Abstract
Objectives
We aimed to determine adherence, virological, and immunological outcomes one year after starting a first combination antiretroviral therapy (ART) regimen.
Design
Observational; synthesis of administrative, laboratory, and pharmacy data. Antiretroviral regimens were divided into efavirenz, nevirapine, boosted protease inhibitor (PI), and single PI categories. Propensity scores were used to control for confounding by treatment assignment. Adherence was estimated from pharmacy refill records.
Setting
Veterans Affairs Healthcare System, all sites.
Participants
HIV-infected individuals starting combination ART with a low likelihood of previous antiretroviral exposure.
Interventions
None.
Outcomes
The proportion of antiretroviral prescriptions filled as prescribed, a change in log HIV-RNA, the proportion with log HIV-RNA viral suppression, a change in CD4 cell count.
Results
A total of 6394 individuals unlikely to have previous antiretroviral exposure started combination ART between 1996 and 2004, and were eligible for analysis. Adherence overall was low (63% of prescriptions filled as prescribed), and adherence with efavirenz (67%) and nevirapine (65%) regimens was significantly greater than adherence with boosted PI (59%) or single PI (61%) regimens (P < 0.001). Efavirenz regimens were more likely to suppress HIV-RNA at one year (74%) compared with nevirapine (62%), boosted PI (63%), or single PI (53%) regimens (all P < 0.001), and this superiority was maintained when analyses were adjusted for baseline clinical characteristics and propensity for treatment assignment. Efavirenz also yielded more favorable immunological outcomes.
Conclusion
HIV-infected individuals initiating their first combination ART using an efavirenz-based regimen had improved virological and immunological outcomes and greater adherence levels.
Keywords: Adherence, resistance, ART, Veterans Affairs Healthcare System
Introduction
Guidelines for the use of antiretroviral agents in HIV-infected individuals support the choice of initiating combination antiretroviral therapy (ART) with either a boosted protease inhibitor (PI) or efavirenz in combination with a nucleoside reverse transcriptase inhibitor backbone [1]. These recommendations are based on randomized controlled trials that have demonstrated their superiority over single PI-based regimens, nevira-pine-based regimens, triple nucleoside regimens, and others [2–6]. Data directly comparing these two preferred regimens are, however, limited [7], and therefore their relative effectiveness and tolerance is unknown.
Whereas large observational databases offer the option of comparing combination ART regimens, it is difficult to ascertain whether observed differences are attributable to the regimens themselves or to differences in patient assignment by regimen type. This danger may be mitigated by limiting analyses to antiretroviral-naive patients in order to reduce the confounding effect of baseline drug resistance, or to studies with objective adherence data in order to control for adherence differences across regimen types, but these restrictions often preclude adequate statistical power for analysis.
We have created and validated a large `virtual' cohort composed of all HIV-infected individuals receiving medical care within the Veterans Affairs Healthcare System. The information available for the cohort includes virological, immunological, and clinical outcome data, antiretroviral regimen history, and adherence measurements. We used the substantial statistical power of this cohort to compare virological and immunological outcomes for patients unlikely to have previous antiretroviral exposure starting efavirenz, boosted PI, and other combination ART regimen types, with and without considering differences in adherence levels and other patient characteristics. Furthermore, we performed analyses using propensity scores in order to reduce the likelihood that differences in outcomes were attributable to confounding by treatment assignment.
Methods
We identified all antiretroviral-naive HIV-infected individuals within the Veterans Affairs Healthcare System who started their first combination ART regimen, comparing virological and immunological outcomes one year after initiating therapy. Because the Veterans Affairs Healthcare System maintains a nationwide database of pharmacy refill information, we were able to measure and control for adherence to combination ART.
The Veterans Affairs virtual cohort
The integrated electronic medical record used for patient care in all Veterans Affairs Medical Centers makes it possible to create a `virtual' cohort of veterans with HIV infection to monitor trends in utilization, toxicity, and clinical outcomes. Our method synthesizes disparate components of the Veterans Affairs Healthcare System's administrative apparatus, and has been shown to identify HIV-infected veterans with a sensitivity of 90% and a specificity of 99.9% [8]. We adapted an algorithm developed by Fasciano et al. [9], using the International Classification of Diseases, 9th Revision (ICD-9-CM) diagnostic codes to identify patients with a first HIV or AIDS diagnosis. Our modifications required veterans to have one or more inpatient or two or more HIV-related outpatient codes. Our program searched for the following ICD-9-CM codes: AIDS (042), asymptomatic HIV (V08), and related diagnostic-related group codes (488–490). This methodology has been described in full elsewhere [8]. Subjects were linked with the Immunology Case Registry (the Veterans Affairs HIV registry, which contains laboratory and clinical data) and the Pharmacy Benefits Management Registry (centralized database of outpatient prescriptions).
Individuals unlikely to have previous antiretroviral exposure
The Veterans Affairs virtual cohort was used to identify all individuals unlikely to have had previous antiretroviral exposure who started their first combination ART regimen. Combination ART was defined as three or more antiretroviral medications prescribed simultaneously. The individuals in our analysis started combination ART between January 1996 and December 2003, and our period of observation extended to 2004. The Pharmacy Benefits Management database was used to identify an individual's history of antiretroviral drug prescriptions within the Veterans Affairs system. Given that some veterans may have initiated antiretroviral therapy outside the Veterans Affairs system, we specified criteria to identify individuals with a low likelihood of previous antiretroviral exposure: (i) each individual must have had HIV prognostic indices (HIV-RNA, CD4 cell count) measured before the first combination ART prescription; (ii) no HIV-RNA level before the first combination ART prescription could have been sufficiently low to suggest antiretroviral exposure (< 500 copies/ml); (iii) the first combination ART regimen must not have been a `salvage' regimen [including both PI and non-nucleoside reverse transcriptase inhibitors (NNRTI), or including five or more drugs]; and (iv) each individual must not have received previous antiretroviral therapy (i.e. monotherapy) within the Veterans Affairs system. In sensitivity analyses, we explored alternative inclusion criteria (requiring HIV-RNA to be above 5000 copies/ml rather than above 500 copies/ml, and requiring each individual to be within the Veterans Affairs system without prescribed antiretroviral therapy for a 120-day `run-in' period before starting combination ART).
