Abstract
Alcohol consumption is associated with decreased antiretroviral adherence, and decreased adherence results in poorer outcomes. However the magnitude of alcohol’s impact on survival is unknown. Our objective was to use a calibrated and validated simulation of HIV disease to estimate the impact of alcohol on survival. We incorporated clinical data describing the temporal and dose-response relationships between alcohol consumption and adherence in a large observational cohort (N = 2,702). Individuals were categorized as nondrinkers (no alcohol consumption), hazardous drinkers (consume ≥5 standard drinks on drinking days), and nonhazardous drinkers (consume <5 standard drinks on drinking days). Our results showed that nonhazardous alcohol consumption decreased survival by more than 1 year if the frequency of consumption was once per week or greater, and by 3.3 years (from 21.7 years to 18.4 years) with daily consumption. Hazardous alcohol consumption decreased overall survival by more than 3 years if frequency of consumption was once per week or greater, and by 6.4 years (From 16.1 years to 9.7 years) with daily consumption. Our results suggest that alcohol is an underappreciated yet modifiable risk factor for poor survival among individuals with HIV.
Introduction
Alcohol consumption is the most prevalent risk factor for poor adherence to Highly Active Antiretroviral Therapy (HAART) among HIV-positive (HIV+) individuals, with approximately half of all persons reporting recent use (Golin et al., 2002; Chesney et al., 2000) While the cross-sectional association is well established (Paterson et al. 2000, d’Arminio et al. 2005; Howard et al. 2002; Brigido et al. 2001; Haubrich et al. 1999), recently it has been shown that there is a tightly coupled temporal association, with nonadherence on drinking days greatly exceeding nonadherence on nondrinking days in a particular patient sample (Braithwaite et al. 2005a). This observation decreases the likelihood that the relationship between alcohol consumption and antiretroviral nonadherence is spurious and merely attributable to confounding. Furthermore, a significant impact was seen even at levels of consumption that did not meet the threshold for ‘at-risk’ drinking.
This relationship may have important survival implications. While greater than 95% adherence to HAART is likely to extend life by 15 to 20 years, even small reductions in adherence may compromise its effectiveness (Karon et al., 2001). In a two-site observational study of HIV outpatients in the US, individuals who missed between 5% and 20% of medication doses were nearly three times less likely to effectively suppress plasma HIV than those who missed fewer than 5% of doses (Paterson et al., 2000). In a cohort of Swiss HIV patients, lapses in adherence preceded more than half of all therapy failures.(Paris et al., 1999) Because the adherence reductions induced by alcohol consumption may decrease survival, it is possible that HIV clinical guidelines should place a greater emphasis on screening for alcohol use disorders and facilitating access to alcohol cessation interventions.
To inform this question, we sought to estimate the impact of alcohol consumption on life expectancy among individuals with HIV. Because primary data analyses have not had sufficient power to investigate this relationship in the current treatment era (deaths have become increasingly rare events with modern therapies and few studies have prospectively measured alcohol consumption), we employed a validated computer simulation of HIV disease (Braithwaite et al., 2005b) to predict survival. This simulation explicitly represents adherence to HAART, and incorporates prospective measures of adherence and alcohol consumption from a sample of 2,702 individuals (Braithwaite et al., 2005a).
Methods
To assess the impact on survival of changes in adherence associated with alcohol consumption, we simulated cohorts of HIV-positive patients with varying clinical characteristics (age, CD4 count, viral load) and varying patterns of alcohol consumption. Alcohol consumption was represented by two variables, one which represented quantity of consumption and another which represented frequency of consumption. We made the simplifying and conservative assumption that alcohol’s only effect was to decrease adherence to HIV medications (i.e. it was not assumed to impact the toxicity of HIV therapies, the immune function of the host, or to have any impact on non-HIV comorbidities such as Hepatitis C or coronary artery disease). Adherence was defined as the proportion of HAART medication doses that were taken as directed, irrespective of the particular reason for which the doses may have been missed (‘pill fatigue’, side effects, life-threatening toxicity, etc). The difference in adherence patterns between alcohol users and nonusers were based on clinical data from Veterans Aging Cohort Study (VACS).
