Abstract
Amelogenesis involves the coordinated expression of a set of molecules that includes enamel matrix proteins and calcium-binding proteins. Msx2 is a member of the divergent homeobox gene family and is instrumental in dental morphogenesis and biomineralization. This study focused on an EF-hand calcium-binding protein, calbindin-D28k, which is highly expressed in dental epithelium. In vivo data showed that calbindin-D28k levels were higher in ameloblasts from Msx2+/− mice than Msx2+/+ mice. Consistent with this finding, calbindin-D28k distribution was affected in transgenic mice with ectopic expression in root epithelium in rests of Malassez in Msx2+/− and more clearly in Msx2−/− mice. In accordance with these in vivo data, calbindin-D28k protein and mRNA levels were decreased in LS8 ameloblast-like cells by exogenous Msx2 overexpression. Furthermore, calbindin-D28k promoter activity (nt-1075/+34) was specifically diminished in the presence of Msx2 overexpression, showing that Msx2 behave as a transcriptional repressor for calbindin-D28k gene expression. In conclusion, Msx2 may control the spatiotemporally restricted frame of calbindin-D28k production in the dental epithelium in relation to enamel mineralization, as previously shown for amelogenin.
Keywords: dental epithelium, calbindin-D28k, Msx2, ameloblast differentiation, calcium
Enamel is unique in the vertebrate skeleton. This exceptionally acellular and highly mineralized tissue is formed by epithelium-derived ameloblasts. The functional differentiation of ameloblasts is driven by several developmental genes, including homeobox genes such as Tbx1 (Catón et al. 2009) and Msx2 (Satokata et al. 2000; Molla et al. 2010). Ameloblasts timely produce specific proteins whose self-assembly lends enamel its unique architecture (Hu et al. 2007). However, dental epithelium also comprises other cells such as the stratum intermedium, the stellate reticulum, the outer dental epithelium, or the root epithelium that are not directly involved in amelogenesis (Bosshardt and Nanci 2004; Tummers and Thesleff 2009). In adult physiology, dental epithelium forms rests of Malassez that also express a limited number of genes involved in enamel formation (e.g., calretinin but not calbindin-D28k; Korkmaz et al. 2010).
In vivo, several pathways driving epithelial stem cell niches, ameloblast cell differentiation, and crown-root transition have so far been deciphered (Tummers and Thesleff 2009). They provide an interpretation of evolutionary changes that lead to tooth type and shape biodiversity. Ameloblast-related gene expression patterns are also under the spotlight in studies of various pathophysiological situations: When crown and root are formed, there is an ectopic and/or abnormally high expression of enamel proteins in non-ameloblastic cells. This pattern has been documented in root epithelial cells during repair (Nishio et al. 2010) and in Msx2−/− mice (Molla et al. 2010).
This study focused on calcium-binding proteins, as some of them are highly expressed in ameloblasts but not significantly expressed in the non-ameloblastic cells (Hotton et al. 1995; Hubbard 1995; Berdal et al. 1996; Korkmaz et al. 2010). Calbindin-D28k is an intracellular EF-hand calcium-binding protein that is highly concentrated in vitamin D–dependent calcium-transporting epithelia, including those in the kidney, intestine, and enamel. Calbindin-D28k represents 1% of total cytosolic proteins in dental epithelium (Berdal et al. 1996). Various studies have focused on transcriptional regulations of calbindin-D28k expression (Gill and Christakos 1993, 1995) but it has yet to be explored in dental cells.
Msx and Dlx transcription factors are expressed in the dental epithelium (Lézot et al. 2000; Ghoul-Mazgar et al. 2005) and have been shown to control amelogenin expression (Lézot et al. 2008; Venugopalan et al. 2011). More specifically, Msx2-null mutant mice exhibit defective amelogenesis (Satokata et al. 2000) due to the decrease in laminin-5α3, which maintains the organized structure of the ameloblast layer (Bei et al. 2004; Molla et al. 2010). In a syndromic case of amelogenesis imperfecta with cleft lip and palate and polycystic kidney disease, a T447C missense mutation was found that results in the conversion of methionine to threonine at position 129 (Suda et al. 2006). This further highlights the important role of Msx2 during amelogenesis. In vitro, Msx2 represses amelogenin expression by interacting with C/EBPα, which activates amelogenin transcription (Zhou et al. 2000; Xu et al. 2007). In vivo, haploinsufficiency in Msx2+/− mice leads to increased amelogenin expression, enamel thickness, and rod size, confirming the repressor role played by Msx2 in enamel morphogenesis (Molla et al. 2010).
