Abstract
1,25-DihydroxyvitaminD3 (1,25(OH)2D3), the active form of vitamin D, mediates antitumor effects in various cancers. The expression of key players in vitamin D signaling in thyroid tumors was investigated. Vitamin D receptor (VDR) and CYP27B1 and CYP24A1 (respectively activating and catabolizing vitamin D) expression was studied (RT-PCR, immunohistochemistry) in normal thyroid, follicular adenoma (FA), differentiated thyroid cancer (DTC) consisting of the papillary (PTC) and follicular (FTC) subtype, and anaplastic thyroid cancer (ATC). VDR, CYP27B1, and CYP24A1 expression was increased in FA and DTC compared with normal thyroid. However, in PTC with lymph node metastasis, VDR and CYP24A1 were decreased compared with non-metastasized PTC. In ATC, VDR expression was often lost, whereas CYP27B1/CYP24A1 expression was comparable to DTC. Moreover, ATC with high Ki67 expression (>30%) or distant metastases at diagnosis was characterized by more negative VDR/CYP24A1/CYP27B1 staining. In conclusion, increased expression of key players involved in local 1,25(OH)2D3 signaling was demonstrated in benign and differentiated malignant thyroid tumors, but a decrease was observed for local nodal and especially distant metastasis, suggesting a local antitumor response of 1,25(OH)2D3 in early cancer stages. These findings advocate further studies with 1,25(OH)2D3 and analogs in persistent and recurrent iodine-refractory DTC.
Keywords: thyroid, cancer, vitamin D, VDR
Thyroid nodules are very prevalent, especially in iodine-deficient regions, and can be benign adenomas (follicular adenoma [FA]) or malignant lesions. Although only 5% to 10% of the thyroid nodules are malignant, thyroid cancer represents the most common endocrine malignancy. Most thyroid cancers are derived from the follicular thyroid cell and are well-differentiated thyroid cancers (DTC) of the papillary or follicular subtype (respectively PTC and FTC), and have good survival rates (>90% 10-year survival) after standard treatment, consisting of thyroidectomy, in most cases followed by treatment with radioactive iodine (Sherman 2003). A minority of thyroid cancers (<5%) are undifferentiated or anaplastic thyroid cancers (ATC), characterized by dedifferentiation, high aggressiveness, lethality due to failure of standard treatment and, most often, resistance to chemotherapy and external radiation (Kondo et al. 2006). Also in DTC, persistent and recurrent disease remains a therapeutic challenge, especially in radio-iodine refractory cases (Stojadinovic et al. 2002).
Besides the “classical” effects on bone and calcium metabolism, the antiproliferative and redifferentiating capacity of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3)—the active form of vitamin D—on several cancer types, including thyroid cancer (cell lines), has been described (Bouillon et al. 2006). We and others have shown that 1,25(OH)2D3 and especially superagonistic analogs exert antiproliferative effects in poorly to undifferentiated thyroid cancer cell lines. Combined treatment with other antineoplastic agents has led to additive and even synergistic antiproliferative effects (Dackiw et al. 2004; Clinckspoor et al. 2011). 1,25(OH)2D3 exerts its biological activity through binding to the vitamin D receptor (VDR), a member of the nuclear receptor family. VDR polymorphisms and serum 25-OHD3 levels have been associated with cancer incidence (Giovannucci et al. 2006; Kostner et al. 2009). Furthermore, VDR knockout mice exhibit a higher incidence rate of carcinogen-induced lesions compared with their wild-type littermates (Zinser et al. 2005). These studies highlight the potential role of 1,25(OH)2D3–VDR signaling in defining sensitivity to carcinogenesis. The metabolism of 1,25(OH)2D3 is tightly regulated through a complex process, involving the vitamin D–activating enzyme CYP27B1, responsible for the final hydroxylation step from 25-OHD3 toward 1,25(OH)2D3, and CYP24A1, the key enzyme in the inactivation of 1,25(OH)2D3.
Several studies have focused on the dynamics of VDR, CYP24A1, and CYP27B1 in different cancer tissues such as breast, prostate, colon, and ovary (Segersten et al. 2005; McCarthy et al. 2009; Horvath et al. 2010), demonstrating altered expression in benign or malignant tumors. The current knowledge on the presence of locally altered vitamin D metabolism in benign and malignant thyroid tissue is limited. The VDR is expressed in normal thyroid tissue (Mangelsdorf 2005), and increased expression of VDR and CYP27B1 protein compared with normal thyroid tissue was demonstrated in human PTC tissues (Khadzkou et al. 2006). Furthermore, in a panel of thyroid cancer cell lines, it has been shown that VDR is differentially present, not correlating to the sensitivity of the thyroid cancer cell lines to 1,25(OH)2D3, contrary to CYP24A1 mRNA levels that were lower in responsive cell lines (Sharma et al. 2010).
The main purpose of this study was to investigate local vitamin D metabolism in a diverse spectrum of human thyroid tumors, ranging from benign FA to highly malignant ATC, and to study the correlation with markers of proliferation and differentiation and with clinicopathological characteristics.
Materials and Methods
Patients and Tumors
Thyroid tissues were obtained from patients undergoing thyroid surgery at the University Hospitals Leuven in Leuven, Belgium, or at the General Hospital Sint-Jan in Bruges, Belgium. The indication for surgery was based on clinical findings, and snap-frozen thyroid tissues (for RT-PCR analysis) from 64 patients and paraffin-embedded tissues (for immunohistochemistry analysis) from 72 patients were studied. All tumors were histologically classified according to the World Health Organization (WHO) classification. The clinical charts were reviewed and the following patient data were extracted: age at diagnosis, gender, primary tumor diameter, presence of immune cell infiltration, multifocality, and TNM (Edge et al. 2010) for malignancy. Patients with abnormal thyroid function, a toxic nodule, histological diagnosis of underlying autoimmune thyroid disease (Hashimoto or Graves), or nodular hyperplasia at histological examination were excluded, as well as those with partially cystic nodules. The work was approved by the Ethics Committee of the University Hospitals Leuven, and patients gave their written informed consent.
