Abstract
Background
Despite the effectiveness of antiretroviral therapy, nearly half of patients entering human immunodeficiency virus (HIV) care have advanced disease. Many attribute this delay to poor access to healthcare. Others argue that delays will persist until routine screening is adopted. The Veterans Health Administration (VA) is a unique laboratory to examine whether access to comprehensive health benefits results in earlier entry into HIV care.
Methods
Retrospective observational study of 4368 HIV-positive patients entering HIV care during 1998–2002 at VA medical centers nationwide. Outcomes of interest: rates of acquired immune deficiency syndrome in year of presentation; duration of VA utilization before HIV presentation; presence of “clinical triggers,” signaling greater risk of HIV infection, before presentation.
Results
Fifty-one percent (n = 2211) of all patients presented with CD4 counts of <200 cells/mm3. Thirty-nine percent (n = 1697) of all patients used other VA services before presentation for HIV care, with median duration of 3.6 years (interquartile range 25–75: 2.2–5.1 year) and 6 physician visits [interquartile range (IQR), 25–75: 2–18 visits] between first utilization and HIV presentation. No difference existed in the percentage presenting with CD4 counts <200 cells/mm3 among those with and without prior VA healthcare (50% vs. 51%, P = 0.76). Only 13% of those with prior VA healthcare demonstrated a clinical trigger before HIV presentation.
Conclusions
More than half of veterans entered HIV care with an acquired immune deficiency syndrome diagnosis at presentation irrespective of whether they had previously established healthcare in the VA. Access to care does not seem to be the primary cause of delayed HIV presentation. Widespread HIV screening is needed to improve rates of early detection.
Keywords: HIV, AIDS, diagnosis, screening, access to care
Antiretroviral therapy (ART) has revolutionized human immunodeficiency virus (HIV) care by decreasing mortality, acquired immune deficiency syndrome (AIDS)-defining illnesses, and transmission of the virus.1,2 To maximally benefit from ART, patients should begin therapy before their CD4 count falls below 200 cells/mm3 or they develop an AIDS-defining illness.3,4 Because the median time between HIV infection and the development of AIDS is 9–11 years,5,6 this goal would seem attainable. Yet, multiple studies have shown that the median CD4 count among patients presenting for HIV care is near or below 200 cells/mm3 (Table 1).7–12
TABLE 1.
Median CD4 Count and Percentage of Patients Presenting With CD4 Count <200 cells/mm3 at Time of Presentation for HIV Care
| Study | Years of Study | Median CD4 Count at HIV Presentation (Cells/mm3) | Percent of Patients With CD4 <200 Cells/mm3 at Time of HIV Presentation |
|---|---|---|---|
| Samet et al8 | 1994–1996 | 280 | 37 |
| Liddicoat et al10 | 1994–2001 | 256 | 44 |
| Klein et al20 | 1998 | 254 | 43 |
| Dybul et al7 | 1999–2000 | Not available | 24–5* |
| Egger11 | 2003–2005 | 53–239† | Not available |
Range among 4 clinic sites.
Range among 176 sites in 42 countries.
For an individual to initiate ART, he must access healthcare (general access), be tested for HIV (HIV testing), and present for HIV care (specialty access). Although debate exists regarding the role of each of these steps in delaying HIV care, the relative importance of access to care (general or specialty) versus HIV testing has not been well described.
The Veterans Health Administration (VA) is a unique laboratory to consider questions of general and specialty access and testing among HIV-infected patients,13–15 because it provides low cost, comprehensive health benefits to over 5 million veterans each year, including 19,000 HIV-infected patients.14,16,17 The VA also uses an electronic medical record, which allows for analysis of nationwide administrative, pharmacy, and laboratory data.13–15
If access to general and specialty healthcare is the major barrier to early entry into HIV care, we would expect veterans to present for HIV care earlier in their course (ie, higher presenting CD4 count). Further, we would expect veterans already receiving general medical care at the VA to present earlier than veterans new to the VA. If, instead, HIV testing or delay in seeking treatment after HIV diagnosis is the major problem, we would expect the proportion of patients presenting with advanced HIV infection to be similar to those outside the VA system.
Methods
This retrospective, observational study examines healthcare utilization patterns of patients identified with HIV at VA medical centers (VAMC) nationwide. The “VA centralized data,” derived from the VA Austin Automation Center, Immunology Case Registry, and Pharmacy Benefits Manager, contain demographics, diagnostic codes, inpatient and outpatient utilization, laboratory tests, and pharmacy prescriptions for all patients receiving care at VAMCs nationwide.