Although calbindins have been shown to be vitamin D-dependent (Berdal et al. 1993), the regulatory effectors controlling calbindin-D28k basal expression remain unknown in dental cells. Mouse calbindin-D28k promoter has been characterized in terms of basal activity and response to 1,25-(OH)2 vitamin D3 (Gill and Christakos 1993), estrogen, and retinoic acid (Wang and Christakos 1995). Here, we investigated the role of Msx2 in the regulation of calbindin-D28k gene expression in dental epithelial cells. This study was carried out in vivo in Msx2 knock-in mice and in vitro by measuring calbindin-D28k promoter activity (nt-1075/+ 34), RNA, and protein levels in mouse ameloblast-like LS8 cells.
Materials and Methods
Collection of Biological Samples
Msx2 gene knock-in (KI) mutant mice were generated by inserting the bacterial lacZ gene into the Msx2 gene coding sequences leading to a dental phenotype (Aïoub et al. 2007). The same litters were used to analyze wild-type (Msx2+/+), heterozygous, and homozygous (Msx2−/−) mice. This study was performed in accordance with the French National Consultative Committee on Bioethics for Health and Life Sciences, following ethical guidelines for animal care. All experiments were performed by staff that had been trained to perform in vivo studies.
Cell Culture and Transfections
The LS8 cell line was previously established by immortalizing primary cultures of enamel organ epithelium with SV40 large T-antigen (gift from M. Snead, Center for Craniofacial Molecular Biology, University of Southern California, Los Angeles). Cells were maintained in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin (all from Sigma; La Verpillière, France). After reaching 40% confluence (24 hr after plating), LS8 cells were transfected according to the manufacturer’s instructions (Qiagen; Courtaboeuf, France). At 48 hr posttransfection, cells were either collected for RNA and protein extractions or lysed for calbindin-D28k promoter activity assays.
Msx2 cDNA cloned into the pcDNA3 expression vector (Dr. Abate-Shen, Cancer Institute of New Jersey) was used for Msx2 overexpression studies. Mouse calbindin-D28k promoter CAT construct (nt-1075/+34) was used for promoter activity investigations (Sylvia Christakos, University of Medicine and Dentistry of New Jersey, Newark), and pGL4.13 (Promega; Madison, WI) was used for transfection efficiency standardization. Final 25 nM Msx2 siRNAs (5-CAGUACCUGUCCAUAGCAG) or scrambled siRNAs used as controls (Eurogentec; Angers, France) were transfected using lipofectamine according to the manufacturer’s procedure (Invitrogen; Saint Aubin, France) to directly and specifically repress Msx2 expression.
Immunohistochemistry
Fourteen-day-old Msx2+/+, Msx2+/−, and Msx2−/− mouse mandibles were collected and fixed overnight in 4% paraformaldehyde. The mandibles were then decalcified in ethylenediaminetetraacetate, dehydrated, and embedded in paraffin. Then, 8-µm sections were cut, deparaffinized, rehydrated, and stained for immunohistochemistry analysis. After a 1-hr incubation with rabbit anti–calbindin-D28k (Swant, Bellinzona, Switzerland; 1/1000) or rabbit anti–β-galactosidase polyclonal antibodies (Abcam, Cambridge, MA; 1/500) (for Msx2 expression), slides were incubated with the goat anti-rabbit horseradish peroxidase (HRP)–conjugated polyclonal antibody (DAKO, Glostrup, Denmark; 1/ 200). All incubations were performed at room temperature.
Western Blot Analysis
Whole-cell lysates were prepared from transfected LS8 cells. After centrifugation at 3000 rpm for 15 min at 4C, protein concentrations of the supernatants were determined using a BCA protein assay kit (Pierce Thermo Scientific, Rockford, IL). Proteins were separated by electrophoresis and electroblotted onto PVDF membranes (Pierce Thermo Scientific). The membranes were first incubated with a primary antibody, that is, either anti–calbindin-D28k 1/1000 (Swant) or anti-actin 1/5000 (Santa Cruz Biotechnology, Santa Cruz, CA; Sigma, St. Louis, MO) and finally with goat anti-rabbit peroxidase-conjugated secondary antibody (1/2000). The Immobilon enhanced chemiluminescent detection system (DAKO) was used to detect bound antibodies quantified in a LAS-4000 Imaging Analysis System (FUJIFILM Medical Systems, Asnières, France).