Real-Time Quantitative RT-PCR
Biopsies were taken centrally from the nodule to obtain a pure tumor sample. The samples were immediately snap-frozen in liquid nitrogen and stored at –80°C. In total, 29 FA, 22 FTC, 15 PTC, and 44 normal thyroid tissue samples (from contralateral macroscopically normal lobes) were analyzed. Snap-frozen tumor tissue from only two ATC cases was available, so this subgroup was excluded from the RT-PCR analysis. Total RNA was extracted with TRIzol reagent (Invitrogen; Merelbeke, Belgium). Briefly, 50 to 100 mg thyroid tissue was homogenized and RNA was isolated following phase separation with chloroform, RNA precipitation with isopropanol, RNA wash with 75% ethanol, and eventually RNA redissolving in water. Using Superscript II RT (Invitrogen), 1 µg RNA was reverse transcribed at 42°C for 80 min in the presence of 5 µM oligo(dT) (Roche; Vilvoorde, Belgium). PCR analysis (StepOnePlus; Applied Biosystems, Halle, Belgium) was performed using delta-Ct. Primer and probe sequences (Eurogentec; Seraing, Belgium) for HPRT, VDR, CYP24A1, CYP27B1, NIS, Ki67, and p21 are available upon request. All real-time RT-PCRs were performed with dual-labeled fluorescent Taqman probes. Each experiment was performed with triplicate samples. Expression levels of target genes were compared with HPRT levels in each sample.
Immunohistochemistry
Immunohistochemistry (IHC) for Ki67, VDR, CYP24A1, and CYP27B1 was performed on paraffin-embedded sections from 17 FA, 18 FTC, 20 PTC, 17 ATC, and 31 normal thyroid tissues (see Table 1). Deparaffinization, rehydration, and epitope retrieval were performed using PT Link (Dakocytomation; Heverlee, Belgium). After blocking of endogenous peroxidase activity by Envision Flex Peroxidase blocking reagent (Dako), the sections were incubated with the primary antibody for VDR and CYP24A1 (Table 1). Incubation with the primary antibody against CYP27B1 was preceded by an incubation period with 10% donkey serum. Antibody binding was detected using a second antibody conjugated to a peroxidase-labeled polymer (EnVision; Dako), and peroxidase activity was revealed by diaminobenzidine (DAB) substrate (Dako). Finally, sections were counterstained with hematoxylin and rinsed and mounted with mounting medium (Dako). Kidney tissue was used as a positive control for VDR, CYP24A1, and CYP27B1, whereas omission of the primary antibody was used as a negative control.
Table 1.
Primary antibody and conditions used
| Antibody | Conditions Used |
|---|---|
| Ki67 (clone MIB-1; Dakocytomation, Heverlee, Belgium) | Undiluted, 20 min |
| VDR (sc-13133; Santa Cruz Biotechnology, Heidelberg, Germany) | 1/500, 20 min |
| CYP24A1 (sc-32166; Santa Cruz Biotechnology) | 1/20, 20 min |
| CYP27B1 (kind gift of Dr. R. Bland, University of Warwick, Coventry, UK) | 1/500, overnight |
The interpretation of the IHC staining was performed by two independent investigators (IC and EH). Ki67 expression was quantified as % stained nuclei/100 cells. For VDR, CYP24A1, and CYP27B1, the following method was used: Staining intensity was ranked either 1 (weak), 2 (modest), or 3 (strong). Staining extension was scored 0 (negative), 1 (<10% of cells stained), 2 (10–50% of cells), and 3 (>50% of cells). The scores for intensity and extension were then multiplied, and the following criterion was applied: Cases with a multiplied score of 4 or higher were considered to have a high level of VDR, CYP24A1, or CYP27B1 expression. In the present study, we have used the H-score for immunostaining, also used by another group (Lopes et al. 2010).
Statistical Analysis
Statistical analysis was performed using the software program Statistica (StatSoft; Tulsa, USA). For continuous variables, the unpaired Student’s t-test or ANOVA test was performed where appropriate. When the ANOVA test was significant, the Fisher’s least significant difference (LSD) multiple-comparison test was applied. For categorical variables, the χ2 test was used. Pearson’s correlation analysis was performed to determine correlations between gene or protein expression. Significance was defined at the 0.05 level.
Results
Increased Local Vitamin D Metabolism in Tumor versus Normal Thyroid Tissue
To characterize the proliferation and differentiation status of the frozen thyroid tissue samples used for RT-PCR analysis, mRNA expression of, respectively the general proliferation marker Ki67 and differentiation marker NIS were studied. As expected, DTC (59% FTC and 41% PTC) was characterized by high Ki67 and very low NIS expression compared with normal thyroid tissue (Fig. 1; p<0.02). FA had an intermediary profile characterized by low Ki67 but also low NIS expression.
Figure 1.