Study Sample
A cohort using VA centralized data was created and validated13 to identify HIV-infected veterans. Patients were assigned a “date of HIV presentation,” reflecting the first evidence of HIV infection in the VA, by identifying the earliest of the following: HIV test, CD4 count, HIV viral load, HIV genotype, HIV ICD-9 diagnostic code, antiretroviral prescription, or entry into the Immunology Case Registry. The date of HIV presentation was validated by chart review in 216 patients at the Atlanta VAMC (see Appendix which can be found on the Medical Care website, www.lww-medicalcare.com).
Patients were eligible if their date of HIV presentation was in fiscal years 1998–2002 (October 1, 1997 to September 30, 2002). Patients were excluded if: (1) their first antiretroviral prescription was before a baseline CD4 count and viral load were measured (suggesting they were already receiving ART), and (2) if their baseline viral load was <400 copies (see Appendix online).
Measurements and Definitions
Patients were categorized by baseline CD4 count and whether or not they used other VA services >1 year before their date of HIV presentation. Patients were considered to have an AIDS-defining illness if they had an ICD-9 code for HIV dementia, cryptococcal infection, cytomegalovirus infection, Kaposi Sarcoma, pneumocystis pneumonia, toxoplasmosis, tuberculosis, other mycobacterial infections, or HIV wasting. These diagnostic codes have been previously validated by comparison with medical records.18
The duration of prior VA utilization was calculated between first VA utilization and the date of HIV presentation. Sites of prior utilization were categorized as primary care, psychiatric, substance abuse, subspecialty, and emergency department visits, based on outpatient utilization codes.
“Clinical triggers” were adapted10 to reflect the clinical conditions, which influence practitioners to perform HIV tests on patients. Patients were considered to have a clinical trigger if they had diagnostic codes for oral or other candidiasis, hepatitis B or C, herpes zoster, endocarditis, any sexually transmitted disease, or an AIDS-defining illness, >1 year before their HIV presentation.
The study was approved by the Institutional Review Boards of Yale University, West Haven VA and Pittsburgh VA.
Outcomes and Analysis
The outcomes of interest were the proportion of patients with AIDS at HIV presentation (CD4 <200 cells/mm3 or AIDS-defining illnesses within a year), median CD4 count at presentation and interquartile range (IQR), proportion with other VA services used before HIV presentation, and proportion with clinical triggers before HIV presentation. t tests and χ2 tests were used to compare demographics, proportions with baseline CD4 counts <200 cells/mm3, AIDS-defining illnesses, and clinical triggers. Logistic regression was used to determine the impact of prior utilization on proportion presenting with CD4 <200 and AIDS-defining illnesses, adjusting for age, sex, and race. Given the large study sample, comparisons with P < 0.01 were considered significant.
Results
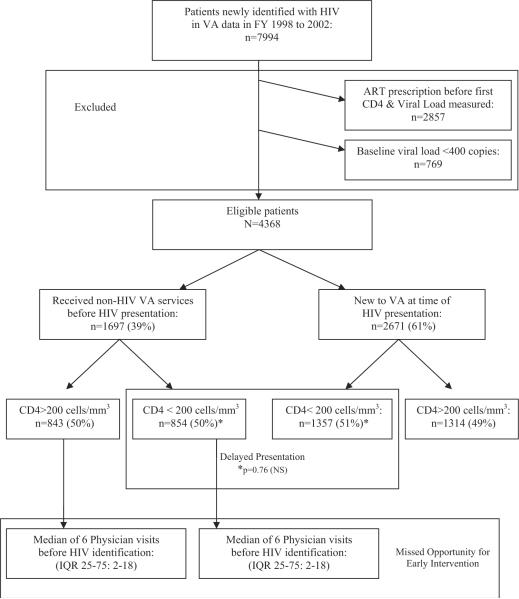
Overall, 7994 veterans were identified with a date of first HIV presentation in the VA centralized data nationwide between fiscal years 1998 and 2002. Of these, 4368 patients were eligible for analysis (Fig. 1). The mean age was 46.3 years (SD, 9.9) and 98% were male. Thirty-two percent were white, 51% African American, 8% Latino (race not recorded for 8%). Study eligible patients were more likely to be African American (P = 0.004); otherwise, no significant demographic differences were found.
FIGURE 1.
Outline of study design and results.