Combination antiretroviral therapy categorization
To evaluate the recommended regimens, we divided combination ART regimens into six mutually exclusive groups: (i) NNRTI-based regimens containing efavirenz; (ii) NNRTI-based regimens containing nevirapine; (iii) PI-based regimens with a single PI; (iv) PI-boosted regimens, in which a PI is paired with ritonavir (boosted PI); (v) triple nucleoside regimens; and (vi) other. Boosted PI regimens were further divided into newer regimens (including lopinavir, fosamprenavir, or atazanavir) or older regimens (including none of these drugs) when power considerations allowed.
Outcomes
Adherence
We used the algorithm of Steiner and colleagues [10,11] to estimate the proportion of medication doses consumed as directed based upon pharmacy refill data from the Veterans Affairs national database. This algorithm keeps a running tally of whether a patient would have run out of medications at any particular time based on the dates and number of medication doses given with previous refills, and determines what proportion of time the patient should have medications available (i.e. has not run out of medications). Assuming that patients do not obtain medications from elsewhere (a reasonable assumption for our study given that it is based on data from all Veterans Affairs sites nationwide), the result of this algorithm may be thought of as an upper bound for possible adherence. We averaged adherence from all antiretroviral medications to yield a summary adherence measure, and performed our analysis over the first year of therapy. The Steiner algorithm has previously been validated by demonstrating that its estimates were highly correlated with drug plasma levels [10]. The small proportion of individuals who `stockpile' drugs, as indicated by a pharmacy refill frequency that consistently exceeds the scheduled dosing interval, are inadequately characterized by this algorithm, and the method did not validate within this particular subgroup [10]. For this reason, we analysed this subgroup separately.
Virological measures
We measured (i) the change in the base-10 logarithm of the HIV-RNA levels between the HIV-RNA level obtained closest to the initiation of treatment and the one-year value, and (ii) the proportion of individuals with HIV-RNA suppression at one year. Viral suppression was defined as any value less than 500 HIV-RNA copies/ml. The less than 500 copies/ml threshold was chosen because the sensitivity of the HIV-RNA assay varied by site and year, and this threshold was sufficiently inclusive to allow all sites to contribute data (i.e. no site used an assay with a detection limit greater than 500 copies/ml). Because it was unlikely that individuals had HIV-RNA levels measured precisely at one-year intervals, we allowed a 6-month `window' for HIV-RNA measurement for both pre-treatment and on-treatment HIV-RNA levels. For all regimens, approximately half of the observations included in our analyses were recorded between 6 and 12 months after starting therapy, and approximately half were recorded between 12 and 18 months after starting therapy. All analyses were performed using intent-to-treat principles (individuals contributed to the analysis regardless of whether they switched regimens).
Immunological measures
Using the same time windows as for virological outcomes, we determined the change in CD4 count between the value obtained closest to the initiation of treatment and the one-year value. We also used intent to treat principles for these analyses.
Duration of treatment regimen
We determined how the likelihood of regimen discontinuation varied by type of regimen. `Discontinuation' was defined as changing or discontinuing two or more of the drugs within a particular regimen.
Statistical methods
All outcomes were examined using crude analyses (unadjusted for potential confounders), multivariate models (adjusted for potential confounders), and multivariate models with propensity scores (adjusting for potential confounders, and also adjusting for potential confounding by treatment assignment).
Crude
We compared dichotomous outcomes (proportion with HIV-RNA suppression) using the chi-squared test for independent proportions, and continuous outcomes (proportion of doses taken as directed, change in HIV-RNA level) using the Student's t-test for independent means.
Multivariate models
We constructed multivariate models of HIV-RNA suppression using stepwise generalized linear regression models (when the outcome measure was decrement in log HIV-RNA) and generalized logistic regression models (when the outcome measure was HIV-RNA suppression to < 500 copies/ml). The predictor variables were age, race/ethnicity, baseline CD4 cell count, baseline HIV-RNA level, combination ART category, type of nucleoside backbone (zidovudine/lamivudine versus stavudine/lamivudine versus other), ICD-9 diagnosis suggestive of past or present illicit drug use, ICD-9 diagnosis suggestive of past or present alcohol use disorder, site of care, and year of care; the criteria for inclusion and exclusion were P = 0.05 and P = 0.10, respectively. We performed separate analyses with and without including the adherence variables as predictor variables, and we performed separate analyses with and without dividing boosted PI regimens into newer and older groups. We used similar analysis strategies for our other outcome measures, employing generalized linear regression models to predict CD4 cell count elevation and Cox proportional hazard models to predict the time to regimen discontinuation.
Multivariate models with propensity scores
Propensity scores can be used in observational studies to adjust for confounding by treatment assignment [12]. A propensity score reflects the likelihood of being assigned to a particular treatment beyond random chance, and is estimated on the basis of patient characteristics that would be expected to influence treatment choice (e.g. patients starting treatment in later calendar years have a greater likelihood of having been assigned to efavirenz regimens, and this is reflected by a higher propensity score for receiving efavirenz). We determined a propensity score for efavirenz assignment by constructing a distinct logistic regression model and evaluating the covariates listed above as possible predictors [12]. The resulting propensity score was highly predictive of treatment assignment, explaining 85% of the variance. We then used this score as a distinct explanatory variable in each of our regression models for virological and immunological outcomes.
Results
Of the 33 420 individuals in the virtual cohort, 10 337 were identified as unlikely to have had previous antiretroviral exposure when they started combination ART. Of these individuals, 2909 (28%) had incomplete virological outcome data and 1034 (10%) had incomplete adherence data, leaving 6394 patients eligible for analysis. There were no clinically significant differences between medication assignments or disease stage (Table 1) among individuals with and without evaluable data. Patients who received efavirenz did not have clinically significant differences in disease stage compared with patients who received boosted PI (Table 1). A majority of individuals was non-Caucasian (68%) and male (98%). Their mean pretreatment CD4 cell count was 243 cells/μl and their mean pretreatment log HIV-RNA level was 4.6 copies/ml.
Table 1.