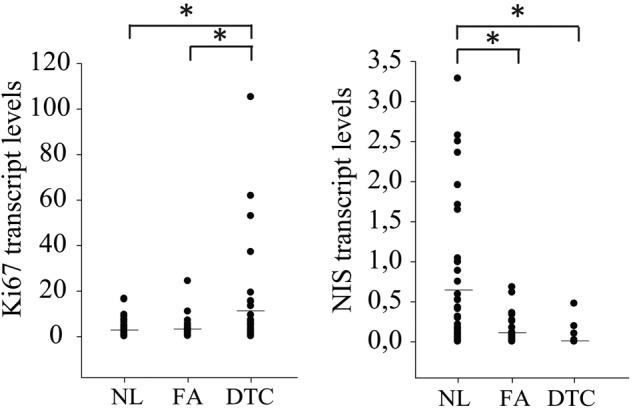
mRNA expression profile of the proliferation marker Ki67 and the differentiation marker NIS in normal thyroid tissue (NL), follicular adenoma (FA), and differentiated thyroid cancer (DTC; consisting of follicular thyroid cancer [FTC] and papillary thyroid cancer [PTC]). Ki67 mRNA levels were clearly enhanced in DTC compared with NL tissue but not in FA. NIS expression was significantly reduced in both DTC and FA compared with NL tissue. Mean mRNA expression is represented by the horizontal bar. *p<0.05.
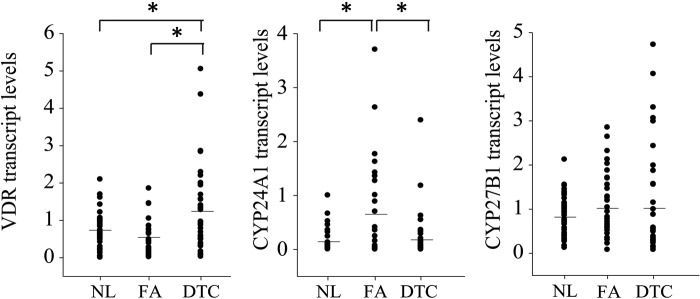
Further study of expression of genes involved in vitamin D metabolism (Fig. 2) first confirmed the presence of VDR in all subgroups, with a 2-fold increased mean expression in DTC compared with normal thyroid tissue and FA. Mean expression of the 1,25(OH)2D3-degrading enzyme CYP24A1 was increased in FA. For the mean expression of CYP27B1, capable of local 1,25(OH)2D3 production, we observed a tendency toward higher expression in FA and DTC. Expression of the three main actors of local vitamin D metabolism in thyroid tissue was thus confirmed but with clearly different dynamics in each histological subgroup. No significant difference in mean VDR, CYP24A1, or CYP27B1 mRNA expressions were observed when comparing the DTC subgroups of PTC and FTC (data not shown).
Figure 2.
mRNA expression profile of vitamin D receptor (VDR), CYP24A1 and CYP27B1 in normal thyroid tissue (NL), follicular adenoma (FA), and differentiated thyroid cancer (DTC). VDR mRNA expression was significantly increased compared with both NL and FA, whereas CYP24A1 mRNA expression was clearly enhanced in FA compared with both NL and DTC. CYP27B1 mRNA expression was slightly higher in FA and DTC compared with NL. Mean mRNA expression is represented by the horizontal bar. *p<0.05.
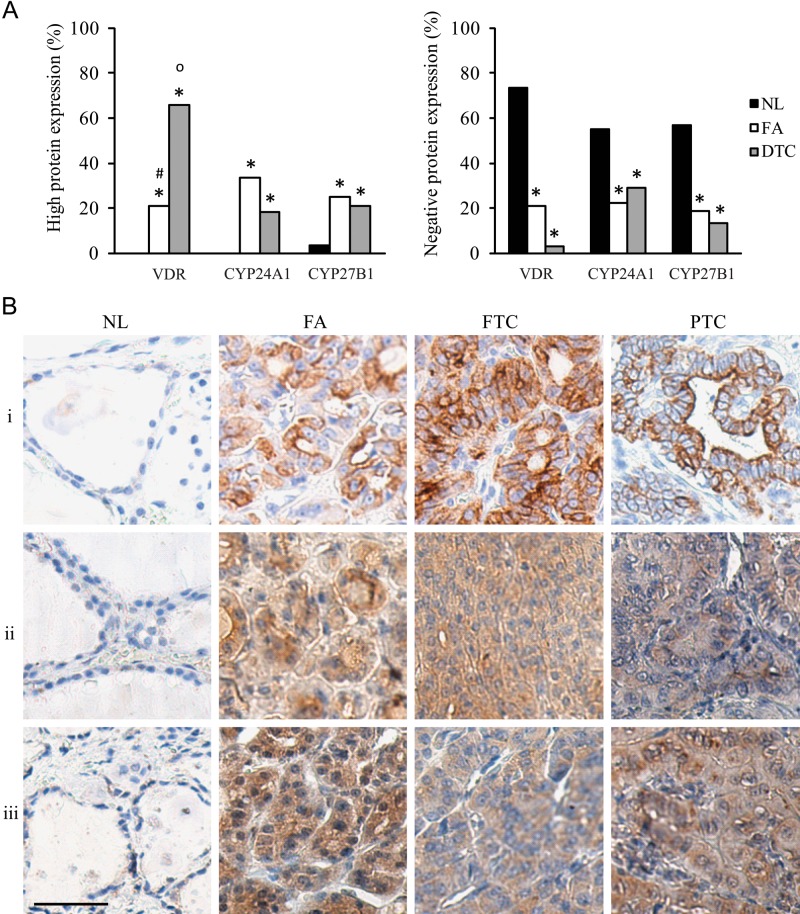
Next, we studied protein expression of VDR, CYP24A1, and CYP27B1 by IHC in identical histological subgroups in a large set of paraffin-embedded tissues, including normal thyroid tissue, FA, and DTC (47% FTC and 53% PTC). Ki67 protein expression was confirmed to be clearly increased in the DTC group (9 ± 0.9% in DTC, 5 ± 2.8% in FA, and 1 ± 0.2% in normal thyroid tissue). As shown in Figure 3A, high VDR expression (multiplied score ≥4; see Materials and Methods) was present in 21% of FA and even in 66% of DTC, but was not detected in normal thyroid tissues. High CYP24A1 and CYP27B1 expressions were also rare in normal tissue (respectively 0% and 2%). However, high CYP24A1 expression was observed in 18% of DTC and even in 33% of FA cases, confirming the mRNA results. High CYP27B1 expression was present in 21% of DTC and 25% of FA. On the other hand, absent VDR, CYP24A1, and CYP27B1 expressions were observed in a lower percentage of FA (respectively 21%, 22%, and 19%) or DTC (3%, 29%, and 13%) compared with normal thyroid tissue (73%, 55%, and 57%). Moreover, within the DTC group, CYP24A1 and CYP27B1 protein expressions were positively correlated (r2 = 0.43, p<0.05), but no correlation was present with tumor diameter or Ki67 expression. Figure 3B shows representative cases of high VDR, CYP24A1, and CYP27B1 expression in the different subgroups.