Real-Time PCR Analysis
Total RNAs from dental epithelial cells were isolated using TriReagent (Sigma) according to the manufacturer’s instructions. Reverse transcription (RT) was performed on 1 µg of RNA template with SuperScript II RNase H Reverse Transcriptase (Invitrogen). All cDNA samples were diluted 100-fold, and quantitative PCR (qPCR) reactions were performed in triplicate using a Roche Light Cycler real-time PCR instrument (Roche Diagnostics, Neuilly-sur-Seine, France; Table 1). Mean, standard deviation (SD), and coefficient of variation (CV) of the triplicate qPCR reactions were calculated for each reaction plate.
Table 1.
Quantitative PCR Primers
| Gene Symbol | Primers | Annealing Temperature | Product Size (bp) | |
|---|---|---|---|---|
| Calbindin-D28k | Forward | 5′-ACGGAAGTGGTTACCTGGAA-3′ | 60 | 552 |
| Reverse | 5′-CACACATTTTGATTCCCTGG-3′ | |||
| GAPDH | Forward | 5′-AACCATAGGAAGGATACGGCTG-3′ | 058 | 450 |
| Reverse | 5′-GGGGTGTGGTAACATCAGCAC-3′ | |||
| Msx2 | Forward | 5′-CCTGAGGAAACACAAGACCA-3′ | 60 | 278 |
| Reverse | 5′-AGTTGATAGGGAAGGGCAGA-3′ |
Chloramphenicol Acetyl-Transferase and Luciferase Assays
Cells were lysed using a chloramphenicol acetyl-transferase (CAT)–ELISA kit according to the manufacturer’s instructions (Roche Diagnostics, Mannheim, Germany). Cell lysates were cleared by centrifugation, frozen, and stored at −80C until use. CAT activity was measured using the CAT-ELISA kit, and the resulting reaction was read at 450 nm on a FLUOstar Omega microplate reader (BMG LABTECH, Offenburg, Germany).
Luciferase activity was used as a transfection standard (1/10 of transfected plasmids) and was measured by mixing 25 µl of protein extract with 75 µl of Luciferase Assay Substrate (Promega).
Statistical Analysis
Unless otherwise stated, all data are presented as mean ± standard error of the mean (SEM). Statistical significance (*p<0.05 and **p<0.01) was determined by Student’s t-test or analysis of variance (ANOVA).
Results
Calbindin-D28k Expression in Msx2+/− and Msx2−/− Compared with Msx2+/+ Mice
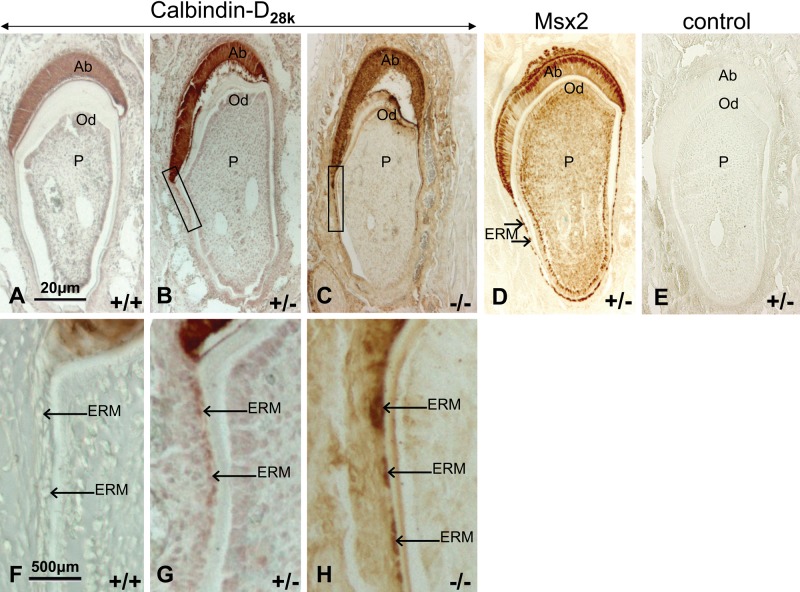
A strong immunoreactivity to calbindin-D28k was localized in the cytoplasm of secretion stage ameloblasts from Msx2+/+, Msx2+/−, and Msx2−/− mice (Fig. 1A–C). Calbindin-D28k protein was also present in hypertrophic epithelial rests of Malassez (ERM) (arrows) from Msx2+/− and Msx2−/− mice and appeared upregulated depending on Msx2 gene dosage (Fig. 1B, C; see Fig. 1G, H for greater magnification). It was not detected in ERM cells in Msx2+/+ mice (Fig. 1A, F). The overall structure of the ameloblast layer was affected in Msx2−/− incisor as previously described (Molla et al. 2010).