Characteristics of all patients meeting inclusion criteria, and patients with evaluable data.
| Patients with evaluable data (N=6394) |
||||||||
|---|---|---|---|---|---|---|---|---|
| All patientsa (N=10337) | All regimens (N=6394) | Efavirenz N=1140 (17.8%) | Nevirapine N=512 (8.0%) | Single PI N=3324 (52.0%) | Boosted PI N=401 (6.3%) | 3 NRTI N=517 (8.1%) | Other N=500 (7.8%) | |
| Mean age (years) | 42.8* | 45.7* | 47.1† | 45.5† | 45.1† | 45.8† | 46.7† | 45.4† |
| Male | 98.0% | 98.0% | 97.2% | 98.2% | 98.2% | 98.8% | 97.9% | 97.2% |
| Caucasian | 32.0%* | 31.1%* | 24.1%† | 30.9%† | 34.4%† | 27.3%† | 31.6%† | 39.0%† |
| African-American | 50.6%* | 51.3%* | 49.7%† | 52.3%† | 53.0%† | 52.5%† | 50.8%† | 41.6%† |
| Hispanic | 6.2%* | 6.0%* | 8.3%† | 5.3%† | 5.5%† | 5.0%† | 4.3%† | 7.4%† |
| Other race/ethnicity | 12.2%* | 10.8%* | 17.9%† | 11.5%† | 7.0%† | 15.3%† | 13.4%† | 12.1%† |
| Baseline CD4 cell count (mean) | 249 | 243 | 232† | 313† | 233† | 211† | 283† | 250† |
| Baseline log HIV-RNA (mean) | 4.5‡ | 4.6‡ | 4.8† | 4.5† | 4.6† | 4.7† | 4.5† | 4.5† |
Among all patients, 18.2% were on efavirenz, 8.2% on nevirapine, 51.2% on a single protease inhibitor (PI), 6.5% on boosted PI, 7.5% on three nucleoside reverse transcriptase inhibitors (NRTI), and 8.4% on other.
P<0.001 for evaluable versus non-evaluable data.
P<0.001 between regimen types.
P=0.04 for evaluable versus non-evaluable data.
Of the combination ART regimen types that were the focus of this analysis, the most prevalent type was single PI (N = 3324), followed by efavirenz (N 1140), nevirapine (N = 512), and boosted PI (N = 401, of which 257 were older regimens and 144 were newer). Of the 1017 individuals who were on other regimen types, 517 were on triple-nucleoside regimens.
Adherence
Overall, the estimated adherence with antiretroviral medications was 63%. Adherence with efavirenz (67%) and nevirapine (65%) regimens was significantly greater than adherence with boosted PI (59%) or single PI (61%) regimens (P < 0.001). Significantly more individuals on either non-nucleoside regimen (33%, efavirenz; 31%, nevirapine) were in the highest adherence stratum (80–100%) compared with individuals on either type of PI-based regimen (23%, single PI; 21%, boosted PI).
Virological outcomes
We report separately the results for the decline in log HIV-RNA and the likelihood of HIV-RNA suppression.
Decline in log HIV-RNA
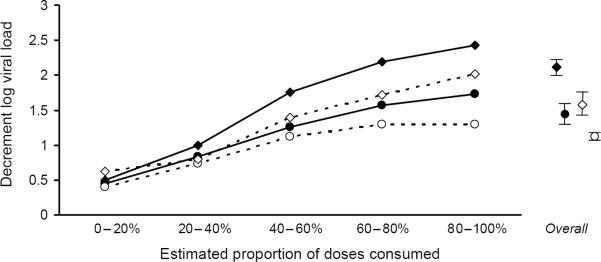
Patients on efavirenz had a greater decline in log HIV-RNA (2.07 log units) than patients on each of the other regimen types (nevirapine, 1.44 log units; single PI, 1.12 log units; boosted PI, 1.58 log units; triple nucleoside, 1.22 log units; other, 1.02 log units; all P < 0.001 compared with efavirenz). For all regimens, the decline in the log HIV-RNA level was greater with higher levels of adherence (Fig. 1). In multivariate analyses that controlled for demographic, behavioral, and baseline clinical characteristics, year of starting therapy, and therapy `backbone'; efavirenz-based regimens remained superior, suppressing HIV-RNA by an additional 0.22–0.49 log units (all P ≤ 0.002; Table 2). Augmenting the model to consider the propensity of having received efavirenz did not greatly impact our results, nor did augmenting the model to consider the level of adherence. When boosted PI were stratified into newer compared with older regimens, efavirenz continued to suppress HIV-RNA levels by an additional 0.34 log units (newer boosted PI) or 0.27 log units (older boosted PI); however, the difference with newer boosted PI did not reach statistical significance (P = 0.058) for this comparatively small subgroup.
Fig. 1. Change in log HIV-RNA level at one year, by adherence stratum.
Adherence was approximated by estimating the maximum proportion of antiretroviral doses that could have been taken as prescribed based on pharmacy refill records. Allthough the regimens differed greatly in their maximum effectiveness, a similar proportion of the maximum effect was observed within each adherence stratum.  Efavirenz;
Efavirenz;  nevirapine;
nevirapine;  boosted protease inhibitor;
boosted protease inhibitor;  single protease inhibitor.
single protease inhibitor.
Table 2.
Multivariate analysis of change in log HIV-RNA level one year after starting antiretroviral therapy.