Figure 3.
“High” (left histogram) and “negative” (right histogram) protein expression profiles of vitamin D receptor (VDR), CYP24A1, and CYP27B1 in normal thyroid tissue (NL), follicular adenoma (FA), and differentiated thyroid cancer (DTC) (A); and representative photographs of “high” VDR, CYP24A1, and CYP27B1 staining in NL, FA, and DTC tissues (B). Immunohistochemistry expression was considered high when the H-score ≥4 (B). (A) High VDR, CYP24A1, and CYP27B1 staining was present in FA and DTC samples, whereas NL tissue was usually characterized by absent staining for the 1,25(OH)2D3 metabolizing proteins. (B) (i) High VDR expression, (ii) high CYP24A1 expression, and (iii) high CYP27B1 expression; staining in NL, FA, and DTC (FTC and PTC). Kidney tissue and omission of the primary antibody provided, respectively, the positive and negative control. Scale bar: 50 µm. *p<0.05 vs NL; op<0.05 vs FA; #p<0.05 vs DTC.
Altered Local 1,25(OH)2D3 Metabolism in N1 versus N0 PTC
Because PTC, by far the most frequent thyroid cancer subtype, frequently locally spreads to the lymph nodes, we further investigated whether differences in local vitamin D metabolism could be observed between PTC with and without lymph node metastases (respectively N1 and N0).
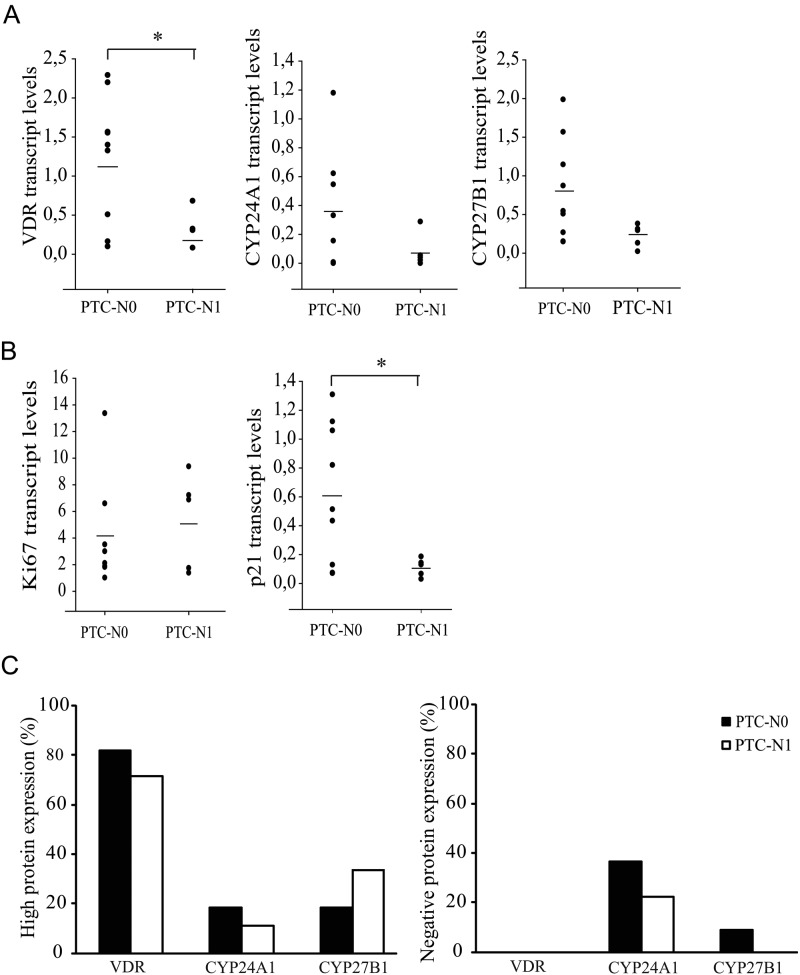
As shown in Figure 4A, mRNA expression of VDR, CYP24A1, and CYP27B1 was lower in PTC N1 compared with N0. Furthermore, PTC N1 tissues were characterized by higher expression of Ki67 and lower expression of the cell cycle inhibitor p21 mRNA compared with PTC N0, indicative of a higher proliferative status (Fig. 4B). Gene expression of VDR was significantly positively correlated with CYP24A1 (r2 = 0.62, p<0.05) and CYP27B1 (r2 = 0.57, p<0.05). Also, the gene expression of the cell cycle inhibitor p21, a well-known target gene of 1,25(OH)2D3, was positively correlated with VDR and CYP24A1 expressions (r2 = 0.76 and r2 = 0.70, respectively; p<0.05).