Figure 1.
Calbindin-D28k expression in mouse incisor. Calbindin-D28k protein was comparatively immunodetected in the incisors of 14-day-old Msx2+/+ (A, F), Msx2+/− (B, G), and Msx2−/− (C, H) mice. Tissue sections (in ameloblast secretion stage) were fixed in 4% paraformaldehyde, decalcified in ethylenediaminetetraacetate, and embedded in paraffin. Then, 8-µm sections were analyzed by immunohistochemistry with rabbit anti–calbindin-D28k or rabbit anti–β-galactosidase polyclonal antibodies (for Msx2) (D). Control sections (E) were incubated without primary antibody. Arrows indicate calbindin-D28k increased staining in epithelial rests of Malassez cells from Msx2+/+ to Msx2−/− incisors. Ab, ameloblasts; Od, odontoblasts; P, pulp; ERM, epithelial rests of Malassez. Top row, scale bar = 20 µm; bottom row, scale bar = 500 µm.
Msx2 protein was located in the nuclei of dental cells of 14-day-old Msx2+/− mouse incisor sections (Fig. 1D). The control (without the primary antibody) was negative (Fig. 1E).
Msx2 Decreased Endogenous Calbindin-D28k mRNA and Protein Levels
LS8 cells were transiently transfected with increasing amounts of Msx2 expression vector to study the effect of Msx2 on endogenous calbindin-D28k expression.
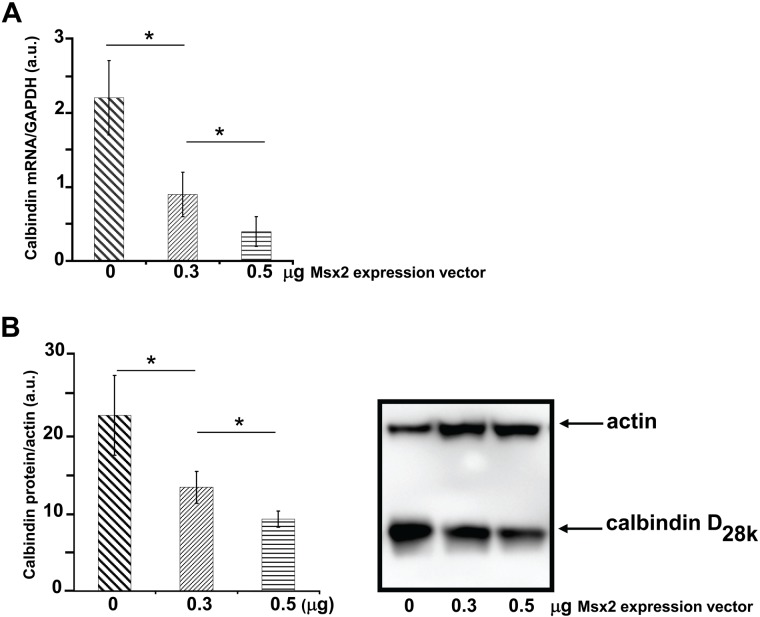
RT-qPCR analysis showed that calbindin-D28k transcript expression was 2.4-fold and 6.0-fold lower in the presence of 0.3 and 0.5 µg Msx2 vector, respectively (both p<0.05) (Fig. 2A).
Figure 2.