| Base case |
With propensity |
With adherence |
||||
|---|---|---|---|---|---|---|
| Effect | P value | Effect | P value | Effect | P value | |
| Nevirapine based | 0.22 | 0.002 | 0.21 | 0.003 | 0.24 | 0.001 |
| Single protease inhibitor based | 0.45 | <0.001 | 0.44 | <0.001 | 0.44 | <0.001 |
| Boosted protease inhibitor baseda | 0.32 | <0.001 | 0.31 | <0.001 | 0.27 | 0.001 |
| Triple nucleoside based | 0.36 | <0.001 | 0.35 | <0.001 | 0.40 | <0.001 |
| Other based | 0.49 | <0.001 | 0.49 | <0.001 | 0.48 | <0.001 |
| Current illicit drug use, compared with none | 0.13 | 0.003 | 0.12 | 0.006 | 0.10 | 0.018 |
| African-American, compared with Caucasian | 0.17 | <0.001 | 0.17 | <0.001 | 0.10 | 0.014 |
| Hispanic, compared with Caucasian | 0.19 | 0.007 | 0.24 | 0.002 | 0.18 | 0.010 |
| Other, compared with Caucasian | 0.05 | 0.442 | 0.07 | 0.294 | −0.06 | 0.348 |
| Baseline log HIV-RNA, per log unit | −0.80 | <0.001 | −0.79 | <0.001 | −0.80 | <0.001 |
| Baseline CD4 cell count, per 100 cells/μl | −0.06 | <0.001 | −0.06 | <0.001 | −0.06 | <0.001 |
| Start in 1998, compared with ≤1997 | −0.30 | <0.001 | −0.29 | <0.001 | −0.30 | <0.001 |
| Start in 1999, compared with ≤1997 | −0.30 | <0.001 | −0.16 | 0.124 | −0.28 | <0.001 |
| Start in 2000, compared with ≤1997 | −0.45 | <0.001 | −0.24 | 0.136 | −0.38 | <0.001 |
| Start in ≥2001, compared with ≤1997 | −0.49 | <0.001 | −0.29 | 0.059 | −0.43 | <0.001 |
| Stavudine/lamivudine backbone, compared with zidovudine/lamivudine | 0.09 | 0.011 | 0.09 | 0.013 | 0.08 | 0.033 |
| Other backbone, compared with zidovudine/lamivudine | 0.20 | <0.001 | 0.19 | <0.001 | 0.19 | <0.001 |
| Propensity score | NAb | 0.53 | 0.153 | NAb | ||
| Adherence 60–80%, compared with 80–100% | NAc | NAc | 0.01 | 0.784 | ||
| Adherence 40–60%, compared with 80–100% | 0.21 | <0.001 | ||||
| Adherence 20–40%, compared with 80–100% | 0.64 | <0.001 | ||||
| Adherence 0–20%, compared with 80–100% | 0.94 | <0.001 | ||||
Efavirenz-based therapy is the referent category. Positive values correspond to smaller reductions in log HIV-RNA levels and are therefore less favorable. Results from three different models are displayed; the first does not adjust for the likelihood of being prescribed efavirenz nor levels of adherence (Base case), the second uses propensity scores to adjust for confounding by treatment selection (With propensity), and the third adjusts for the level of medication regimen adherence (With adherence).
Older boosted protease inhibitors (PI): 0.34, P<0.001 (base case), 0.33, P<0.001 (with propensity), 0.28, P=0.001 (with adherence). Newer boosted PI: 0.27, P=0.058 (base case), 0.26, P=0.065 (with propensity), 0.24, P=0.100 (with adherence).
Not applicable because analysis did not attempt to control for propensity.
Not applicable because analysis did not attempt to control for adherence.
Virological suppression
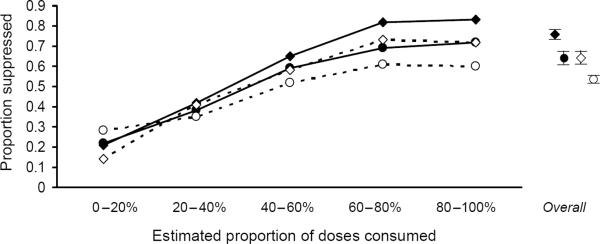
Patients on efavirenz had a greater likelihood of HIV-RNA suppression at one year (74%) compared with each of the other regimen types (nevirapine, 62%; single PI, 53%; boosted PI, 63%; triple nucleoside, 55%; other, 52%; all P < 0.001 compared with efavirenz). For all regimens, the likelihood of viral suppression was greater with higher levels of adherence (Fig. 2). In multivariate analyses that controlled for demographic, behavioral, and baseline clinical characteristics, year of starting therapy, and therapy `backbone', efavirenz-based regimens remained superior, with odds ratios of viral suppression for alternative regimens ranging from 0.45 to 0.60 (all P < 0.001; Table 3). Augmenting the model to consider the propensity of having received efavirenz did not greatly impact our results, nor did augmenting the model to consider the level of adherence. When boosted PI were stratified into newer versus older regimens, odds ratios were 0.57 for both older and newer subgroups of boosted PI (P < 0.05).
Fig. 2. Proportion with HIV-RNA suppression at one year, by adherence stratum.
Adherence was approximated by estimating the maximum proportion of antiretroviral doses that could have been taken as prescribed based on pharmacy refill records. Although the regimens differed greatly in their maximum effectiveness, a similar proportion of the maximum effect was observed within each adherence stratum.  Efavirenz;
Efavirenz;  nevirapine;
nevirapine;  boosted protease inhibitor;
boosted protease inhibitor;  single protease inhibitor.
single protease inhibitor.
Table 3.
Multivariate analysis of the odds of HIV-RNA suppression one year after starting antiretroviral therapy.