Figure 4.
mRNA expression profiles of vitamin D receptor (VDR), CYP24A1, and CYP27B1 (A); the proliferation markers Ki67 and p21 (B); and “high” (left histogram) and “negative” (right histogram) protein expression profiles of VDR, CYP24A1, and CYP27B1 in papillary thyroid cancer (PTC) with (N1) or without metastasis (N0) (C). (A) VDR, CYP24A1, and CYP27B1 mRNA expressions were all decreased in PTC N1 compared with PTC N0. (B) PTC N1 expressed higher Ki67 and decreased p21 mRNA levels compared with PTC N0. (C) Decreased VDR and CYP24A1 protein expressions were present in PTC N1, whereas CYP27B1 protein expression was slightly enhanced compared with PTC N0. Mean mRNA expression is represented by a horizontal bar. *p<0.05.
At the protein level (Fig. 4C), 71% of PTC N1 showed high VDR expression versus 82% of PTC N0, and 11% of PTC N1 showed high CYP24A1 expression versus 18% of PTC N0. On the other hand, negative VDR staining was not observed in any PTC case, and negative CYP24A1 staining was present in a lower percentage of the PTC N1 cases (22% vs 36%). For CYP27B1, however, more “high” (33% vs 18%) and no “negative” (0% vs 9.0%) protein expression was observed in the PTC N1 group contrary to the gene expression data. A positive correlation was present between CYP24A1 and CYP27B1 protein expression and was higher than in the more heterogeneous DTC group (r2 = 0.49, p<0.05).
Compared to DTC, Decreased Vitamin D Metabolism Is Present in ATC, Especially in Case of High Ki67 Expression or Distant Metastasis at Diagnosis
As local vitamin D metabolism was increased in FA and DTC, respectively representing mostly slowly growing benign and malignant thyroid tumor tissue, we finally studied local vitamin D metabolism along with Ki67 expression at the protein level in ATC (17 cases), characterized by aggressive growth (with the presence of distant metastases already at diagnosis in about half of the cases), in comparison with the DTC group (32 cases, 47% FTC and 53% PTC).
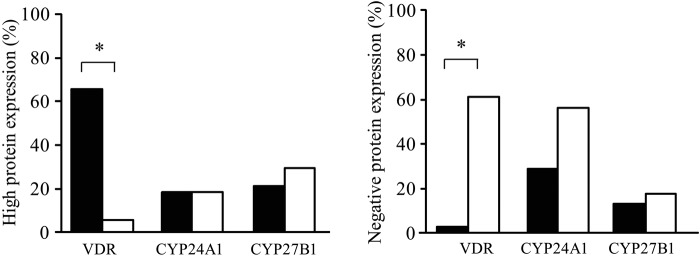
ATC was characterized by more than 3-fold increased Ki67 protein expression as compared with DTC (33 ± 3.9% and 9 ± 0.9%, respectively; p<1E–10). As shown in Figure 5, high VDR expression was rare in ATC patients (6%) in contrast to DTC (66%), and the opposite was observed for the percentage of VDR-negative tissues (61% vs 3%). However, the proportion of high CYP24A1 expression was comparable in ATC and DTC (respectively 19% and 18%), whereas high CYP27B1 expression was even slightly higher in ATC compared with DTC (respectively 29% and 21%). But again, proportionally more ATC cases were observed with negative CYP24A1 and CYP27B1 staining (56% and 18%) compared with DTC cases (29% and 13%). A strong positive correlation was present between CYP24A1 and CYP27B1 protein expression (r2 = 0.80, p<0.05).
Figure 5.
“High” (left histogram) and “negative” (right histogram) protein expression profiles of vitamin D receptor (VDR), CYP24A1, and CYP27B1 in differentiated thyroid cancer (DTC) and anaplastic thyroid cancer (ATC). VDR protein expression was often lost in ATC compared with DTC. High CYP24A1 and CYP27B1 protein expressions were respectively similar and even slightly higher in ATC compared with DTC, but also more cases of absent CYP24A1 and CYP27B1 staining were found in ATC. *p<0.05.
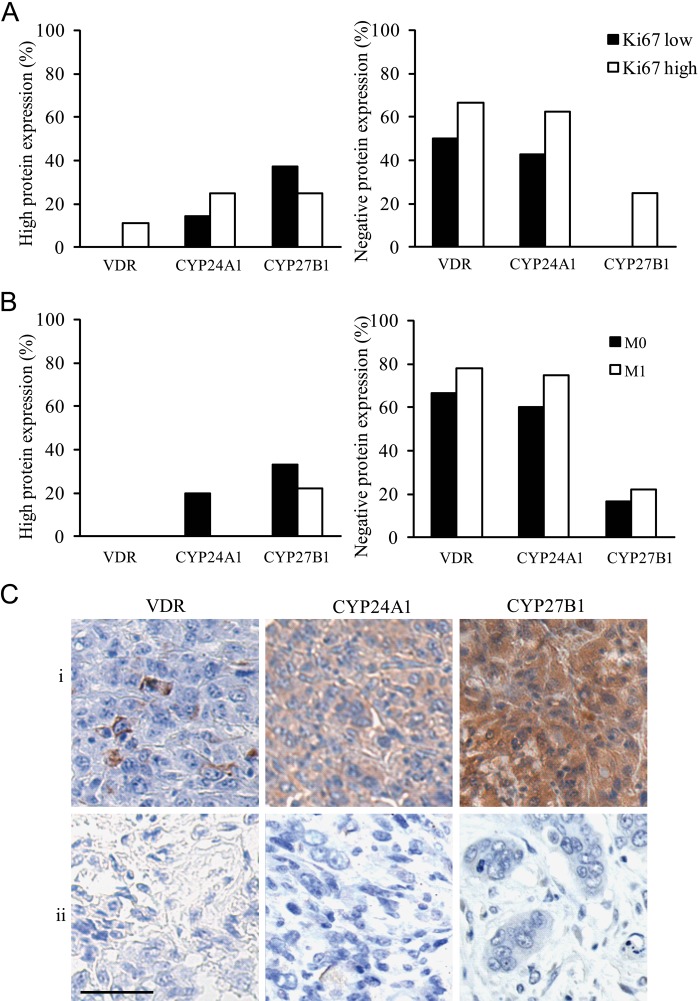
Within the ATC group, a different VDR, CYP24A1, and CYP27B1 expression profile was observed in Ki67 “high” (>30%, n=8) versus “low” (≤30%, n=8) cases, especially when observing the percentage of negative staining. As shown in Figure 6A, in the Ki67 “high” group, more cases lost VDR (66.7%), CYP24A1 (63%), and CYP27B1 (25%) protein expressions compared with the Ki67 “low” group (respectively 50%, 43%, and 0%).