Characterization of alcohol-adherence relationship
Assumptions regarding the relationship between alcohol consumption and adherence were based on the study of Braithwaite et al. (2005a) in which adherence on days when alcohol was consumed (drinking days) and on days immediately after alcohol was consumed (postdrinking days) were compared with adherence on days temporally separated from alcohol consumption (nondrinking days), among 2,702 patients in the Veterans Aging Cohort Study. Hazardous, nonhazardous, and nondrinkers were evaluated separately and longitudinally, which permitted the impact of alcohol consumption to be separated from the impact of known or unknown confounders that may have been disproportionately prevalent in alcohol consumers, such as depression or drug abuse (Table I).
Table I.
Relative risk for not adhering to medications by temporal proximity to time of alcohol consumption and by type of drinker. Referent case is nondrinkers. Hazardous drinkers have higher nonadherence even on nondrinking days because of factors that exacerbate adherence and are unrelated to alcohol consumption, yet are more prevalent among hazardous drinkers (depression, drug abuse, etc). Results adapted from Braithwaite et al. (2005a).
| Non-drinking days | Drinking days | Post-drinking days | |
|---|---|---|---|
| Nondrinkers | 1.0 | – | – |
| Non-hazardous drinkers | 1.0 | 1.5 | 1.3 |
| Hazardous drinkers | 1.7 | 4.6 | 2.9 |
Representation of alcohol consumption in the simulation
Individuals were categorized as nondrinkers, non-hazardous drinkers, or hazardous drinkers. Non-drinkers were assumed to have consumed no alcohol whatsoever during the time horizon of the computer simulation. Consistent with accepted definitions of hazardous or ‘at risk’ drinking (Samet et al., 2003) nonhazardous drinkers were assumed to drink between 1 and 4 standard drinks on each drinking day, and hazardous drinkers were assumed to consume 5 or more standard drinks on each drinking day. For each group of drinkers, we explored varying frequencies of consumption (never, once per month, once per week, twice per week, three times per week, and every day).
Representation of adherence in the simulation
Most individuals were assigned a baseline probability of nonadherence (25%) that corresponded to a typical prevalence of nonadherence observed across cohorts of individuals with HIV (Haubrich et al., 1999; Paterson et al., 2000; Howard et al., 2002; Arnsten et al., 2002). This was also the particular level of nonadherence that resulted in the closest calibration of observed with expected simulation results (Braithwaite et al., 2006). All individuals except for hazardous drinkers were assumed to have this baseline probability of nonadherence on non-drinking days. Hazardous drinkers were assumed to have a 1.7 times higher probability of nonadherence on nondrinking days (Table I), based on the study of Braithwaite et al. (2005a) which found that these individuals had a higher probability of not adhering to medications even on days when no alcohol was consumed. This increase was unlikely to be attributable to alcohol consumption, but rather was likely to be attributable to characteristics that were more prevalent among hazardous drinkers and that also may have led to nonadherence, such as drug abuse and mental illness. On drinking days and postdrinking days, individuals were assumed to have greater levels of nonadherence than on nondrinking days, with risk increases specified by the relationships observed previously (Table I) (Braithwaite et al., 2005a). We used relative rather than absolute risk increases in our analyses because baseline nonadherence rates may vary greatly between patient samples (from 10% to 59% of doses not consumed on time) (Howard et al., 2002; Paterson et al., 2000; Haubrich et al., 1999; Arnsten et al., 2002) whereas the relative risks for nonadherence associated with alcohol consumption in the era of HAART have been more consistent (Arnsten et al., 2002; Cook et al., 2001; Paterson et al., 2000); however, we explored the impact of using absolute risk increases in sensitivity analyses.
Estimating the impact of alcohol consumption
To estimate the impact of alcohol consumption on time to treatment failure and survival, we used the probabilistic HIV computer simulation developed by Braithwaite et al. (2005b) which predicts survival and time to failure of antiretroviral regimens. This simulation differs from other HIV simulations because of its more detailed representations of the biological and clinical processes that underlie treatment failure (accumulation of genotypic mutations, and patterns of nonadherence to antiretroviral medications), which eliminates the need to assume a particular number of possible antiretroviral therapy rounds (Figure 1). It also separately tracks deaths from HIV-related and non-HIV-related events. Its internal validity has been demonstrated by the agreement of predicted and observed times to treatment failure and survival in the 3,545 patient sample on which it was calibrated, and its external validity has been demonstrated by the agreement of predicted and observed mortality rates on an almost entirely distinct patient sample of 12,574 composed of 13 cohorts from disparate geographic locations and care settings.(Egger et al., 2002).