| Base case |
With propensity |
With adherence |
||||
|---|---|---|---|---|---|---|
| Odds ratio | P value | Odds ratio | P value | Odds ratio | P value | |
| Nevirapine based | 0.60 | <0.001 | 0.61 | <0.001 | 0.56 | <0.001 |
| Single protease inhibitor based | 0.48 | <0.001 | 0.49 | <0.001 | 0.47 | <0.001 |
| Boosted protease inhibitor baseda | 0.57 | <0.001 | 0.57 | <0.001 | 0.63 | 0.004 |
| Triple nucleoside based | 0.45 | <0.001 | 0.46 | <0.001 | 0.44 | <0.001 |
| Other based | 0.45 | <0.001 | 0.46 | <0.001 | 0.41 | <0.001 |
| Current illicit drug use, compared with none | 0.79 | 0.003 | 0.81 | 0.007 | 0.81 | 0.014 |
| African-American, compared with Caucasian | 0.81 | 0.004 | 0.81 | 0.003 | 0.93 | 0.368 |
| Hispanic, compared with Caucasian | 0.59 | <0.001 | 0.52 | <0.001 | 0.61 | 0.001 |
| Other, compared with Caucasian | 0.84 | 0.152 | 0.80 | 0.065 | 0.95 | 0.714 |
| Baseline log HIV-RNA, per log unit | 0.82 | <0.001 | 0.80 | <0.001 | 0.80 | <0.001 |
| Baseline CD4 cell count, per 100 cells/μl | 1.06 | <0.001 | 1.05 | <0.001 | 1.07 | <0.001 |
| Start in 1998, compared with ≤1997 | 1.37 | <0.001 | 1.29 | 0.003 | 1.38 | <0.001 |
| Start in 1999, compared with ≤1997 | 1.19 | 0.068 | 0.84 | 0.382 | 1.20 | 0.083 |
| Start in 2000, compared with ≤1997 | 1.62 | <0.001 | 0.92 | 0.779 | 1.54 | <0.001 |
| Start in ≥2001, compared with ≤1997 | 1.72 | <0.001 | 1.00 | 0.998 | 1.63 | <0.001 |
| Stavudine/lamivudine backbone, compared with zidovudine/lamivudine | 0.79 | <0.001 | 0.79 | 0.001 | 0.78 | 0.001 |
| Other backbone, compared with zidovudine/lamivudine | 0.78 | 0.004 | 0.79 | 0.006 | 0.79 | 0.014 |
| Propensity score | NAb | 4.08 | 0.049 | NAb | ||
| Adherence 60–80%, compared with 80–100% | NAc | NAc | 1.07 | 0.448 | ||
| Adherence 40–60%, compared with 80–100% | 0.71 | <0.001 | ||||
| Adherence 20–40%, compared with 80–100% | 0.36 | <0.001 | ||||
| Adherence 0–20%, compared with 80–100% | 0.17 | <0.001 | ||||
Efavirenz-based therapy is the referent category. Odds ratios (OR) greater than 1 are favorable, whereas odds ratios less than 1 are unfavorable. Results from three different models are displayed; the first does not adjust for the likelihood of being prescribed efavirenz nor levels of adherence (Base case), the second uses propensity scores to adjust for confounding by treatment selection (With propensity), and the third adjusts for the level of medication regimen adherence (With adherence).
Older boosted protease inhibitors (PI): OR 0.57, P=0.001 (base case), OR 0.57, P=0.001 (with propensity), OR 0.62, P=0.009 (with adherence).Newer boosted PI: OR 0.57, P=0.046 (base case), OR 0.58, P=0.052 (with propensity), OR 0.63, P=0.137 (with adherence).
Not applicable because analysis did not attempt to control for propensity.
Not applicable because analysis did not attempt to control for adherence.
Immunological outcomes
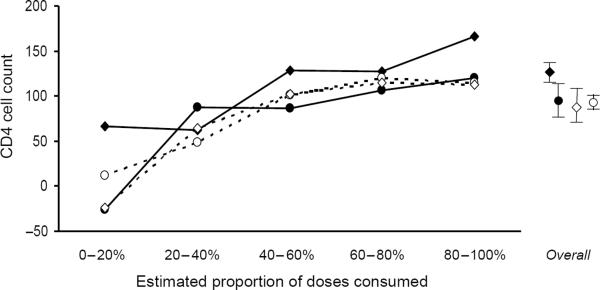
Whereas immunological outcomes were impacted less than virological outcomes by the choice of regimen, there were still significant differences. Patients on efavirenz had a greater increase in CD4 cell count at one year (126 cells/μl) compared with each of the other regimen types (nevirapine, 97 cells/μl; single PI, 97 cells/μl; boosted PI, 94 cells/μl; triple nucleoside, 81 cells/μl; other, 78 cellsμl; all P < 0.001 compared with efavirenz; Table 4). For all regimens, the elevation in CD4 cell count was greater with higher levels of adherence (Fig. 3). In multivariate analyses that controlled for demographic, behavioral, and baseline clinical characteristics, year of starting therapy, and therapy `backbone', efavirenz-based regimens increased the CD4 cell count by an additional 17–32 cells/μl compared with alternative regimens. The statistical significance of these relationships was, however, less robust than for virological outcomes, and in the case of nevirapine did not reach statistical significance at all (P = 0.091). Augmenting the model to consider the propensity of having received efavirenz did not greatly impact our results, nor did augmenting the model to consider the level of adherence. When boosted PI were stratified into newer versus older regimens, efavirenz continued to elevate the CD4 cell count by greater amounts (27 cells/μl, older boosted PI; 44 cells/μl, newer boosted PI; P < 0.05 for both).
Table 4.
Multivariate analysis of change in CD4 cell count (cells/μl) one year after starting antiretroviral therapy.
| Base case |
With propensity |
With adherence |
||||
|---|---|---|---|---|---|---|
| Effect | P value | Effect | P value | Effect | P value | |
| Nevirapine based | −17 | 0.091 | −15 | 0.139 | −22 | 0.038 |
| Single protease inhibitor based | −22 | 0.003 | −20 | 0.006 | −21 | 0.008 |
| Boosted protease inhibitor baseda | −31 | 0.005 | −30 | 0.007 | −23 | 0.043 |
| Triple nucleoside based | −22 | 0.047 | −20 | 0.069 | −31 | 0.009 |
| Other based | −32 | 0.003 | −31 | 0.004 | −33 | 0.003 |
| Current illicit drug use, compared with none | −18 | 0.002 | −17 | 0.004 | −15 | 0.013 |
| African-American, compared with Caucasian | −8 | 0.133 | −9 | 0.109 | −1 | 0.856 |
| Hispanic, compared with Caucasian | −27 | 0.006 | −34 | 0.001 | −28 | 0.007 |
| Other, compared with Caucasian | 2 | 0.831 | −1 | 0.924 | 11 | 0.237 |
| Baseline log RNA, per log unit | 14 | <0.001 | 13 | <0.001 | 13 | <0.001 |
| Baseline CD4 cell count, per 100 cells/μl | −6 | <0.001 | −6 | <0.001 | −5 | <0.001 |
| Start in 1998, compared with ≤1997 | 11 | 0.074 | 7 | 0.289 | 10 | 0.131 |
| Start in 1999, compared with ≤1997 | 25 | 0.001 | −1 | 0.952 | 25 | 0.001 |
| Start in 2000, compared with ≤1997 | 21 | 0.008 | −19 | 0.406 | 17 | 0.050 |
| Start in ≥2001, compared with ≤1997 | 14 | 0.075 | −23 | 0.270 | 7 | 0.404 |
| Stavudine/lamivudine backbone, compared with zidovudine/lamivudine | −7 | 0.155 | −7 | 0.172 | −7 | 0.187 |
| Other backbone, compared with zidovudine/lamivudine | −16 | 0.011 | −16 | 0.016 | −14 | 0.033 |
| Propensity score | NAb | 97 | 0.062 | NAb | ||
| Adherence 60–80%, compared with 80–100% | NAc | NAc | −5 | 0.428 | ||
| Adherence 40–60%, compared with 80–100% | −21 | 0.001 | ||||
| Adherence 20–40%, compared with 80–100% | −66 | <0.001 | ||||
| Adherence 0–20%, compared with 80–100% | −104 | <0.001 | ||||
Efavirenz-based therapy is the referent category. Positive numbers represent higher elevations in the CD4 cell count, and are therefore more favorable. Results from three different models are displayed; the first does not adjust for the likelihood of being prescribed efavirenz nor levels of adherence (Base case), the second uses propensity scores to adjust for confounding by treatment selection (With propensity), and the third adjusts for the level of medication regimen adherence (With adherence).
