatin, paclitaxel, and topotecan. No significant correlations were found between resistance to these anticancer agents and expression of TIMP-2 in the analyzed cell lines. Contrastingly, in vivo, in cases with complete response to cisplatin- and paclitaxel-based chemotherapy, the TIMP-2 expression in the stromal compartment was higher than in patients with progressive disease, suggesting the stromal influences on tumor behavior in a physiological environment. TIMPs, being the secreted factors, most likely have paracrine effects on the surrounding stroma, thereby affecting tumor growth, angiogenesis, and sensitivity to anticancer factors. Studying TIMP-1 in MDA-MB-231 cells and in SCID mice, Bigelow et al. (2009) observed that TIMP-1 stimulates changes in gene expression in vivo distinct from those observed in vitro: TIMP-1’s effect on gene expression levels was more pronounced and affected more genes in vivo as compared with the in vitro analysis (approximately 600 genes vs. approximately 200 genes, respectively). Thus, studying the in vitro effects of TIMPs will not accurately reflect their true function when those proteins are overexpressed in a physiological environment (Bigelow et al. 2009).
Our study revealed that expression of TIMP-2 in the tumor and stromal compartments in postoperative specimens was not associated with clinicopathological parameters, such as histological tumors type, grade, and CA-125 serum level at primary laparotomy. Interestingly, we demonstrated a borderline significant association of enhanced stromal expression of TIMP-2 and a better clinical response to chemotherapy. The Kaplan-Meier analysis confirmed these results, as stromal immunoreactivity of TIMP-2 had a significant impact on overall survival and progression-free survival time (overexpression strongly correlated with a better outcome). Intriguingly, the analysis revealed a tendency toward there being a positive correlation between Ki67 overexpression and increased TIMP-2 expression in ovarian cancer cells (tumor compartment).
To the best of our knowledge, this is the first study that describes TIMP-2 overexpression in the stromal compartment of ovarian cancer as a favorable prognostic and predictive factor. It supports the assumption that TIMP-2 has a pluripotential impact on the behavior of cancer cells. Studies of the TIMP-2 expression pattern and its significance in the prognosis of clinical outcome have been discrepant and seem to depend on the type of tissue material and detection methods used (Kikkawa et al. 1997; Sakata et al. 2000; Furuya et al. 2000; Yoshida et al. 2001; Okamoto et al. 2003).
Davidson et al. (2002) demonstrated that overexpression of TIMP-2 in the stroma and in cancer cells, as detected by colorimetric non-radioactive in situ mRNA hybridization, is strongly correlated with a poor outcome. Their findings point to the central role of TIMP-2 in determining the outcome in ovarian cancer patients, but the main function of TIMP-2 is probably related to activation of MMP-2, together with MT1-MMP, rather than in inhibiting the activation of MMP-2. Interestingly, Kim et al. (2006) examined the immunohistochemical expression of TIMP-2 in 52 serous ovarian tumors (12 benign, 20 borderline, and 20 malignant) and observed intracytoplasmic and membranous staining for TIMP-2. Furthermore, their analysis revealed stromal TIMP-2 expression in some cases, especially in the serous type of ovarian cancer, but this study did not correlate immunohistochemical parameters with the patient’s survival. Using immunohistochemistry, Sakata et al. (2000) suggested that overexpression of TIMP-2 and the downregulation of TIMP-1 might promote the development of ovarian carcinogenesis.
By contrast, Furuya et al. (2000) examined the neoplastic cysts of ovarian mucinous tumors for the presence of MMPs and TIMPs and reported that TIMP-2 concentration in cystic fluids is less prevalent in cancer/borderline fluids than in adenoma fluids. In addition, Okamoto et al. (2003) showed that TIMP-2 levels were markedly decreased in malignant tumors, as compared with normal ovarian cells. In this experiment, ovarian clear cell carcinomas were the exception because, in these cases, TIMP-2 reactivity was significantly elevated. Furthermore, Okamoto et al. (2003) also demonstrated that in the ovarian clear cell carcinoma, the expression of TIMP-2 was predominantly located in the cancer cells within the ovary. On the other hand, in the serous and mucinous type of ovarian cancer, the topography of TIMP-2 was seen mainly in the stromal compartment of the tumor. Our study did not reveal any significant correlations between TIMP-2 expression (in the stromal or tumor compartment) and histological type of tumor. These contradicting results regarding the role of TIMP-2 expression in the stromal and tumor compartments of OC may reflect the analysis of both early and advanced-stage tumors and, in addition, reflect the use of different methods to detect the studied protein.