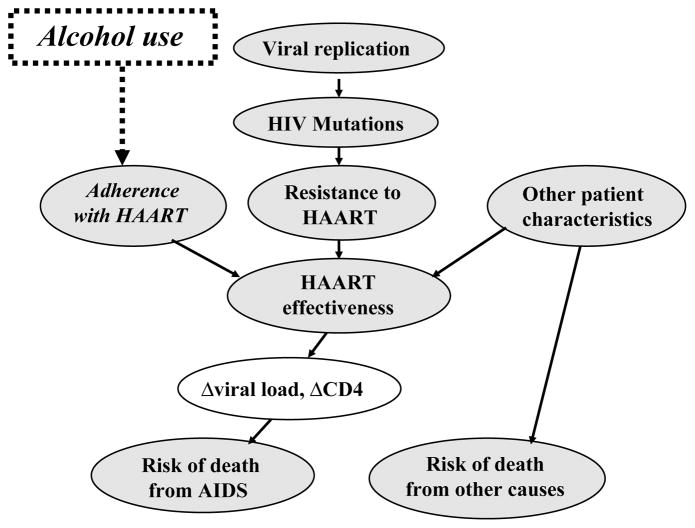
Figure 1. Influence diagram of selected constructs in computer simulation.
Clinical characteristics are assumed to affect the probability of dying from HIV-related or non–HIV-related causes and also to affect viral genetic characteristics by altering viral replication rates and selection pressures for new mutations. Viral replication and selection pressures may give rise to HIV mutations. Each HIV mutation may or may not give rise to resistance to one or more HAART drugs. Adherence to HAART combined with resistance to HAART and other patient characteristics determine the level of HAART effectiveness. The effectiveness of HAART influences changes in viral load and CD4 count, and also feeds back to influence changes in the viral replication rate. Changes in CD4 and viral load influence the risk of death from AIDS. The risk of death from other causes may be influenced by patient characteristics including age, sex, and race. Alcohol use is assumed to impact adherence with HAART, and subsequently to influence HAARTeffectiveness, and ultimately the risk of death from AIDS. Although it is not depicted in this diagram, alcohol also was assumed to directly impact the risk of death from other causes during the ‘J curve’ sensitivity analyses.
This simulation permits the probability of non-adherence to be specified as an independent variable. We programmed the simulation such to ensure that the probability of nonadherence would fluctuate temporally with alcohol consumption on a daily basis according to the relationships that were observed in our temporal analyses in the Veterans Aging Cohort Study (Table I).
For our baseline analyses, we simulated a hypothetical patient cohort of initially equivalent individuals who are newly diagnosed with chronic HIV infection, each 40 years old, with a CD4 count of 350 and a viral load of 100,000. Other combinations of baseline characteristics were explored in sensitivity analyses. The cohort was assumed to start treatment immediately after diagnosis, consistent with current recommendations for antiretroviral treatment initiation (Anonymous, 2005). In sensitivity analyses, we explored cohorts defined by other combinations of age, CD4 counts, and viral loads.
Time to treatment failure was defined as the median time until the patient was intolerant of or resistant to all antiretroviral drugs that were FDA-approved at the start of the calendar year 2005. Survival was defined as the median time until death.
Because modest levels of alcohol consumption have been associated with decreased overall mortality in multiple large studies among individuals uninfected with HIV (Gaziano et al., 2000; Camargo et al., 1997; Thun et al., 1997; Hart et al., 1999) we performed all analyses both with and without an assumption incorporating this mortality reduction (J curve assumption). With the J curve assumption, nonhazardous drinking was assumed to decrease non-HIV-related mortality by a proportion in accord with these observations (a relative risk of 0.8) (Gaziano et al., 2000; Camargo et al., 1997; Thun et al., 1997; Hart et al., 1999). This impact was assumed to occur on all days, not just on drinking days, a generous assumption that biases the analysis in favour of alcohol consumption. Without the J curve assumption, drinking was assumed to have no impact, beneficial or otherwise, on non-HIV-related mortality.
Results
Alcohol consumption was observed to have substantial and deleterious impacts on time to treatment failure and survival. The impact of hazardous alcohol consumption was far greater than the impact of nonhazardous consumption.