Msx2-mediated dose-dependent inhibition of calbindin-D28k expression. (A) Calbindin-D28k transcript levels were quantified by reverse transcriptase quantitative polymerase chain reaction (RT-qPCR). RNAs were isolated from LS8 cells after transient transfection with the Msx2 expression vector. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level was used for normalization. Differences were considered significant at p<0.05 (*). Mean ± SEM from a representative set of three independent experiments are shown. (a.u. for arbitrary units). (B) Whole-cell lysates were prepared from LS8 cells transiently transfected with Msx2 vector. Comparable amounts of cell lysate proteins were loaded into each lane and immunoblotted with a goat anti-actin antibody (top) and a rabbit anti–calbindin-D28k antibody (bottom). The numbers indicate the molecular masses of marker proteins (in kilodaltons). The left panel shows the graphical representation of calbindin signal quantification (related to actin) of three independent experiments.
The polyclonal antibody used in Western blotting specifically recognises the 28-kDa protein. Calbindin-D28k protein levels appeared lower in LS8 cells overexpressing Msx2 compared with controls (Fig. 2B). Quantification analysis showed that transfecting 0.3 µg and 0.5 µg of Msx2 expression vector decreased calbindin-D28k protein expression by 59.1% and 40.9%, respectively, compared with control cells transfected with the same amounts of the empty vector pcDNA3 (Fig. 2B).
The presence of Msx2 and its ability to inhibit endogenous calbindin-D28k expression in the ameloblast-like LS8 cells clearly indicated that Msx2 is a repressor of calbindin-D28k expression.
Msx2 Dose-Dependent and Specific Repression of Mouse Calbindin-D28k Promoter Activity
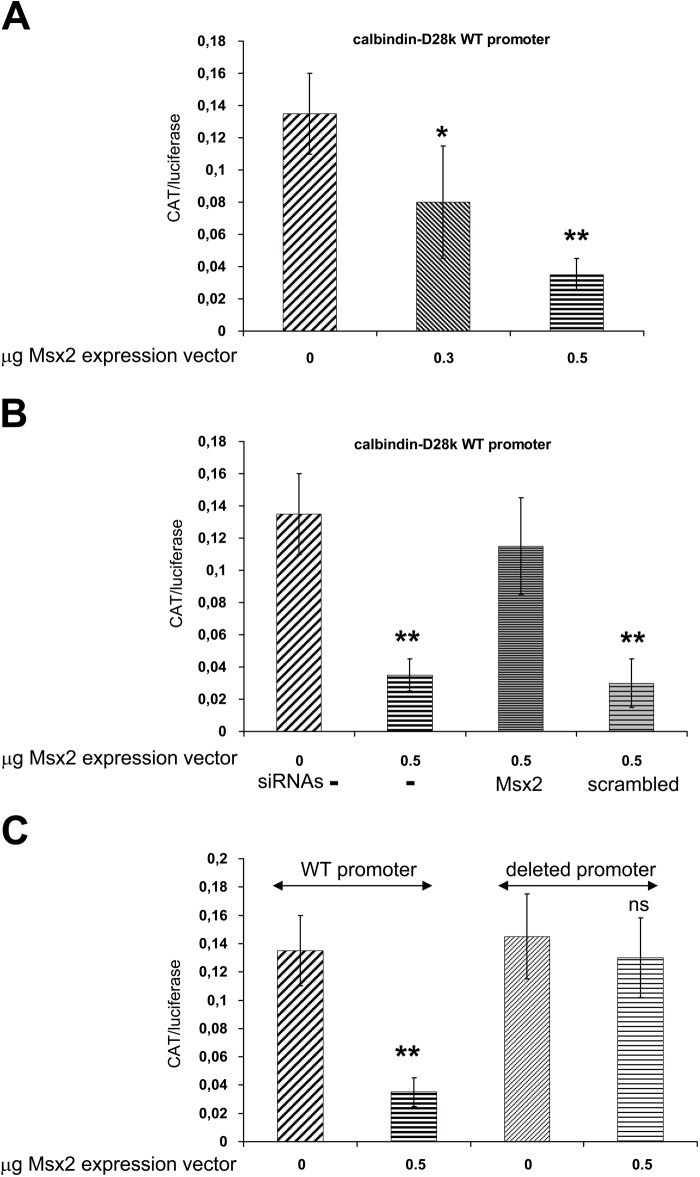
To test whether Msx2 can function as a transcriptional modulator of the mouse calbindin promoter, LS8 cells were transiently co-transfected with calbindin-D28k promoter (nt-1075/+34) CAT (Gill and Christakos 1993), with either pcDNA3 (empty vector used as negative control) or increasing amounts of Msx2 expression vector. Reporter CAT expression was significantly lower in the presence of 0.3 to 0.5 µg Msx2 (p<0.03) (Fig. 3A). This inhibitory effect of Msx2 was dose-dependent and specific to the wild-type (WT) promoter, as the Msx2 inhibitory effect was not observed in the deleted promoter without the Msx2 responsive element (nt-1003/+34) (Fig. 3C). The Msx2-mediated inhibition of calbindin-D28k promoter activity was specific, as demonstrated by concomitant transfection of Msx2 siRNAs, which reduced Msx2 expression by 62.5%. In the presence of 25 nM Msx2 siRNAs, the Msx2 effect was abolished, whereas it remained intact in the presence of scrambled siRNAs (Fig. 3B).
