Abstract
Context
Estrogen deprivation therapy with aromatase inhibitors (AI) has been hypothesized to paradoxically sensitize hormone-receptor-positive breast cancer tumor cells to low-dose estradiol therapy.
Objective
To determine if estradiol 6-mg daily is a viable endocrine therapy for postmenopausal women with advanced AI-resistant hormone-receptor-positive breast cancer.
Design, Setting and Patients
A randomized Phase 2 trial of 6-mg versus 30-mg oral estradiol daily opened in April 2004 and was closed to enrollment in February 2008 (NCT00324259). Eligible patients had metastatic breast cancer treated with an AI with at least 24 weeks progression-free survival, or relapse after two or more years of adjuvant AI. Patients at high risk of estradiol-related adverse events were excluded.
Main Outcome Measures
The primary endpoint was clinical benefit rate – CBR (response plus stable disease at 24 weeks). Secondary outcomes included toxicity, progression-free survival (PFS), time to treatment failure (TTF), quality of life (QOL) and the predictive properties of the FDG-PET metabolic flare reaction.
Results
66 patients were enrolled. The grade 3+ adverse event rate on the 30-mg arm (11/32; 95% CI: 23%–47%) was higher than that in 6-mg arm (4/34; 95% CI: 5%–22%) (P=.03). CBRs were 28% (9/32; 95% CI: 18% – 41%) on the 30-mg arm and 29% (10/34; 95% CI: 19% – 42%) on the 6-mg arm. An estradiol44 stimulated increase in FDG uptake of ≥12% (prospectively defined) was predictive of response (positive predictive value of 80%; 95% CI: 61%–92%). Seven patients with estradiol-sensitive disease were retreated with AI upon estradiol progression, with two PR and one SD, suggesting resensitization to estrogen deprivation.
Conclusions
In women with advanced breast cancer and acquired resistance to AI, an estradiol dose of 6-mg daily provided a similar CBR as 30-mg daily, with fewer serious adverse events. The efficacy of treatment with the lower dose should be further examined in phase 3 clinical trials
Introduction
The efficacy of a synthetic estrogen, diethylstilbestrol (DES) 1 in the treatment of breast cancer was first described by Haddow in the 1940’s, who discussed DES in terms of the wider paradox that certain organic compounds both induce cancer and can be used in cancer treatment 2. Importantly, efficacy was restricted to postmenopausal women, suggesting that the decline in estrogen levels associated with the menopause may sensitize breast cancer cells to DES 3. Interestingly, some patients could even be treated with intermittent therapy, with repeated regressions upon reintroduction of DES 4. In the early 1980’s tamoxifen was shown to be less toxic, although not more effective 5 and DES was eventually withdrawn from human use in the USA. As an alternative to DES, estradiol became an uncommonly used therapy after the failure of more contemporary endocrine agents, with 30 mg (10 mg p.o. three times daily) in the prescribing label for proprietary formulations. More recently high-dose DES (15 mg daily) was reported in a European study to be an effective although relatively poorly tolerated treatment after the development of resistance to aromatase inhibition (AI) 6. Song et al reported that prolonged estrogen deprivation primed MCF7 cells for estradiol induced apoptosis at concentrations more typical of hormone replacement therapy (HRT)7. Furthermore, the unexpected decrease in breast cancer incidence seen in women receiving equine estrogens alone in the Women’s Health Initiative (WHI) Trial has stimulated interest in the possibilities of low dose estrogen therapy for breast cancer 8. We therefore conducted a randomized phase 2 trial in postmenopausal women with hormone receptor-positive, AI-resistant advanced disease to compare 30-mg estradiol daily (10 mg t.i.d.) with 6-mg daily (2-mg t.i.d.) to specifically address whether exposure to third-generation AI treatment sensitizes advanced ER+ breast cancer to lower, better tolerated and safer doses of estradiol.
Methods
Patients
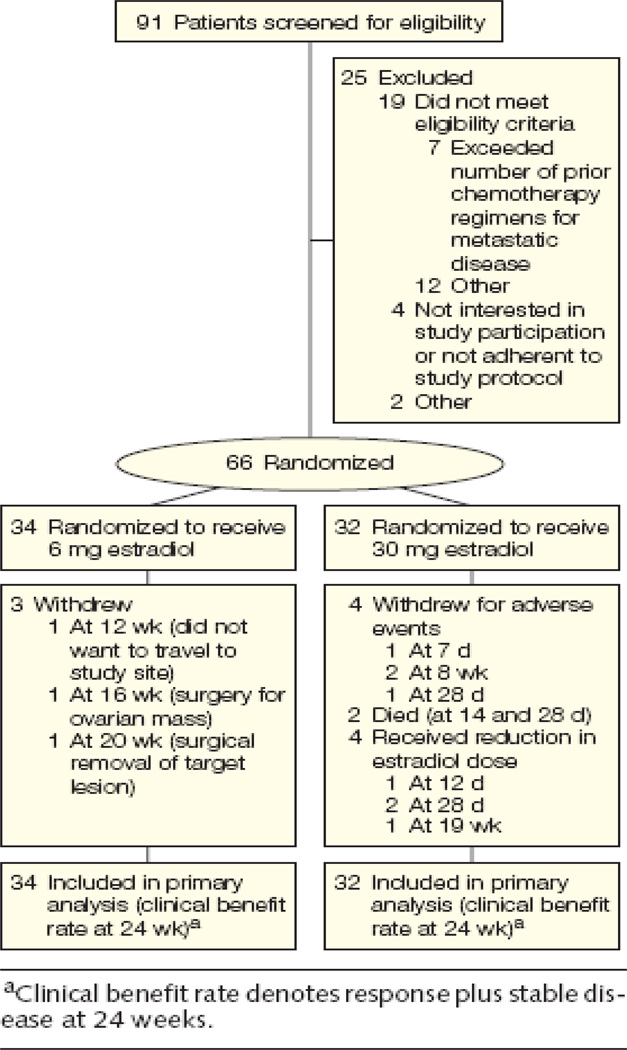