Impact of nonhazardous alcohol consumption
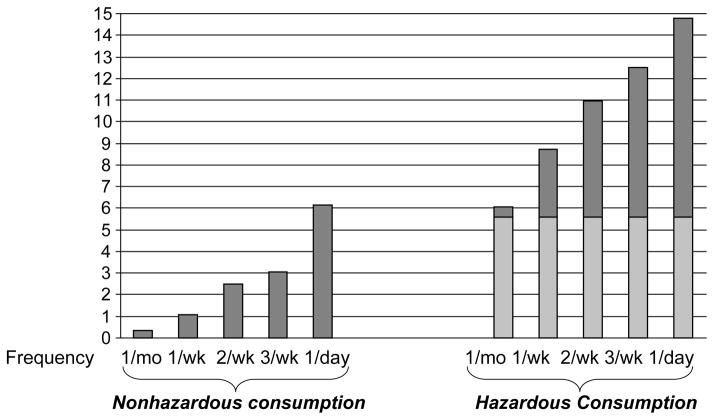
Nonhazardous alcohol consumption hastened time to treatment failure by more than one year if the frequency of consumption was once per week or greater (Figure 2). At a frequency of twice per week, it hastened time to treatment failure by 2.5 years (from 18.3 years to 15.8 years). With daily drinking, time to treatment failure was hastened by 6.1 years (to 12.2 years), a 33% decrease.
Figure 2. Premature treatment failure from alcohol consumption.
Lightly shaded regions denote the effect that is attributable to alcohol consumption itself, and darkly shaded regions denote the effect that is attributable to confounding factors rather than to alcohol consumption.
Increased nonadherence among drinkers leads to more rapid accumulation of genotypic resistance and regimen intolerance, with corresponding decrements in the time to failure of antiretroviral drugs. While a substantial proportion of the decrement experienced by hazardous drinkers arises from confounding factors rather than from the hazardous drinking itself, the decrement attributable to hazardous drinking is greater than the decrement attributable to nonhazardous drinking at each frequency.
Survival was comparably impacted (Figure 3). Any drinking frequency of once per week or greater diminished median survival by more than one year. At a frequency of twice per week, it reduced survival by 2.1 years (from 21.7 years to 19.6 years). With daily drinking, survival was reduced by 3.3 years (to 18.4 years), a 15% decrease.
Figure 3. Premature death from alcohol consumption.
Lightly shaded regions denote the effect that is attributable to alcohol consumption itself, and darkly shaded regions denote the effect that is attributable to confounding factors rather than to alcohol consumption. Decreased time to treatment failure among drinkers leads to decrements in the survival time. While a substantial proportion of the decrement experienced by hazardous drinkers arises from confounding factors rather than from the hazardous drinking itself, the decrement attributable to hazardous drinking is greater than the decrement attributable to nonhazardous drinking at each frequency.
Impact of hazardous alcohol consumption
Hazardous alcohol consumption decreased time to treatment failure and survival by substantially greater amounts than nonhazardous alcohol consumption. These decrements were large even though we only considered nonadherence to be attributable to alcohol consumption if it occurred in close proximity to alcohol consumption and was above and beyond the nonadherence that was observed on days when no alcohol was consumed (a conservative assumption).
Hazardous alcohol consumption hastened time to treatment failure by 3 years or more if the frequency of consumption was once per week or greater (Figure 2). At a frequency of twice per week, it reduced time to treatment failure by 5.4 years (from 12.7 years to 7.3 years). With daily drinking, time to treatment failure was reduced by 9.2 years (to 3.5 years), a 72% decrease.
Survival was commensurately impacted (Figure 3). Any drinking frequency of once per week or greater diminished median survival by more than two years. At a frequency of twice per week, it reduced survival by 4.0 years (from 16.1 years to 12.1 years). With daily drinking, survival was reduced by 6.4 years (to 9.7 years), a 40% decrease.
Variance of alcohol’s impact with patient characteristics
Nonhazardous alcohol consumption had a deleterious impact on survival for all age, CD4, and viral load groupings examined. This impact was relatively uniform as long as the frequency of consumption was held constant (e.g. at twice weekly consumption, all survival decrements ranged between 1.5 years and 2.7 years).