The majority of patients presented late in the course of HIV disease. Fifty-one percent (n = 2211) presented with a CD4 count <200 cells/mm3 (median, 198 cells/mm3; IQR, 59–368); 24% (n = 1029) presented with a CD4 count <50 cells/mm3 (Table 2). Patients with lower CD4 counts were more likely to suffer an AIDS-defining illness within a year of presentation: 49% of patients with CD4 counts <50 cells/mm3 had an AIDS-defining illness, compared with 24% among CD4 counts 50–200, 10% among 200–350, and 7% among >350 cells/mm3 (Table 3, P < 0.0001 for trend).
TABLE 2.
Baseline CD4 Count Among HIV Patients With Prior VA Utilization and Those New to the VA Stratified by Baseline CD4 Count (P = 0.30)
| Baseline CD4 Count (Cells/mm3) | Prior VA Utilization % (n) | New to VA % (n) | Total % (n) |
|---|---|---|---|
| <50 | 23 (387) | 24 (642) | 24 (1029) |
| 51–200 | 28 (467) | 27 (715) | 27 (1182) |
| 201–350 | 23 (395) | 21 (568) | 22 (963) |
| >350 | 26 (448) | 28 (746) | 27 (1194) |
| Total | 100 (1697) | 100 (2671) | 100 (4368) |
TABLE 3.
Rate of AIDS-Defining Illnesses Among HIV Patients With Prior VA Utilization and Those New to the VA Stratified by Baseline CD4 Count
| Baseline CD4 Count (Cells/mm3) | Prior VA Utilization* (n = 1697) % (n) | New to VA* (n = 2671) % (n) | Total† (n = 4368) % (n) |
|---|---|---|---|
| <50 (n =1029) | 52 (201) | 47 (301) | 49 (502) |
| 51–200 (n = 1182) | 25 (119) | 22 (159) | 24 (278) |
| 201–350 (n = 963) | 10 (41) | 10 (57) | 10 (98) |
| >350 (n = 1194) | 10 (43) | 5 (39) | 7 (82) |
| Total | 24 (404) | 21 (556) | 22 (960) |
Statistical comparisons: (1) Trend of proportion with AIDS-defining illnesses is greatest among patients with lowest CD4 counts (χ2 for trend P < 0.0001); (2) Comparison of proportion with AIDS-defining illnesses between patients with prior VA utilization and those new to VA.
P = 0.30.
χ2 for trend P < 0.0001.
Presentation for HIV care was delayed even among patients already receiving medical care within the VA system. Among HIV-positive veterans, 39% (n = 1697) were already receiving other VA medical care >1 year before their HIV presentation (Fig. 1). The median time between first VA service used and HIV presentation was 3.6 years (IQR, 2.2–5.1), with a median of 6 physician visits (IQR, 2–18) before HIV presentation. Fifty-six percent (n = 949) of these patients were seen in primary care clinic before HIV presentation; 50% (n = 850) were seen in a subspecialty clinic; 31% (n = 528) in a psychiatry clinic; and 16% (n = 274) in a substance abuse clinic. No significant differences existed in the sites and number of visits, or time of prior VA utilization among patients with baseline CD4 counts above and below 200 cells/mm3, except that patients with CD4 counts >200 were more likely to be seen in psychiatry (35% vs. 27%, P = 0.0003) or substance abuse clinic (18% vs. 13%, P = 0.004).
Despite extensive use of the VA healthcare system before HIV presentation, patients with prior VA utilization did not differ with respect to baseline CD4 count or AIDS-defining illnesses from those new to the VA at HIV presentation. Fifty percent (n = 854) of patients with prior VA utilization had a baseline CD4 <200 cells/mm3, compared with 51% (n = 1357) of those new to the VA (P = 0.76) (Fig. 1 and Table 2). Twenty-four percent (n = 404) of patients with prior VA utilization had an AIDS-defining illness compared with 21% (n = 556) of those new to the VA (P = 0.21) (Table 3). Although patients with prior VA utilization were more likely to be African American (63% vs. 56%, P < 0.0001), no significant difference in baseline CD4 count or AIDS defining illnesses existed between those with and without prior VA utilization, after adjusting for race in multivariable analysis (P = 0.13).
Analysis of “clinical triggers” revealed that most patients did not have a condition suggesting increased risk for HIV infection before HIV presentation. Among patients with CD4 count <200, only 12.5% (n = 107) had a clinical trigger before HIV presentation. The most prevalent codes were herpes zoster, hepatitis C, hepatitis B, other sexually transmitted diseases (eg, chlamydia, condyloma), herpes simplex, and gonorrhea. The prevalence of clinical triggers was not significantly different between patients with CD4 counts less than and greater than 200 cells/mm3 (12.5% vs. 10.4%, P = 0.18).