Older boosted protease inhibitors (PI): −27, P=0.028 (base case), −26, P=0.036 (with propensity), −17, P=0.179 (with adherence). Newer boosted PI: −44, P=0.030 (base case), −42, P=0.036 (with propensity), −43, P=0.043 (with adherence).
Not applicable because analysis did not attempt to control for propensity.
Not applicable because analysis did not attempt to control for adherence.
Fig. 3. Change in CD4 cell count at one year, by adherence stratum.
Adherence was approximated by estimating the maximum proportion of antiretroviral doses that could have been taken as prescribed based on pharmacy refill records. The improved effectiveness of efavirenz was most evident in the highest adherence stratum.  Efavirenz;
Efavirenz;  nevirapine;
nevirapine;  boosted protease inhibitor;
boosted protease inhibitor;  single protease inhibitor.
single protease inhibitor.
Duration of treatment regimen
In unadjusted analyses, patients on efavirenz had a median time to regimen discontinuation of 1.4 years, shorter than some regimen types (nevirapine, 1.7 years; single PI, 1.6 years; boosted PI, 1.5 years) and longer than others (triple nucleoside, 0.9 years; other, 1.2 years). In multivariate analyses that controlled for demographic, behavioral, and baseline clinical characteristics, year of starting therapy, and therapy `backbone', the duration of efavirenz was statistically indistinguishable from the duration of all other regimen types except for single PI regimens, which were inferior (hazard ratio for treatment discontinuation 1.16; P=0.002). In analyses that controlled for the propensity of receiving efavirenz or for treatment adherence, efavirenz regimens remained superior to single PI regimens, and were also statistically superior to triple-nucleoside regimens and other regimens (Table 5).
Table 5.
Multivariate analysis of duration of treatment regimen.
| Base case |
With propensity |
With adherence |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | Hazard ratio | P value | |
| Nevirapine based | 1.09 | 0.150 | 1.10 | 0.152 | 1.06 | 0.367 |
| Single protease inhibitor based | 1.16 | 0.002 | 1.16 | 0.002 | 1.16 | 0.003 |
| Boosted protease inhibitor baseda | 0.92 | 0.065 | 0.92 | 0.248 | 0.95 | 0.471 |
| Triple nucleoside based | 1.27 | 0.090 | 1.26 | 0.001 | 1.22 | 0.011 |
| Other based | 1.22 | 0.083 | 1.22 | 0.004 | 1.17 | 0.036 |
| Current illicit drug use, compared with none | 1.01 | 0.826 | 1.01 | 0.820 | 1.01 | 0.731 |
| African-American, compared with Caucasian | 0.95 | 0.111 | 0.95 | 0.128 | 0.99 | 0.700 |
| Hispanic, compared with Caucasian | 1.02 | 0.710 | 1.02 | 0.752 | 1.08 | 0.251 |
| Other, compared with Caucasian | 0.86 | 0.009 | 0.87 | 0.014 | 0.89 | 0.061 |
| Baseline log RNA, per log unit | 1.06 | 0.002 | 1.06 | 0.003 | 1.06 | 0.008 |
| Baseline CD4 cell count, per 100 cells/μl | 0.96 | <0.001 | 0.96 | <0.001 | 0.96 | <0.001 |
| Start in 1998, compared with ≤1997 | 1.04 | 0.397 | 1.04 | 0.367 | 1.06 | 0.192 |
| Start in 1999, compared with ≤1997 | 1.31 | <0.001 | 1.31 | 0.004 | 1.34 | <0.001 |
| Start in 2000, compared with ≤1997 | 1.55 | <0.001 | 1.56 | 0.002 | 1.64 | <0.001 |
| Start in ≥2001, compared with ≤1997 | 2.19 | <0.001 | 2.19 | <0.001 | 2.35 | <0.001 |
| Stavudine/lamivudine backbone, compared with zidovudine/lamivudine | 1.06 | 0.057 | 1.06 | 0.057 | 1.08 | 0.032 |
| Other backbone, compared with zidovudine/lamivudine | 1.19 | <0.001 | 1.19 | <0.001 | 1.20 | <0.001 |
| Propensity score | NAb | 1.01 | 0.976 | NAb | ||
| Adherence 60–80%, compared with 80–100% | NAc | NAc | 0.86 | <0.001 | ||
| Adherence 40–60%, compared with 80–100% | 0.77 | <0.001 | ||||
| Adherence 20–40%, compared with 80–100% | 0.88 | 0.010 | ||||
| Adherence 0–20%, compared with 80–100% | 1.01 | 0.942 | ||||
Efavirenz-based therapy is the referent category. Positive numbers represent a greater hazard for changing regimens, and are therefore less favorable. Results from three different models are displayed; the first does not adjust for the likelihood of being prescribed efavirenz nor levels of adherence (Base case), the second uses propensity scores to adjust for confounding by treatment selection (With propensity), and the third adjusts for the level of medication regimen adherence (With adherence).
Subgroups of boosted protease inhibitor regimens not evaluated because no effect was apparent in the larger group.