Hazardous alcohol consumption had a far greater impact on survival than nonhazardous alcohol consumption for all age, CD4, and viral load groupings examined. This impact was also relatively uniform as long as the frequency of consumption was held constant (e.g. at twice weekly consumption, all survival decrements ranged between 4.0 years and 6.3 years).
Sensitivity analysis of baseline adherence
Decreasing the likelihood of nonadherence diminished the impact of alcohol consumption on survival, but it still remained substantial for the majority of situations examined. With the most optimistic assumption regarding baseline adherence (a 5% probability of nonadherence, in which case the absolute risk increase conferred by the model matched the absolute risk increase that was empirically observed), daily nonhazardous alcohol consumption decreased survival from 29.2 years to 27.0 years, an 8% decrease, and daily hazardous alcohol consumption decreased survival from 28.1 to 21.4 years, a 24% decrease. Less frequent nonhazardous alcohol consumption did not have a substantial impact on survival. Less frequent hazardous alcohol consumption decreased survival by at least one year at all frequencies of once weekly or greater.
Sensitivity analysis of J curve assumption
For individuals with the clinical characteristics of our base case analysis (age 40, viral load 100,000, CD4 count 350), the J curve assumption, in which nonhazardous alcohol consumption was assumed to decrease non-HIV-related mortality, only had a modest impact on the survival decrements that were associated with alcohol consumption. Once-weekly nonhazardous drinkers had a survival decrement of 0.9 years (with J curve assumption) compared to 1.6 years (without J curve assumption). Because the J curve hypothesis applies only to light or moderate alcohol consumption, we did not evaluate this assumption for hazardous drinkers.
In sensitivity analyses where we varied clinical characteristics, individuals with high competing risks from non-HIV-related mortality (individuals age 50 or greater with baseline viral loads of 10,000 or less) did have a modest (1 year or less) mortality benefit from nonhazardous alcohol consumption if the J curve assumption was assumed to hold, as long as the frequency of alcohol consumption remained was once per week or less. However, these benefits were abrogated as the frequency of alcohol consumption rose beyond this level.
Discussion
This is the first report that aims to estimate the impact of alcohol consumption on survival in HIV disease. We estimated that the decrement in adherence found among nonhazardous drinkers compared to abstainers in the Veterans Aging Cohort Study would lead to a reduction in median life expectancy of 2.1 years for weekend drinkers and 3.3 years for daily drinkers, 10% and 15% decreases in life expectancy overall. We estimated that the decrement in adherence found among hazardous drinkers compared to abstainers in the Veterans Aging Cohort Study would lead to a reduction in median life expectancy of 4.0 years for weekend drinkers and 6.4 years for daily drinkers, 25% and 40% decreases in life expectancy overall. These results are even more striking because they do not consider the 5.6 years that hazardous HIV+ drinkers forgo compared to nondrinkers because of confounders such as drug abuse and smoking that are not directly related to alcohol consumption but are associated with it.
These findings were robust, remaining clinically significant regardless of assumptions regarding age, CD4 count, and viral load at the time of diagnosis. These findings also persisted even when we assumed that alcohol consumption led to a decrease in non-HIV-related mortality. However, in sensitivity analyses that involved the J-curve assumption, there were rare circumstances when nonhazardous alcohol consumption could extend survival (older individuals with early stage HIV disease and once-weekly nonhazardous alcohol consumption). Nonetheless, it is unclear whether the J curve phenomenon may be generalized to individuals with HIV, who often have multiple comorbidities, less physiological reserve, and therefore may be more susceptible to alcohol-induced injury (Brinkman et al., 1998; Brinkman et al., 1999; Cossarizza et al., 2001; Cote et al., 2002; Bortz, 2002).
We found a substantial survival difference even though the current analyses only captured one of the many deleterious ways in which alcohol may impact survival, namely the impact of alcohol on HIV disease. Additionally, our analyses only considered those aspects of this relationship that were mediated through changes in adherence to HIV therapies. If alcohol were to directly facilitate the progression of HIV disease, as perhaps may occur by decreasing cell-mediated immunity, it may decrease life expectancy more than the current results suggest. Similarly, because alcohol consumption is likely to increase sources of morbidity and mortality that are unrelated to HIV, such as worsening the transaminitis associated with HAART (Montaner et al., 2003; Ogedegbe & Sulkowski, 2003) or hastening the progression of Hepatitis C (Rockstroh & Spengler, 2004) our results are likely to be underestimates.