Figure 3.
Calbindin-D28k promoter activity. Varying amounts of Msx2 expression plasmid were used (A) with or without appropriate siRNAs (B) and were transiently co-transfected into LS8 cells with 0.5 µg reporter construct. The involvement of Msx2 binding element was shown with the loss of inhibition of the deleted promoter activity (C). Each bar represents the mean ± SEM of three independent experiments. **p<0.01, *p<0.05. CAT, chloramphenicol acetyl-transferase; WT, wild-type.
Discussion
This study focused on the regulation of calbindin-D28k gene expression in ameloblasts and dental epithelium by the Msx2 transcription factor. The Msx2 homeoprotein is required for numerous cellular processes as well as for the development of several tissues and organs. Msx2 is a potent transcriptional repressor of many genes involved in mineralized bone and tooth tissues (Marijanović et al. 2009; Molla et al. 2010), including osteocalcin (Hassan et al. 2004) and amelogenin (Zhou et al. 2000; Xu et al. 2007). Msx2 can interact directly with its DNA target sequence as the rat osteoclacin promoter (–84 to −92 nt), thus decreasing its activity (Towler et al. 1994). Msx2 could also act indirectly on target genes by binding other transcription factors such as C/EBPα for amelogenin (Xu et al. 2007) or Runx2 for osteocalcin (Hassan et al. 2004; Sierra et al. 2004). Otherwise, Msx2 and Dlx homeoproteins can recognize the same response elements (AC/TAATTGG) and compete for common target-gene promoters (Bendall and Abate-Shen 2000; Diamond et al. 2006, Lézot et al. 2008). Here, Msx2-induced repression was demonstrated at both protein and RNA levels. Msx2 was shown to specifically repress calbindin-D28k promoter activity as demonstrated by co-transfection of Msx2 siRNAs. The involvement of the putative Msx2 responsive element (–1068 to −1061 nt) on the mouse calbindin-D28k promoter was evident by the loss of Msx2 inhibition on the deleted promoter. These data raise the question of putative Msx2 functions in other systems, such as Purkinje cells of the cerebellar cortex. These cells, where calbindin-D28k is also selectively concentrated in the basal situation, are altered in Msx2−/− mice (Satokata et al. 2000).
The findings reported here could explain at least in part the physiological expression pattern of calbindin-D28k in teeth. Indeed, during amelogenesis, the expression patterns of Msx2 (Molla et al. 2010) and Dlx (Lézot et al. 2008) mirror the stage-specific regulation of enamel protein and certain ion-handling molecules. Although in Msx2+/− ameloblasts, calbindin-D28k level increased compared with Msx2+/+, this was not observed in Msx2−/− incisors. This may be related to reduced laminin-5α3 and cytokeratin 5 expression levels that leads to the loss of ameloblast intercellular attachment and finally to their disorganization and disappearance (Bei et al. 2004; Aïoub et al. 2007; Molla et al. 2010). In parallel, calbindin-D28k was upregulated in the rests of Malassez of Msx2+/− mice and even more in Msx2−/−. This pattern is not physiological (Korkmaz et al. 2010), as confirmed here in Msx2+/+ control mice. The same observation was also repeated for amelogenin in the same pathophysiological context. Indeed, amelogenin gene transcription, which is also repressed by Msx2 (Zhou et al. 2000; Xu et al. 2007), was overexpressed in rests of Malassez in Msx2−/− mice (Molla et al. 2010). These convergent in vivo results on amelogenin and calbindin-D28k and the present findings led us to propose a new concept, where in addition to its developmental function, Msx2 may also limit the expression of its target genes in differentiated cells of dental epithelium within a defined frame related to amelogenesis.