Figure 6.
“High” (left histogram) and “negative” (right histogram) protein expression profile of vitamin D receptor (VDR), CYP24A1, and CYP27B1 in anaplastic thyroid cancer (ATC) subdivided according to their Ki67 status (low, high) (A) and according to the presence of metastasis (M0, M1) at diagnosis (B) and representative photographs of a triple-negative (absent VDR, CYP24A1, and CYP27B1) and triple-positive staining in ATC subdivided according to their Ki67 status (C). (A) More cases of absent VDR, CYP24A1, or CYP27B1 staining were present in ATC cases with a Ki67 “high” status compared with a Ki67 “low” status. (B) Also, more cases of absent VDR, CYP24A1, or CYP27B1 staining were found in ATC M1 compared with ATC M0. (C) Representative photographs of two ATC cases with either a triple-positive or a triple-negative staining for VDR, CYP24A1, and CYP27B1 in, respectively the (i) Ki67 low and (ii) Ki67 high ATC subgroup. Scale bar: 50 µm.
Also, when comparing the ATC cases with and without distant metastases at diagnosis (respectively M1 and M0; for 3 patients, no information on metastatic status was known due to short survival), we observed the tendency toward a further decrease of local vitamin D metabolism in the ATC M1 group (Fig. 6B). More specifically, in ATC M1 tissues, VDR, CYP24A1, and CYP27B1 expressions were negative in a higher proportion (respectively 77.8%, 75.0%, and 22.2%) compared with ATC M0 tissues (respectively 66.7%, 60.0%, and 16.7%). In Figure 6C, a representative case of a Ki67 low triple-positive and a Ki67 high triple-negative ATC is shown.
Discussion
To investigate the role of 1,25(OH)2D3 in tumorigenesis and its potential as an anticancer treatment, the presence of 1,25(OH)2D3-regulating proteins has been studied in several malignancies. In the present study, we described and confirmed for the first time the gene and protein expression profiles of VDR, CYP24A1, and CYP27B in a diverse spectrum of human thyroid tumor and normal tissues. Compared with normal thyroid tissue, the three key players involved in the regulation of 1,25(OH)2D3 were present or even enhanced in all cancer subtypes from the thyroid follicular cell origin, suggesting increased local 1,25(OH)2D3 metabolism. However, this enhanced metabolism is partially or completely lost in thyroid cancers metastasizing regionally or at distance. The gene expression mostly, but not always, paralleled the protein expression results.
The presence of VDR in the normal thyroid was previously described (Mangelsdorf 2005). Later, enhanced VDR and CYP27B1 protein expression was demonstrated in PTC compared with normal human thyroid tissue (Khadzkou et al. 2006). In our study, we largely extended the investigation to other thyroid tumor subtypes by comparing the expression profile of VDR, CYP24A1, and CYP27B1 in normal thyroid tissue, follicular adenoma, DTC (papillary and follicular subtypes), and ATC.
Both FA and DTC demonstrated enhanced VDR, CYP24A1, and CYP27B1 expressions compared with normal thyroid tissue. Increased vitamin D metabolism has also been described in several other benign and malignant tumors (ovarium, endometrium, colon, breast) (Segersten et al. 2005; Agic et al. 2007; McCarthy et al. 2009; Horvath et al. 2010; Silvagno et al. 2010), and 1,25(OH)2D3 is known to exert antiproliferative effects on several cancer types (Dackiw et al. 2004; Bouillon et al. 2006; Clinckspoor et al. 2011). However, other groups reported decreased VDR, CYP24A1, or CYP27B1 expression in cancerous tissue (benign/malignant) compared with normal tissue, such as for breast, colon, and kidney cancer (Lurie et al. 2007; Wada et al. 2009; Blomberg et al. 2010). The expression of VDR, CYP24A1, and CYP27B1 in tumors might be tissue-specific, and variation even exists between research groups studying the same cancer type. Protein instability, protein half-life, or methodological differences can result in discrepancies between gene and protein expression and variation between research groups (Horvath et al. 2010).
Comparing well-differentiated malignant thyroid tumors with benign tumors, we observed significantly higher VDR and lower CYP24A1 expressions, whereas CYP27B1 expression was similar. The high VDR/low CYP24A1 profile in DTC might suggest a stronger antitumor response, increasing local availability of 1,25(OH)2D3.
A pivotal step in cancer progression is metastasis. Because PTC not only represents the most prevalent thyroid cancer but also frequently locally spreads to the lymph nodes, we investigated differential expression of VDR, CYP24A1, and CYP27B1 in primary PTC with or without lymph node metastasis at diagnosis. First, we demonstrated decreased VDR expression in PTC N1, which is in line with the findings of Khadzkou et al. (2006), who observed a decreased VDR protein expression in tumor tissue from lymph node metastasis compared with primary PTC. Together, these data suggest a decreased 1,25(OH)2D3 sensitivity in primary tumors prone to metastasis. On the other hand, the decreased CYP24A1 and increased CYP27B1 protein expressions in the primary PTC lesions with lymph node metastasis might suggest a local antitumor response resulting in elevated local 1,25(OH)2D3 levels.