Given the high prevalence of recent alcohol consumption (37% to 68%) and hazardous alcohol consumption (5% to 28%) among individuals with HIV (Table II), interventions to decrease alcohol use may have great potential to prolong life and to be cost-effective. As an illustrative example, a brief intervention (two 15 minute counselling sessions) for hazardous alcohol consumption may decrease the probability of excessive drinking 12 months later by up to 15% (Fleming et al., 2000). If we assume that at least half of that effect endures over longer time periods and that it may be generalized to HIV+ populations, our simulation results suggest that it could prolong the lives of HIV+ hazardous drinkers by 0.3 years for weekend drinkers and 0.5 years for daily drinkers. As studies suggest that the cost of brief interventions are between $205 and $632 per person screened (Mundt et al., 2005; Kunz et al., 2004; Fleming et al., 2000) its cost is likely to be less than $2000 per life-year saved. By comparison, providing current therapies to HIV patients has a cost-effectiveness of $13,000 to $23,000 per quality-adjusted life year (Freedberg et al., 2001). Therefore, the cost-effectiveness of alcohol interventions for alcohol using HIV+ populations have the potential to compare favourably with many accepted medical practices.
Table II.
Prevalence of alcohol consumption among US HIV+ samples in the era of Highly Active Antiretroviral Therapy.
| Author | N | Year | Study | Prevalence of recent alcohol consumption | Prevalence of hazardous alcohol consumption |
|---|---|---|---|---|---|
| Braithwaite (Braithwaite et al., 2005a) | 2,762 | 2005 | Veterans Aging Cohort Study (VACS) | 46% | 9%* |
| Tucker (Tucker et al., 2003) | 1,910 | 2003 | HIV Cost and Services Utilization Study (HCSUS) | 52% | 14%* |
| Lucas (Lucas et al., 2002) | 695 | 2002 | Hopkins | NR | 5% |
| Kleeberger (Kleeberger et al., 2001) | 539 | 2001 | Multicenter AIDS Cohort Study (MACS) | NR | 6% |
| Samet (Samet et al., 2003) | 349 | 2003 | Boston | 43% | 19% |
| Stein (Stein et al., 2005) | 262 | 2005 | Brown | 48%† | 28%† |
| Cook (Cook et al., 2001) | 212 | 2001 | Pennsylvania | 46% | 19% |
| Golin (Golin et al., 2002) | 140 | 2002 | Adherence and Efficacy to Protease Inhibitor Therapy (ADEPT) | 37% | NR |
| Arnsten (Arnsten et al., 2001) | 67 | 2001 | Bronx HIV Epidemiologic Research on Outcomes Study (HEROS) | NR | 22%‡ |
| Chesney (Chesney et al., 2000) | 65 | 2000 | Adult AIDS Clinical Trials Group (AACTG) | 68% | NR |
NR = Not reported.
Only considers those hazardous drinkers who are also binge drinkers (> = 5 drinks in at least one day during previous month); therefore these are likely underestimates.
All individuals had a history of alcohol problems.
‘Alcohol use more than several days per week’ was used as proxy for hazardous alcohol consumption.
Limitations
Our analyses have several limitations. The data describing the temporal relationship between alcohol consumption and adherence in VACS relied on self-report. The recorded proportion of doses missed or late (approximately 5% to 15%) was lower than would be expected based on methods that do not rely on self-report or based on results from other cohorts (10% to 59%). Because the absolute estimates were likely to be affected by under-reporting, we used the relative rather than the absolute risks associated with alcohol consumption for our base case analysis. However, if the absolute risks are more accurate, the base case analysis may have overestimated the deleterious effects of alcohol consumption on adherence, and our sensitivity analysis incorporating absolute risk is likely to be more accurate. This analysis suggested that the decrease in survival with daily nonhazardous drinking would be 8% (from 29.2 years to 27.0 years) rather than 15% as in our base case analysis, and the decrease in survival with daily hazardous drinking would be 24% (from 16.1 years to 9.7 years) rather than 40% as in our base case analysis.