Discussion
Nationwide, half of the 4368 veterans presenting to VAMCs for HIV care between 1998 and 2002 had AIDS, with baseline CD4 counts <200 cells/mm3. The proportion presenting with AIDS did not differ based on whether or not they had previously accessed VA healthcare, despite the fact that veterans who previously accessed VA healthcare had a median of 6 physician visits over 3.7 years before presenting for HIV care. Given the median CD4 count of approximately 200 cells/mm3 at presentation, it is likely that most of these veterans were infected with HIV during this period of prior VA care,19 yet remained undiagnosed. Our analysis of clinical triggers suggests that patients were not identified earlier because they did not manifest obvious signs and symptoms suggesting an increased risk for HIV infection.
If access to care were the major barrier preventing timely initiation of treatment for HIV, we would expect eligible veterans to present earlier (ie, at higher CD4 cell counts) than non-veterans, because the VA provides comprehensive health benefits nationwide. Moreover, we would expect veterans already receiving medical care within the VA to present earlier than veterans who have not previously accessed VA care. Instead, we found that half the veterans presented for care with CD4 cell counts below 200 cells/mm3—a proportion at least as high as that reported outside the VA (Table 1).7,8,10,12,20 This proportion did not vary by whether or not veterans had previously used VA healthcare. Our results suggest that access to healthcare is not a significant barrier to timely treatment for HIV. Instead, efforts to improve identification of unrecognized HIV, and linkage to HIV care, are necessary.
The US Centers for Disease Control and Prevention have long advocated for early identification of HIV infection by routine HIV screening,21,22 which has also been shown to be cost-effective.23,24 If these veterans had been screened for HIV as part of routine medical care at first contact with the VA, their HIV might have been identified several years earlier. Extrapolating from previous data on the decline of CD4 cell counts in untreated HIV infected patients (Fig. 2),25 identification of HIV 3–4 years earlier would have resulted in baseline CD4 counts 100–200 cells/mm3 greater than the median CD4 count of 200 observed in our study. Initiation of ART at these higher CD4 counts may have delayed AIDS-defining illnesses and facilitated maximal response to ART.
FIGURE 2.
The effect of routine screening at first contact with VA, compared with previously published rate of decline of CD4 counts in natural history of HIV infection (CD4 decline; Reprinted with permission from N Engl J Med. 1993;328:327–33525). Among the nearly 40% of patients in our cohort, the time between their first contact with the VA for other medical care and their HIV presentation was a median of 3.7 years. If patients were screened for HIV as part of routine medical care when they first sought care for non-HIV related illnesses, their HIV would have been detected years earlier and the median baseline CD4 count would have been greater than 350 cells/mm3, instead of the observed median baseline CD4 count of 200 cells/mm3.
Our study has several limitations. As HIV-infected veterans are older and more likely male, our study is limited in its generalizability. Our findings, however, are similar to those in non-VA HIV cohorts.7,8,10,12,20,26 Another limitation is that access to health care for individual patients was not measured. The advantages of receiving comprehensive healthcare benefits at VAMCs were considered as a whole and compared with non-VA populations, rather than by specific patient-level measurement. It also was not possible to determine the exact date of HIV diagnosis for each patient in this large study population. Instead, an algorithm for identifying treatment-naive patients was used and validated in a subset of patients from the Atlanta VA. Finally, our clinical triggers analysis relied on diagnostic codes, which may be insensitive. However, a similar study of newly diagnosed HIV patients also found a low prevalence of triggers (28%).26 Triggers would need to be substantially more prevalent to be considered sensitive markers HIV infection. Inclusion of behavioral risk factors, such as multiple sexual partners or IV drug use, may improve sensitivity of triggers for signaling HIV infection, although their ascertainment in routine medical care before HIV diagnosis is poor.20 The VA centralized data do not capture these data, and thus, we were unable to include them in our analysis.
In summary, patients continue to present for HIV care too late to maximally benefit from ART. This delay exists even in populations with access to comprehensive health benefits and in those already navigating the healthcare system for other illnesses. A delay in identifying HIV, due to insufficient testing, is likely a significant barrier to earlier initiation of ART. Our findings support the notion that widespread screening is needed to affect timely identification and optimal treatment of HIV infection.
ACKNOWLEDGMENTS
The authors recognize and thank Larry Mole, Lisa Backus, and Public Health Strategic Health Care Group which developed and maintains the Immunology Case Registry, without their work this project would not have been possible.