In conclusion, the evaluation of TIMP-2 expression in the stromal compartment of OC may be a useful prognostic marker that can also predict the response to cisplatin- and paclitaxel-based chemotherapy. Overall, the significance of TIMP-2 immunoreactivity and the impact of its tissue topography on patients’ survival are still controversial. Further studies are needed to fully determine the clinicopathological implications of TIMP-2 expression in stromal and tumor compartments and its role in ovarian carcinogenesis.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Baker AH, Edwards DR, Murphy G. 2002. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 115:3719–3727 [DOI] [PubMed] [Google Scholar]
- Bigelow RL, Williams BJ, Carroll JL, Daves LK, Cardelli JA. 2009. TIMP-1 overexpression promotes tumorigenesis of MDA-MB-231 breast cancer cells and alters expression of a subset of cancer promoting genes in vivo distinct from those observed in vitro. Breast Cancer Res Treat. 117:31–44 [DOI] [PubMed] [Google Scholar]
- Bourboulia D, Jensen-Taubman S, Rittler MR, Han HY, Chatterjee T, Wei B, Stetler-Stevenson WG. 2011. Endogenous angiogenesis inhibitor blocks tumor growth via direct and indirect effects on tumor microenvironment. Am J Pathol. 179:2589–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G, Butler M, Atkinson S, Will H, Tamura T, Schade van Westrum S, Crabbe T, Clements J, d’Ortho MP, Murphy G. 1998. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A: a kinetic study. J Biol Chem. 273:871–880 [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Ben-Baruch G, Nesland JM, Reich R. 2002. The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Mol Cell Endocrinol. 187:39–45 [DOI] [PubMed] [Google Scholar]
- Fernandez CA, Butterfield C, Jackson G, Moses MA. 2003. Structural and functional uncoupling of the enzymatic and angiogenic inhibitory activities of tissue inhibitor of metalloproteinase-2 (TIMP-2): loop 6 is a novel angiogenesis inhibitor. J Biol Chem. 278:40989–40995 [DOI] [PubMed] [Google Scholar]
- Furuya M, Ishikura H, Kawarada Y, Ogawa Y, Sakuragi N, Fujimoto S, Yoshiki T. 2000. Expression of matrix metalloproteinases and related tissue inhibitors in the cyst fluids of ovarian mucinous neoplasms. Gynecol Oncol. 78:106–112 [DOI] [PubMed] [Google Scholar]
- Gershtein ES, Levkina NV, Digayeva MA, Laktionov KP, Tershkina IV, Kushlinsky NE. 2010. Matrix metalloproteinases 2, 7 and 9 and tissue inhibitor of metalloproteinases-1 in tumors and serum of patients with ovarian neoplasms. Bull Exp Biol Med. 149:628–631 [DOI] [PubMed] [Google Scholar]
- Györffy B, Surowiak P, Kiesslich O, Denkert C, Schäfer R, Dietel M, Lage H. 2006. Gene expression profiling of 30 cancer cell lines predicts resistance towards 11 anticancer drugs at clinically achieved concentrations. Int J Cancer. 118:1699–1712 [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. 1992. Growth promoting activity of tissue inhibitor of metalloproteinase-1 (TIMP-1) for a wide range of cells: a possible new growth factor in serum. FEBS Lett. 298:29–32 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Goldberg ID, Shi YE. 2002. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 21:2245–2252 [DOI] [PubMed] [Google Scholar]
- Kikkawa F, Tamakoshi K, Nawa A, Shibata K, Yamagata S, Yamagata T, Suqanuma N. 1997. Positive correlation between inhibitors of matrix metalloproteinases 1 and matrix metalloproteinases in malignant ovarian tumor tissues. Cancer Lett. 120:109–115 [DOI] [PubMed] [Google Scholar]
- Kim TJ, Rho SB, Choi YL, Choi CH, Lee JW, Bae DS, Ahn G, Lee JH, Kim BG. 2006. High expression of tissue inhibitor of metalloproteinase-2 in serous ovarian carcinomas and the role of this expression in ovarian tumorigenesis. Hum Pathol. 37:906–913 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Sato H, Okada A, Ohuchi E, Imai K, Okada Y, Seiki M. 1998. TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J Biol Chem. 273:16098–16103 [DOI] [PubMed] [Google Scholar]