Acknowledgments
We thank Mrs. Benoit Robert, Institut Pasteur Paris, for generating the Msx2 mutant strain. We also thank Sylvia Christakos, University of Medicine and Dentistry of New Jersey, Newark, for the plasmid construct containing calbindin-D28k promoter; Malcolm Snead, Center for Craniofacial Molecular Biology, University of Southern California, Los Angeles, for the LS8 ameloblast cell line; Cory Abate-Shen, Columbia University, New York, for the Msx2 expression vector; and Elodie Gérard for technical assistance.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the University Paris-Diderot and INSERM (the French National Institute of Health and Medical Research).
References
- Aïoub M, Lézot F, Molla M, Castaneda B, Robert B, Goubin G, Nefussi JR, Berdal A. 2007. Msx2 −/– transgenic mice develop compound amelogenesis imperfecta, dentinogenesis imperfecta and periodental osteopetrosis. Bone. 41:851–859 [DOI] [PubMed] [Google Scholar]
- Bei M, Stowell S, Maas R. 2004. Msx2 controls ameloblast terminal differentiation. Dev Dyn. 231:758–765 [DOI] [PubMed] [Google Scholar]
- Bendall AJ, Abate-Shen C. 2000. Roles for Msx and Dlx homeoproteins in vertebrate development. Gene. 247:17–31 [DOI] [PubMed] [Google Scholar]
- Berdal A, Hotton D, Pike JW, Mathieu H, Dupret JM. 1993. Cell- and stage-specific expression of vitamin D receptor and calbindin genes in rat incisor: regulation by 1,25-dihydroxyvitamin D3. Dev Biol. 155:172–179 [DOI] [PubMed] [Google Scholar]
- Berdal A, Hotton D, Saffar JL, Thomasset M, Nanci A. 1996. Calbindin-D9k and calbindin-D28k expression in rat mineralized tissues in vivo. J Bone Min Res. 11:768–779 [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, Nanci A. 2004. Hertwig’s epithelial root sheath, enamel matrix proteins, and initiation of cementogenesis in porcine teeth. J Clin Periodontol. 31:184–192 [DOI] [PubMed] [Google Scholar]
- Catón J, Luder HU, Zoupa M, Bradman M, Bluteau G, Tucker AS, Klein O, Mitsiadis TA. 2009. Enamel-free teeth: Tbx1 deletion affects amelogenesis in rodent incisors. Dev Biol. 328:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond E, Amen M, Hu Q, Espinoza HM, Amendt BA. 2006. Functional interactions between Dlx2 and lymphoid enhancer factor regulate Msx2. Nucleic Acids Res. 34:5951–5965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoul-Mazgar S, Hotton D, Lézot F, Blin-Wakkach C, Asselin A, Sautier J-M, Berdal A. 2005. Expression pattern of Dlx3 during cell differentiation in mineralized tissues. Bone. 37:799–809 [DOI] [PubMed] [Google Scholar]
- Gill RK, Christakos S. 1993. Identification of sequence elements in mouse calbindin-D28k gene that confer 1,25-dihydroxyvitamin D3- and butyrate inducible responses. Biochem. 90:2984–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RK, Christakos S. 1995. Regulation by estrogen through the 5′-flanking region of the mouse calbindin-D28k gene. Mol Endocrinol. 9:319–326 [DOI] [PubMed] [Google Scholar]
- Hassan MQ, Javed A, Morasso M, Karlin J, Montecino M, van Wijnen A, Stein G, Stein J, Lian J. 2004. Dlx3 transcriptional regulation of osteoblast differentiation: temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol Cel Biol. 24:9248–9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotton D, Davideau JL, Bernaudin JF, Berdal A. 1995. In situ hybridization of calbindin-D28k transcripts in undecalcified sections of the rat continuously erupting incisor. Connect Tissue Res. 32:137–143 [DOI] [PubMed] [Google Scholar]
- Hu JC, Chun YH, Al Hazzazzi T, Simmer JP. 2007. Enamel formation and amelogenesis imperfecta. Cells Tissues Organs. 186:78–85 [DOI] [PubMed] [Google Scholar]