In ATC tissues, VDR expression is lost in the majority of the cases, whereas CYP24A1 and CYP27B1 expressions were comparable with the DTC tissues. Negative staining for VDR, CYP24A1, and CYP27B1 was more pronounced in ATC tissues with high Ki67 expression and ATC cases with the presence of distant metastases at diagnosis. Thus, tumor progression to highly proliferative and/or metastasizing ATC is characterized by the absence of key players of local vitamin D metabolism or sensitivity for exogenous 1,25(OH)2D3. Similarly, Cross et al. (1996) demonstrated decreased VDR and CYP27B1 gene expression in the progression of low- to high-grade colon cancer. Also, in breast cancer, evidence for reduced local vitamin D metabolism was present in invasive compared with in situ breast cancer tissue (Lopes et al. 2010). On the other hand, increased VDR expression was observed in metastatic or angioinvasive clear cell renal cell carcinoma (Liu et al. 2006).
Thyroid tumor progression is affected by defects in cell cycle regulators (cyclin D1, retinoblastoma protein, p21) and adhesion molecules (fibroblast growth factor [FGF] and its receptors) (Kondo et al. 2006). These molecules are also well known to be targeted by 1,25(OH)2D3. Specifically in thyroid cancer, we and others have shown that 1,25(OH)2D3 inhibits cell proliferation through decreased expression of E2F1 and accumulation of p27, resulting in an increased percentage of cells in the G0–G1 phase of the cell cycle and decreased Ki67 expression (Liu et al. 2002; Dackiw et al. 2004; Liu et al. 2005; Clinckspoor et al. 2011). Similarly, in the present study, the decreased VDR and CYP24A1 mRNA expression in PTC N1 was accompanied by a decreased p21 expression, and we observed a significant positive correlation between VDR and CYP24A1 with p21, a cyclin-dependent kinase inhibitor. Others have described diminished p27 (another cyclin-dependent kinase) and enhanced cyclin D1 in PTC N1. Furthermore, they showed that p27 underexpression and cyclinD1 overexpression were predictors of lymph node metastasis (Khoo et al. 2002). In other cancer types, such as colon cancer, a positive correlation between CYP24A1 and Ki67 was described (Horvath et al. 2010). All these observations suggest an association between genes regulating local 1,25(OH)2D3 signaling and genes involved in proliferation, with the potential of increased local 1,25(OH)2D3 arresting or retarding tumor progression in a differentiation-specific and tissue-specific manner.
Increased CYP24A1 expression, a key target of 1,25(OH)2D3, indicates intensive 1,25(OH)2D3–VDR signaling. However, high CYP24A1 also results in a faster 1,25(OH)2D3 breakdown. Over the past years, a wide array of 1,25(OH)2D3 analogs have been designed, including non-hypercalcemic analogs with CYP24A1-resistant capacities, encouraging the study of 1,25(OH)2D3 as a potential antiproliferative agent in the treatment of thyroid cancer. Also, the correlation between the altered profiles of 1,25(OH)2D3 signaling genes and cell cycle genes reinforces the potential of 1,25(OH)2D3 to halt early thyroid cancer progression.
In conclusion, we demonstrated enhanced presence of key players of 1,25(OH)2D3 signaling in benign and well-differentiated thyroid tumors, suggesting a local antitumor response by 1,25(OH)2D3. The presence of lymph node metastases in PTC was associated with decreased local vitamin D metabolism in the primary tumor, and the presence of distant metastases in ATC was associated with complete loss of local vitamin D metabolism in the primary tumor. In particular, patients with more aggressive forms of DTC (e.g., with extrathyroidal extension, multiple bilateral lymph node metastases, locally recurrent disease, iodine refractory) and possibly a small subgroup of patients with Ki67 “low” anaplastic thyroid cancer might therefore benefit from treatment with vitamin D analogs.
Acknowledgments
We thank Dr. Tom Vauterin and Dr. Catherine Dick (General Hospital Sint Jan, Bruges, Belgium) and Josiane Brebels, Katrien Konings, Gerda Luyckx, and Leen Janssen (University Hospitals Leuven) for their contribution to the thyroid tissue sample collection. We are grateful to Martine Gilis, Emily Bittoun, Wim Cockx, and Ine Beullens (Laboratory for Experimental Medicine and Endocrinology) for their invaluable technical assistance.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Flanders Research Foundation (clinical FWO fellowship for CM and BD and FWOG.0587.09; G.0859.11) and by the University of Leuven (GOA/04/10).