Our analysis is based on observational data describing the relationship of alcohol consumption to adherence, and therefore causality can not be proven. While the temporal design of the source study (Braithwaite et al., 2005a) controlled for known and unknown confounders that were stable over brief time intervals (e.g. socioeconomic status, demographic factors, history of drug abuse, known psychiatric disorders), it did not control for confounders that may have varied from day to day in synchrony with alcohol consumption (e.g. stressors, mood fluctuations, active drug abuse). Our analysis only considers alcohol’s impact on adherence to therapy. Lastly, all computer simulations, no matter how carefully validated, are subject to error. Nonetheless, by basing our results on a well validated simulation, we have sought to maximize the likelihood that our predictions may resemble future observations.
Conclusion
In the Veterans Aging Cohort Study, patients who consumed alcohol were less adherent to anti-retroviral medications than patients who did not consume alcohol. Our computer simulation suggests that these differences in adherence patterns may have an important impact on survival. Based on the magnitude of this survival impact, alcohol interventions aimed at HIV+ hazardous drinkers may have the potential to prolong life and to be cost-effective.
Acknowledgments
Funding Source: National Institute of Alcohol Abuse and Alcoholism, National Institutes of Health Grant # 1 K23 AA14483-01
References
- Anonymous. Panel on Clinical Practices for Treatment of HIV Infection convened by. Department of Health and Human Services (DHHS); 2005. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Electronic Citation. [Google Scholar]
- Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, Buono D, Eckholdt H, Howard AA, Schoenbaum EE. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clinical Infectious Diseases. 2001;33(8):1417–23. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of General Internal Medicine. 2002a;17(5):377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz WM. A conceptual framework of frailty: a review. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2002;57(5):M283–288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, Cook RL, Gordon A, Bridges MW, Seiler JFS, Justice AC. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcoholism: Clinical & Experimental Research. 2005a;29(7):1190–97. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Justice AC, Chang CC, Fusco JS, Raffanti SR, Wong JB, Roberts MS. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. American Journal of Medicine. 2005b;118(8):890–98. doi: 10.1016/j.amjmed.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Shechter SM, Chang CC, Schaefer AJ, Roberts MS. Estimating the rate of accumulating drug resistance mutations in the HIV genome. Value in Health. 2006 doi: 10.1111/j.1524-4733.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- Brigido LF, Rodrigues R, Casseb J, Oliveira D, Rossetti M, Menezes P, Duarte AJ. Impact of adherence to antiretroviral therapy in HIV-1-infected patients at a university public service in Brazil. AIDS Patient Care STDS. 2001;15(11):587–93. doi: 10.1089/108729101753287685. [DOI] [PubMed] [Google Scholar]
- Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354(9184):1112–15. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12(14):1735–44. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- Camargo CA, Jr, Hennekens CH, Gaziano JM, Glynn RJ, Manson JE, Stampfer MJ. Prospective study of moderate alcohol consumption and mortality in US male physicians. Archives of Internal Medicine. 1997b;157(1):79–85. [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW & Patient Care Committe & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000a;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. Journal of General Internal Medicine. 2001b;16(2):83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossarizza A, Mussini C, Vigano A. Mitochondria in the pathogenesis of lipodystrophy induced by anti-HIV antiretroviral drugs: actors or bystanders? Bioessays. 2001;23(11):1070–80. doi: 10.1002/bies.1152. [DOI] [PubMed] [Google Scholar]
- Cote HC, Brumme ZL, Craib KJ, Alexander CS, Wynhoven B, Ting L, Wong H, Harris M, Harrigan PR, O’Shaughnessy MV, Montaner JS. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. New England Journal of Medicine. 2002;346(11):811–20. doi: 10.1056/NEJMoa012035. [DOI] [PubMed] [Google Scholar]
- d’Arminio MA, Sabin CA, Phillips A, Sterne J, May M, Justice A, Dabis F, Grabar S, Ledergerber B, Gill J, Reiss P, Egger M The Antiretroviral Therapy Cohort Collaboration. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Archives of Internal Medicine. 2005;165(4):416–23. doi: 10.1001/archinte.165.4.416. [DOI] [PubMed] [Google Scholar]
- Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, Costagliola D, d’Arminio MA, de WF, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA, Art CC. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Mundt MP, French MT, Manwell LB, Stauffacher EA, Barry KL. Benefit-cost analysis of brief physician advice with problem drinkers in primary care settings. Medical Care. 2000a;38(1):7–18. doi: 10.1097/00005650-200001000-00003. [DOI] [PubMed] [Google Scholar]
- Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, Craven DE, Zhang H, Kimmel AD, Goldie SJ. The cost effectiveness of combination antiretroviral therapy for HIV disease. New England Journal of Medicine. 2001;344(11):824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- Gaziano JM, Gaziano TA, Glynn RJ, Sesso HD, Ajani UA, Stampfer MJ, Manson JE, Hennekens CH, Buring JE. Light-to-moderate alcohol consumption and mortality in the Physicians’ Health Study enrollment cohort. Journal of the American College of Cardiology. 2000b;35(1):96–105. doi: 10.1016/s0735-1097(99)00531-8. [DOI] [PubMed] [Google Scholar]
- Golin CE, Liu H, Hays RD, Miller LG, Beck CK, Ickovics J, Kaplan AH, Wenger NS. A prospective study of predictors of adherence to combination antiretroviral medication. Journal of General Internal Medicine. 2002a;17(10):756–65. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Smith GD, Hole DJ, Hawthorne VM. Alcohol consumption and mortality from all causes, coronary heart disease, and stroke: results from a prospective cohort study of Scottish men with 21 years of follow up. BMJ. 1999a;318(7200):1725–29. doi: 10.1136/bmj.318.7200.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich RH, Little SJ, Currier JS, Forthal DN, Kemper CA, Beall GN, Johnson D, Dube MP, Hwang JY, McCutchan JA. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. California Collaborative Treatment Group. AIDS. 1999a;13(9):1099–107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- Howard AA, Arnsten JH, Lo Y, Vlahov D, Rich JD, Schuman P, Stone VE, Smith DK, Schoenbaum EE HER Study Group. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002a;16(16):2175–82. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- Karon JM, Fleming PL, Steketee RW, De Cock KM. HIV in the United States at the turn of the century: an epidemic in transition. American Journal of Public Health. 2001;91(7):1060–68. doi: 10.2105/ajph.91.7.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kings-ley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2001;26(1):82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- Kunz FM, Jr, French MT, Bazargan-Hejazi S. Cost-effectiveness analysis of a brief intervention delivered to problem drinkers presenting at an inner-city hospital emergency department. Journal of Studies on Alcohol. 2004;65(3):363–70. doi: 10.15288/jsa.2004.65.363. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16(5):767–74. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Montaner JS, Cote HC, Harris M, Hogg RS, Yip B, Chan JW, Harrigan PR, O’Shaughnessy MV. Mitochondrial toxicity in the era of HAART: evaluating venous lactate and peripheral blood mitochondrial DNA in HIV-infected patients taking antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2003;34(Suppl 1):S85–90. doi: 10.1097/00126334-200309011-00013. [DOI] [PubMed] [Google Scholar]
- Mundt MP, French MT, Roebuck MC, Manwell LB, Barry KL. Brief physician advice for problem drinking among older adults: an economic analysis of costs and benefits. Journal of Studies on Alcohol. 2005;66(3):389–394. doi: 10.15288/jsa.2005.66.389. [DOI] [PubMed] [Google Scholar]
- Ogedegbe AO, Sulkowski MS. Antiretroviral-associated liver injury. Clinics in Liver Disease. 2003;7(2):475–99. doi: 10.1016/s1089-3261(03)00023-0. [DOI] [PubMed] [Google Scholar]
- Paris D, Ledergerber B, Weber R, Jost J, Flepp M, Opravil M, Ruef C, Zimmerli S. Incidence and predictors of virologic failure of antiretroviral triple-drug therapy in a community-based cohort. AIDS Research & Human Retro-viruses. 1999;15(18):1631–38. doi: 10.1089/088922299309676. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000c;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Rockstroh JK, Spengler U. HIV and hepatitis C virus co-infection. The Lancet Infectious Diseases. 2004;4(7):437–44. doi: 10.1016/S1473-3099(04)01059-X. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: are they related? Alcoholism: Clinical & Experimental Research. 2003b;27(5):862–67. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- Stein M, Herman DS, Trisvan E, Pirraglia P, Engler P, Anderson BJ. Alcohol use and sexual risk behavior among human immunodeficiency virus-positive persons. Alcoholism: Clinical & Experimental Research. 2005;29(5):837–843. doi: 10.1097/01.alc.0000164363.40533.e0. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr, Doll R. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. New England Journal of Medicine. 1997a;337(24):1705–14. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. American Journal of Medicine. 2003;114(7):573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]