Not applicable because analysis did not attempt to control for propensity.
Not applicable because analysis did not attempt to control for adherence.
Impact of covariates
Whereas some covariates were consistently associated with poorer virological and immunological outcomes (current illicit drug use, Hispanic ethnicity, stavudine/lamivudine backbone, and `other' backbone), other covariates had more complex relationships. African-American ancestry was associated with poorer virological suppression only when adherence was left out of the model, suggesting that adherence levels contributed to the poorer outcomes. Whereas virological outcomes were improved in later calendar years, this trend was less evident when the propensity for choosing efavirenz was considered in the model, suggesting that the calendar year improvement was partly attributable to changes in preferred regimens. There was no comparable calendar year trend for the improvement in immunological outcomes, but later calendar years were associated with shorter times to regimen discontinuation, possibly because of an increase in the number of alternative regimens. Finally, although adherence had a profound impact on both virological and immunological outcomes, the more favorable results of efavirenz were not explained wholly by higher adherence levels, as the results remained clinically and statistically significant even when adherence was included in the models.
Sensitivity analyses
Our inclusion criteria attempted to isolate antiretroviral-naive individuals; however, they reflect the limitations of administrative and pharmacy databases within a particular care system, and may have included some individuals with previous antiretroviral exposure. To explore the robustness of these criteria, we reran all analyses using two alternative and stricter specifications, first mandating no HIV-RNA level lower than 5000 copies/ml before the first combination ART prescription (instead of 500 copies/ml, as in our base-case criteria), and the second requiring each individual to have spent 120 days in the Veterans Affairs system without receiving any antiretroviral prescriptions before the first combination ART prescription (instead of no time restrictions, as in our base-case criteria). These alternative criteria had minimal impact on our results. The only statistically significant finding that was altered by these sensitivity analyses was the HIV-RNA decrement of nevirapine compared with efavirenz (smaller and no longer statistically significant).
Discussion
In the Veterans Affairs system, individuals initiating their first combination ART regimen using an efavirenz-based regimen had improved virololgical outcomes compared with those starting nevirapine, PI or boosted PI-based regimens. Immunological outcomes may also have been improved in the efavirenz group, although these data were less robust. The clinical implications of these results may be substantial because the prognostic value of the HIV-RNA level and CD4 cell count for predicting clinical outcomes (AIDS and death) appears not to vary by regimen type [13]. Our results are consistent with preliminary data from ACTG 5142, which demonstrated a more favorable time to treatment failure for efavirenz compared with an older boosted PI-based regimen (lopinavir/ritonavir) [14].
Despite obtaining these results from one type of care setting, our results may be more generalizable to everyday practice than results from clinical trials, which often enroll highly selected individuals who are particularly likely to adhere to prescribed therapies. The large sample size came from all 50 states and Puerto Rico, all patients were included, and the Veterans Affairs Healthcare System allowed practitioners to choose from any of the approved antiretroviral agents. Furthermore, the ranges of HIV-RNA suppression observed by regimen type were generally within the ranges observed in previous studies [1].
Our results further confirm that adherence is an important issue in HIV care, even with the advent of newer regimens with lower pill burdens and fewer lifestyle impositions. Whereas adherence to efavirenz regimens was greater than either of the PI-based regimen types, only 35% had adherence in the highest stratum (> 80%). In contrast to Bangsberg et al. [15], we did not find that regimens with NNRTI were more `forgiving' of non-adherence compared with regimens with PI (Fig. 1 and Fig. 2). The proportion of maximum effect we observed in each adherence stratum (i.e. the shape of the curve) was remarkably similar across medication regimens, whereas the maximum effect (i.e. the amplitude of the curve) differed greatly by the type of medication regimen. Although our study was not intended to determine why adherence to efavirenz regimens was more favorable, the possible explanations include less common or less burdensome side effects, or decreased pill burdens.
Our results further document that most individuals are non-adherent in a range (60–80%) that is maximally conducive to the development of resistance mutations [16–18]. Therefore, increasing combination ART adherence should remain one of the highest priorities in HIV care, and may be facilitated by interventions that increase adherence directly [19,20] or that mitigate major risk factors for poor adherence such as alcohol use [21–25], illicit drug use [23,24], and mental illness [24,26,27]. This emphasis may also serve to decrease disparities in HIV care, as our results suggest that the poorer virological suppression of African-American individuals appears to be partly mediated by adherence levels, and may ultimately originate from factors such as education, health literacy, or provider trust.
Even though initial combination ART regimens using efavirenz had better virological and immunological one-year outcomes than other types of combination ART among patients in routine care, our results should not be interpreted to suggest that efavirenz regimens should necessarily be preferred to boosted PI regimens as first-line agents. The treatment of HIV-infected patients is a marathon not a sprint, and the ultimate goal of a combination ART is to extend the life of a patient and improve quality of life. A treatment strategy success depends not upon the effectiveness of a single regimen considered in isolation, but rather on the effectiveness of an entire sequence of regimens. Because our results do not evaluate long-term clinical outcomes, it is possible that any benefit conferred by starting with efavirenz is no longer evident after several years, when most individuals are on their second or third regimens.
Our results underscore the formidable improvements in treatment success since the beginning of the combination ART era. Individuals who started combination ART in later calendar years were far more likely to have HIV-RNA suppression than individuals who started combination ARTearlier. Much of this improvement probably resulted from a stepwise improvement in the effectiveness of regimen types. Boosted PI regimens were more likely to suppress HIV-RNA levels than older, unboosted PI regimens, and newer boosted PI were more likely to be suppressive than older boosted PI regimens. Similarly, efavirenz was more likely to be suppressive than nevirapine.
There is no gold standard for measuring adherence. Estimating adherence based on pharmacy refill records has the advantage of correlating more closely with virological suppression than self-report measures [28], and offers a consistent adherence metric that can be compared across patient populations as long as the same interpretation algorithm is used. Methods such as medication event monitoring system caps [26] or unanticipated pill counts [16] are arguably likely to give more accurate estimates of adherence than pharmacy refill records. Nonetheless, the formidable expense and respondent burden of these methods renders them unsuitable for studies with very large patient samples or for usual care settings.