- Kowalski P, Surowiak P, Lage H. 2005. Reversal of different drug-resistant phenotypes by an autocatalytic multitarget multiribozyme directed against the transcripts of the ABC transporters MDR1/P-gp, MRP2, and BCRP. Mol Ther. 11:508–522 [DOI] [PubMed] [Google Scholar]
- Lu KV, Jong KA, Rajasekaran AK, Cloughesy TF, Mischel PS. 2004. Upregulation of tissue inhibitor of metalloproteinases (TIMP)–2 promotes matrix metalloproteinase (MMP)–2 activation and cell invasion in a human glioblastoma cell line. Lab Invest. 84:8–20 [DOI] [PubMed] [Google Scholar]
- Manenti L, Paganoni P, Floriani I, Landoni F, Torri V, Buda A, Taraboletti G, Labianca R, Belotti D, Giavazzi R. 2003. Expression levels of vascular endothelial growth factor, matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 and 2 in the plasma of patients with ovarian carcinoma. Eur J Cancer. 39:1948–1956 [DOI] [PubMed] [Google Scholar]
- Materna V, Liedert B, Homale J, Lage H. 2005. Protection of platinum-DNA adduct formation and reversal of cisplatin resistance by anti-MRP2 hammerhead ribozymes in human cancer cells. Int J Cancer. 115:393–402 [DOI] [PubMed] [Google Scholar]
- Materna V, Stege A, Surowiak P, Priebsch A, Lage H. 2006. RNA interference-triggered reversal of ABCC2-dependent cisplatin resistance in human cancer cells. Biochem Biophys Res Commun. 348:153–157 [DOI] [PubMed] [Google Scholar]
- Materna V, Surowiak P, Markwitz E, Spaczynski M, Drag-Zalesinska M, Zabel M, Lage H. 2007. Expression of factors involved in regulation of DNA mismatch repair- and apoptosis pathways in ovarian cancer patients. Oncol Rep. 17:505–516 [PubMed] [Google Scholar]
- Okamoto T, Nui R, Yamada S. 2003. Increased expression of tissue inhibitor of metalloproteinase-2 in clear cell carcinoma of the ovary. Mol Hum Reprod. 9:569–575 [DOI] [PubMed] [Google Scholar]
- Rauvala M, Puistola U, Turpeenniemi-Hujanen T. 2005. Gelatinases and their tissue inhibitors in ovarian tumors; TIMP-1 is a predictive as well as a prognostic factor. Gynecol Oncol. 99:656–663 [DOI] [PubMed] [Google Scholar]
- Remmele W, Stegner HE. 1987. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 8:138–140 [PubMed] [Google Scholar]
- Sakata K, Shigemasa K, Nagai N, Ohama K. 2000. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int J Oncol. 17:673–681 [PubMed] [Google Scholar]
- Shimizu Y, Kamoi S, Amada S, Akiyama F, Silverberg SG. 1998. Toward the development of a universal grading system of ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 82:893–901 [DOI] [PubMed] [Google Scholar]
- Sobin LH, Wittekind C. editors. 1997. UICC TNM classification of malignant tumors. New York: Wiley-Liss [Google Scholar]
- Sternlicht MD, Werb Z. 2001. How matrix metalloproteinases regulate cell behavior. Ann Rev Cell Dev Biol. 17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dietel M, Lage H, Zabel M. 2006. Topoisomerase 1A, HER/2neu and Ki67 expression in paired primary and relapsed ovarian cancer tissue samples. Histol Histopathol. 25:131–139 [DOI] [PubMed] [Google Scholar]
- Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dolinska-Krajewska B, Gebarowska E, Dietel M, Zabel M, Lage H. 2006. ABCC2 (MRP2, cMOAT) can be localized in the nuclear membrane of ovarian carcinoma and correlates with resistance to cisplatin and clinical outcome. Clin Cancer Res. 12:7149–7158 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. 2000. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 92:205–216 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Ishiko O, Sumi T, Matsumoto Y, Oqita S. 2001. Survivin, bcl-2 and matrix metalloproteinase-2 enhance progression of clear cell- and serous-type ovarian carcinomas. Int J Oncol. 19:537–542 [DOI] [PubMed] [Google Scholar]