Supported by grants from the Robert Wood Johnson Clinical Scholars Program (to N.R.G.), the National Institute of Alcoholism and Alcohol Abuse grant number UO1 AA 13566-01 (to A.C.J.), and the Veterans Affairs Office of Research and Development (to A.C.J.). Also supported by the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by the National Institutes of Health grant number NIH AI-51519.
The funding sources had no role in the design and conduct of study; collection, management, analysis, and interpretation of data; or preparation of manuscript.
Dr. Gandhi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 3.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. 1904 2006. Available at: aidsinfo.nih.gov.
- 5.Bacchetti P, Moss AR. Incubation period of AIDS in San Francisco. Nature. 1989;338:251–253. doi: 10.1038/338251a0. [DOI] [PubMed] [Google Scholar]
- 6.Morgan D, Mahe C, Mayanja B, et al. HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries? Aids. 2002;16:597–603. doi: 10.1097/00002030-200203080-00011. [DOI] [PubMed] [Google Scholar]
- 7.Dybul M, Bolan R, Condoluci D, et al. Evaluation of initial CD4+ T cell counts in individuals with newly diagnosed human immunodeficiency virus infection, by sex and race, in urban settings. J Infect Dis. 2002;185:1818–1821. doi: 10.1086/340650. [DOI] [PubMed] [Google Scholar]
- 8.Samet JH, Freedberg KA, Stein MD, et al. Trillion virion delay: time from testing positive for HIV to presentation for primary care. Arch Intern Med. 1998;158:734–740. doi: 10.1001/archinte.158.7.734. [DOI] [PubMed] [Google Scholar]
- 9.Katz MH, Bindman AB, Keane D, et al. CD4 lymphocyte count as an indicator of delay in seeking human immunodeficiency virus-related treatment. Arch Intern Med. 1992;152:1501–1504. [PubMed] [Google Scholar]
- 10.Liddicoat RV, Horton NJ, Urban R, et al. Assessing missed opportunities for HIV testing in medical settings. J Gen Intern Med. 2004;19:349–356. doi: 10.1111/j.1525-1497.2004.21251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egger M. Outcomes of ART in resource-limited and industrialized countries. Presented at: 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. February 27, 2007. [Google Scholar]
- 12.Castilla J, Sobrino P, De La FL, et al. Late diagnosis of HIV infection in the era of highly active antiretroviral therapy: consequences for AIDS incidence. Aids. 2002;16:1945–1951. doi: 10.1097/00002030-200209270-00012. [DOI] [PubMed] [Google Scholar]
- 13.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44:25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 14.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44:13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Justice AC, Erdos J, Brandt C, et al. The Veterans Affairs Healthcare System: a unique laboratory for observational and interventional research. Med Care. 2006;44:7–12. doi: 10.1097/01.mlr.0000228027.80012.c5. [DOI] [PubMed] [Google Scholar]
- 16.Backus L, Mole L, Chang S, et al. The Immunology Case Registry. J Clin Epidemiol. 2001;54(Suppl 1):12–15. doi: 10.1016/s0895-4356(01)00442-5. [DOI] [PubMed] [Google Scholar]
- 17.Public Health Strategic Health Care Group Reports from the Immunology Case Registry. VA HIV Report. 2004;1(1):2–3. [Google Scholar]
- 18.Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care. 2006;44:52–60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 19.Samet JH, Freedberg KA, Savetsky JB, et al. Understanding delay to medical care for HIV infection: the long-term non-presenter. Aids. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 20.Klein D, Hurley LB, Merrill D, et al. Review of medical encounters in the 5 years before a diagnosis of HIV-1 infection: implications for early detection. J Acquir Immune Defic Syndr. 2003;32:143–152. doi: 10.1097/00126334-200302010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 22.CDC Recommendations for HIV testing services for inpatients and outpatients in acute-care hospital settings. Center for Disease Control and Prevention. MMWR Recomm Rep. 1993;42:1–6. [PubMed] [Google Scholar]
- 23.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 24.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo G, Graziosi C, Fauci AS. New concepts in the immunopatho-genesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins TC, Gardner EM, Thrun MW, et al. Risk-based human immunodeficiency virus (HIV) testing fails to detect the majority of HIV-infected persons in medical care Settings. Sex Transm Dis. 2006;33:329–333. doi: 10.1097/01.olq.0000194617.91454.3f. [DOI] [PubMed] [Google Scholar]