Our results have several limitations. They were based on a patient sample that was overwhelmingly male. We could not control for every important patient characteristic (e.g. mental illness was not considered in our analyses). Our results also have the potential limitation of confounding by treatment assignment. Anecdotally, providers sometimes choose PI-based regimens preferentially over efavirenz-based regimens for individuals who have more advanced disease or who are less likely to adhere. However, patients starting efavirenz in our sample had pre-treatment CD4 cell counts and viral loads that did not differ greatly from patients starting PI-based regimens. Furthermore, because we were able to control for adherence to therapy, and because we included a propensity score that reflected an individual's likelihood of assignment to efavirenz based on disease stage and other characteristics (i.e. drug abuse), confounding by treatment assignment is less likely to have impacted our results. Because our sample had a low probability of previous antiretroviral exposure, it is unlikely that our results were explained by disparities in pre-existing resistance mutations. NNRTI mutations predominate in treatment-naive populations, and therefore any preexisting mutations would have probably caused an underestimation of efavirenz effectiveness, a bias in the opposite direction of our findings.
In summary, among antiretroviral-naive individuals initiating combination ART in the Veterans Affairs system, those starting an efavirenz-based regimen had more favorable adherence levels, virological responses, and immunological outcomes than among those starting PI-based regimens. Adherence levels were, however, generally poor across all combination ART regimens, frequently occurring at levels that maximize the accumulation of resistance mutations. Future work should focus on methods to improve adherence, regardless of regimen type.
Acknowledgments
Sponsorship: This work was funded by grants K23 AA14483-01 and 2U10 AA13566 from the National Institute of Alcohol Abuse and Alcoholism. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 10 October 2006. The Panel on Clinical Practices for the Treatment of HIV Infection convened by the Department of Health and Human Services. Available at: http://AIDSinfo.nih.gov. Accessed: March 2007.
- 2.Robbins GK, De Gruttola V, Shafer RW, Smeaton LM, Snyder SW, Pettinelli C, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:>2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staszewski S, Morales-Ramirez J, Tashima KT, Rachlis A, Skiest D, Stanford J, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;241:>1865–1873. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 4.Walmsley S, Bernstein B, King M, Arribas J, Beall G, Ruane P, et al. Lopinavir–ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:>2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-French A, Boghossian J, Gray GE, Nadler JP, Quinones AR, Sepulveda GE, et al. The NEAT Study: a 48-week open-label study to compare the antiviral efficacy of GW433908 versus nelfinavir in antiretroviral naive HIV-1 infected patients. J Acquir Immune Defic Syndr. 2004;35:22–32. doi: 10.1097/00126334-200401010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Gathe JC, Ive P, Wood R, Schurmann D, Bellos NC, DeJesus E, et al. SOLO: 48 week efficacy and safety comparison of once-daily fosamprenavir/ritonavir versus twice daily nelfinavir in naive HIV-1-infected patients. AIDS. 2004;18:>1529–1537. doi: 10.1097/01.aids.0000131332.30548.92. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JA, Johnson J, Herrera G, Sosa N, Rodriguez AE, Shaefer MS. XIVth International AIDS Conference. Barcelona; Spain: Jul 7–12, 2002. Abacavir/lamivudine (ABC/3TC) in combination with efavirenz (NNRTI), amprenavir/ritonavir (PI) or stavudine (NRTI): ESS4001 (CLASS) preliminary 48 week results. [Abstract TuOrB 1189] [Google Scholar]
- 8.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the national VA health information system. Med Care. 2006;44(8 Suppl. 2):S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 9.Fasciano NJ, Cherlow AL, Turner BJ, Thornton CV. Profile of Medicare beneficiaries with AIDS: application of an AIDS casefinding algorithm. Health Care Financ Rev. 1998;19:>19–38. [PubMed] [Google Scholar]
- 10.Steiner JF, Kopesell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records: description and validation. Med Care. 1988;26:>814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Steiner JF, Prochanska AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:>105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 12.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:>757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 13.Olson CH, Gatell J, Ledergerber B, Katlama C, Friis-Moller N, Weber J, et al. Risk of AIDS and death at given HIV-RNA and CD4 cell counts, in relation to specific antiretroviral drugs in the regimen. AIDS. 2005;19:>319–330. [PubMed] [Google Scholar]
- 14.Riddler SA, Haubrich R, DiRienzo G, Peeples L, Powderly WG, Klingman KL, et al. XVIth International AIDS Conference. Toronto; Canada: Aug 13–18, 2006. A prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regiments for initial treatment of HIV infection-ACTG 5142. [Abstract ThLB0204] [Google Scholar]
- 15.Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother. 2004;53:>696–699. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- 16.Bangsberg DR, Charlebois ED, Grant RM, Holodniy M, Deeks SG, Perry S, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS. 2003;17:>1925–1932. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- 17.Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191:>339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 18.Braithwaite RS, Shechter S, Roberts MS, Schaefer A, Bangsberg D, Harrigan R, Justice A. Explaining variability in the relationship between antiretroviral adherence and HIV mutation accumulation. J Antimicrob Chemother. 2006;58:>1036–1043. doi: 10.1093/jac/dkl386. [DOI] [PubMed] [Google Scholar]
- 19.Altice FL, Mezger JA, Hodges J, Bruce RD, Marinovich A, Walton M, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis. 2004;38(Suppl. 5):S376–S387. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- 20.Goldie SJ, Paltiel AD, Weinstein MC, Losina E, Seage GR, III, Kimmel AD, et al. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection. Am J Med. 2003;115:>632–641. doi: 10.1016/j.amjmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal C, Day N, et al. The temporal association between alcohol consumption and adherence in a cohort of HIVR and matched HIV– patients. Alcoholism: Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 22.Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:>83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:>767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 24.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114:>573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 25.Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcoholism: Clin Exp Res. 2004;28:>572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 26.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez JS, Penedo FJ, Antoni MH, Duran RE, McPherson-Baker S, Ironson G, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychol. 2004;23:>413–418. doi: 10.1037/0278-6133.23.4.413. [DOI] [PubMed] [Google Scholar]
- 28.Grossberg R, Zhang Y, Gross R. A time-to-prescription refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57:>1107–1110. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]