- Hubbard MJ. 1995. Calbindin28kDa and calmodulin are hyperabundant in rat dental enamel cells: identification of the protein phosphatase calcineurin as a principal calmodulin target and of a secretion-related role for calbindin28kDa. Eur J Biochem. 230:68–79 [DOI] [PubMed] [Google Scholar]
- Korkmaz Y, Klinz FJ, Beikler T, Blauhut T, Schneider K, Addicks K, Bloch W, Raab WH. 2010. The Ca(2+)-binding protein calretinin is selectively enriched in a subpopulation of the epithelial rests of Malassez. Cell Tissue Res. 342:391–400 [DOI] [PubMed] [Google Scholar]
- Lézot F, Thomas B, Greene S, Hotton D, Yuan Z, Castaneda B, Bolanos A, Depew M, Sharpe P, Gibson C, et al. 2008. Physiological implications of DLX homeoproteins in enamel formation. J Cell Physiol. 216:688–697 [DOI] [PubMed] [Google Scholar]
- Lézot F, Thomas B, Hotton D, Forest N, Orestes-Cardoso S, Robert B, Sharpe P, Berdal A. 2000. Biomineralization, life-time of odontogenic cells and differential expression of the two homeobox genes MSX-1 and DLX-2 in transgenic mice. J Bone Miner Res. 15:430–441 [DOI] [PubMed] [Google Scholar]
- Marijanović I, Kronenberg MS, Erceg Ivkosić I, Lichtler AC. 2009. Comparison of proliferation and differentiation of calvarial osteoblast cultures derived from Msx2 deficient and wild type mice. Coll Antropol. 33:919–924 [PubMed] [Google Scholar]
- Molla M, Descroix V, Aïoub M, Simon S, Castañeda B, Hotton D, Bolaños A, Simon Y, Lezot F, Goubin G, et al. 2010. Enamel protein regulation and dental and periodontal physiopathology in MSX2 mutant mice. Am J Pathol. 177:2516–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio C, Wazen R, Kuroda S, Moffatt P, Nanci A. 2010. Disruption of periodontal integrity induces expression of apin by epithelial cell rests of Malassez. J Periodontal Res. 45:709–713 [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, et al. 2000. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 24:391–395 [DOI] [PubMed] [Google Scholar]
- Sierra OL, Cheng SL, Loewy AP, Charlton-Kachigian N, Towler DA. 2004. MINT, the Msx2 interacting nuclear matrix target, enhances Runx2-dependent activation of the osteocalcin fibroblast growth factor response element. J Biol Chem. 279:32913–32923 [DOI] [PubMed] [Google Scholar]
- Suda N, Kitahara Y, Ohyama K. 2006. A case of amelogenesis imperfecta, cleft lip and palate and polycystic kidney disease. Orthod Craniofac Res. 1:52–56 [DOI] [PubMed] [Google Scholar]
- Towler DA, Rutledge SJ, Rodan GA. 1994. Msx-2/Hox 8.1: a transcriptional regulator of the rat osteocalcin promoter. Mol Endocrinol. 8:1484–1493 [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. 2009. The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol. 4:309–319 [DOI] [PubMed] [Google Scholar]
- Venugopalan SR, Li X, Amen MA, Florez S, Gutierrez D, Cao H, Wang J, Amendt BA. 2011. Hierarchical interactions of homeodomain and forkhead transcription factors in regulating odontogenic gene expression. J Biol Chem. 286:21372–21383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YZ, Christakos S. 1995. Retinoic acid regulates the expression of the calcium binding protein, calbindin-D28K. Mol Endocrinol. 9:1510–1521 [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhou YL, Erickson RL, Macdougald OA, Snead MI. 2007. Physical dissection of the CCAAT/enhancer-binding protein alpha in regulating the mouse amelogenin gene. Biochem Biophys Res Commun. 354:56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lei Y, Snead M. 2000. Functional antagonism between Msx2 and CCAAT/enhancer binding protein α in regulating the mouse amelogenin gene expression is mediated by protein-protein interaction. J Biol Chem. 275:29066–29075 [DOI] [PubMed] [Google Scholar]