References
- Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, Friedrich M, Taylor RN, Hornung D. 2007. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci. 14:486–497 [DOI] [PubMed] [Google Scholar]
- Blomberg JM, Andersen CB, Nielsen JE, Bagi P, Jorgensen A, Juul A, Leffers H. 2010. Expression of the vitamin D receptor, 25-hydroxylases, 1alpha-hydroxylase and 24-hydroxylase in the human kidney and renal clear cell cancer. J Steroid Biochem Mol Biol. 121:376–382 [DOI] [PubMed] [Google Scholar]
- Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. 2006. Vitamin D and cancer. J Steroid Biochem Mol Biol. 102:156–162 [DOI] [PubMed] [Google Scholar]
- Clinckspoor I, Verlinden L, Overbergh L, Korch C, Bouillon R, Mathieu C, Verstuyf A, Decallonne B. 2011. 1,25- Dihydroxyvitamin D3 and a superagonistic analog in combination with paclitaxel or suberoylanilide hydroxamic acid have potent antiproliferative effects on anaplastic thyroid cancer. J Steroid Biochem Mol Biol. 124:1–9 [DOI] [PubMed] [Google Scholar]
- Cross HS, Bajna E, Bises G, Genser D, Kallay E, Potzi R, Wenzl E, Wrba F, Roka R, Peterlik M. 1996. Vitamin D receptor and cytokeratin expression May be progression indicators in human colon cancer. Anticancer Res. 16:2333–2337 [PubMed] [Google Scholar]
- Dackiw AP, Ezzat S, Huang P, Liu W, Asa SL. 2004. Vitamin D3 administration induces nuclear p27 accumulation, restores differentiation, and reduces tumor burden in a mouse model of metastatic follicular thyroid cancer. Endocrinology. 145:5840–5846 [DOI] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. editors. 2010. AJCC cancer staging manual. 7th ed. New York: Springer [Google Scholar]
- Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. 2006. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 98:451–459 [DOI] [PubMed] [Google Scholar]
- Horvath HC, Lakatos P, Kosa JP, Bacsi K, Borka K, Bises G, Nittke T, Hershberger PA, Speer G, Kallay E. 2010. The candidate oncogene CYP24A1: a potential biomarker for colorectal tumorigenesis. J Histochem Cytochem. 58:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadzkou K, Buchwald P, Westin G, Dralle H, Akerstrom G, Hellman P. 2006. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D receptor expression in papillary thyroid carcinoma. J Histochem Cytochem. 54:355–361 [DOI] [PubMed] [Google Scholar]
- Khoo ML, Beasley NJ, Ezzat S, Freeman JL, Asa SL. 2002. Overexpression of cyclin D1 and underexpression of p27 predict lymph node metastases in papillary thyroid carcinoma. J Clin Endocrinol Metab. 87:1814–1818 [DOI] [PubMed] [Google Scholar]
- Kondo T, Ezzat S, Asa SL. 2006. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 6:292–306 [DOI] [PubMed] [Google Scholar]
- Kostner K, Denzer N, Muller CS, Klein R, Tilgen W, Reichrath J. 2009. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 29:3511–3536 [PubMed] [Google Scholar]
- Liu W, Asa SL, Ezzat S. 2005. 1alpha,25-Dihydroxyvitamin D3 targets PTEN-dependent fibronectin expression to restore thyroid cancer cell adhesiveness. Mol Endocrinol. 19:2349–2357 [DOI] [PubMed] [Google Scholar]
- Liu W, Asa SL, Fantus IG, Walfish PG, Ezzat S. 2002. Vitamin D arrests thyroid carcinoma cell growth and induces p27 dephosphorylation and accumulation through PTEN/akt-dependent and -independent pathways. Am J Pathol. 160:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Tretiakova M, Kong J, Turkyilmaz M, Li YC, Krausz T. 2006. Expression of vitamin D3 receptor in kidney tumors. Hum Pathol. 37:1268–1278 [DOI] [PubMed] [Google Scholar]
- Lopes N, Sousa B, Martins D, Gomes M, Vieira D, Veronese LA, Milanezi F, Paredes J, Costa JL, Schmitt F. 2010. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer. 10:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie G, Wilkens LR, Thompson PJ, McDuffie KE, Carney ME, Terada KY, Goodman MT. 2007. Vitamin D receptor gene polymorphisms and epithelial ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 16:2566–2571 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf TJ. 2005. Tissue-specific expression patterns of nuclear receptors. www.nursa.org/10.1621/datasets.02001
- McCarthy K, Laban C, Bustin SA, Ogunkolade W, Khalaf S, Carpenter R, Jenkins PJ. 2009. Expression of 25-hydroxyvitamin D-1-alpha-hydroxylase, and vitamin D receptor mRNA in normal and malignant breast tissue. Anticancer Res. 29:155–157 [PubMed] [Google Scholar]
- Segersten U, Holm PK, Bjorklund P, Hessman O, Nordgren H, Binderup L, Akerstrom G, Hellman P, Westin G. 2005. 25-Hydroxyvitamin D3 1alpha-hydroxylase expression in breast cancer and use of non-1alpha-hydroxylated vitamin D analogue. Breast Cancer Res. 7:R980–R986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Fretwell D, Crees Z, Kerege A, Klopper JP. 2010. Thyroid cancer resistance to vitamin D receptor activation is associated with 24-hydroxylase levels but not the ff FokI polymorphism. Thyroid. 20:1103–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SI. 2003. Thyroid carcinoma. Lancet. 361:501–511 [DOI] [PubMed] [Google Scholar]
- Silvagno F, Poma CB, Realmuto C, Ravarino N, Ramella A, Santoro N, D’Amelio P, Fuso L, Pescarmona G, Zola P. 2010. Analysis of vitamin D receptor expression and clinical correlations in patients with ovarian cancer. Gynecol Oncol. 119:121–124 [DOI] [PubMed] [Google Scholar]
- Stojadinovic A, Shoup M, Nissan A, Ghossein RA, Shah JP, Brennan MF, Shaha AR. 2002. Recurrent differentiated thyroid carcinoma: biological implications of age, method of detection, and site and extent of recurrence. Ann Surg Oncol. 9:789–798 [DOI] [PubMed] [Google Scholar]
- Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, Kubo N, Muguruma K, Yamada N, Yashiro M, et al. 2009. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 22:1021–1025 [DOI] [PubMed] [Google Scholar]
- Zinser GM, Suckow M, Welsh J. 2005. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 97:153–164 [DOI] [PubMed] [Google Scholar]