The study was approved by the ethics committees at each of the participating institutions and registered with the National Cancer Institute (NCT00324259). The CONSORT diagram is provided as Figure 1. Between April 2004 and February 2008, 66 postmenopausal women with advanced estrogen-receptor (ER) and/or progesterone-receptor-(PgR) positive breast cancer (defined as at least 10% of malignant cells with positive nuclear staining) and ECOG performance status 0–2 were enrolled into the protocol after providing written consent. Eligible patients had received prior treatment with an AI in the advanced disease setting, with at least 24 weeks of progression-free-survival (PFS) before disease progression. A patient remained eligible even if further lines of endocrine therapy had been unsuccessfully employed. Eligibility also included relapses at least two years after initiation of adjuvant AI therapy. In this instance, estradiol therapy was offered as first-line endocrine treatment. Menopausal status was defined as age ≥50 years and amenorrhea for 1 year or bilateral oophorectomy, or serum FSH and estradiol levels in the postmenopausal range before the initiation of AI therapy. One line of chemotherapy for advanced disease was permissible. Adequate hematological, renal, hepatic function was required and treatment with an i.v. bisphosphonate was mandatory for all patients with bone metastasis. Patients were excluded on the basis of CNS involvement, a history of deep venous thrombosis, pulmonary embolism, stroke, acute myocardial infarction, congestive cardiac failure, untreated hypertension, ischemic changes on a baseline electrocardiogram, undiagnosed abnormal vaginal bleeding, untreated cholelithiasis, previous malignancy not treated with curative intent or with an estimated recurrence risk of greater than 30% and untreated metabolic disturbances (glucose ≥ 200 mg/dL and triglycerides >400 mg/dL or an elevated calcium (local laboratory limit). Treatment with fulvestrant within 12 months of study initiation was also an exclusion criterion as this agent had been shown to antagonize estrogen-induced apoptosis in vitro 7.
Figure 1.
Patient Enrollment, Randomization, and Treatment Flow
Procedures and definitions
A randomization table was created using the SAS program PROC PLAN. To better ensure the balance of potential risk factors in two arms, treatment assignment was implemented in small blocks of 4 to 6 patients. Patients were randomized to receive either one oral 2-mg generic estradiol tablet (commercial stock) three times daily (total daily dose 6-mg) or five 2 mg tablets (10-mg) three times daily (total daily dose 30-mg). Patients were reviewed after one and two weeks for clinical and laboratory toxicities and flare reactions and thereafter every four weeks. Tumor radiological assessment occurred every 12 weeks. At least one measurable lesion defined by RECIST criteria was followed or, in the case of bone-only disease, at least four measurable lesions on CT scan bone windows were assessed by WHO response criteria and an elevation in a baseline tumor marker greater than two fold the upper limit was required to assist in the evaluation of tumor response 9. No evidence of disease progression at the 24 week evaluation defined stable disease. The clinical benefit rate (CBR) defined the fraction of patients with either RECIST response or stable disease.
PET/CT imaging and analysis
Patients underwent baseline clinical imaging by FDG-PET/CT up to 4 weeks before study initiation; FDG-PET/CT was repeated on the same scanner 24 hours after initiation of the assigned dose of estradiol. The third dose was taken typically 1–3 hours before the expected time of injection of FDG. The fasting glucose was required to be < 200 mg/dL immediately prior to injection of 10-15 mCi (370–555 MBq) of FDG. After one hour, a spiral CT scan (typically 95-111 effective mAs, 130 kvP and 5-mm-slice thickness) was performed followed by pelvis to skull emission images. The PET emission images were corrected for measured attenuation and reconstructed using an ordered-subset estimation-maximization (OSEM) iterative algorithm. All PET images were evaluated semi-quantitatively by determining the standardized uptake value (SUV). The percent changes in maximum SUV for FDG were determined. Baseline FDG-PET/CT studies were reviewed to select metastatic lesion(s) for analysis. In patients with multiple lesions, the average SUV of up to six lesions was determined. An increase in tumor SUV of ≥ 12% was prospectively defined as the threshold for a positive estradiol stimulation test 10.
Quality-of-life analysis
Participants were surveyed at baseline and at 28 days using the multidimensional Functional Assessment of Cancer Therapy-Breast (FACT-B) questionnaire 11 and a 6-item estrogen adverse side-effect questionnaire (headaches, bloating, breast tenderness, retaining fluid, nausea and vomiting). Both the FACT-B and estrogen side-effects questionnaires used a 5-point scale ranging from 0 “not at all” to 4 “very much”). Cronbach’s alpha was reported to be .90 on the FACT-B, indicating high internal consistency of items on this measure 11. Some of the items on the FACT-B (measuring physical well being, social well being, functional well being, emotional well being and additional breast cancer concerns) were summed to create a total FACT-B score, with higher scores indicating better quality of life.
Estradiol and insulin-like growth factor 1 (IGF1) levels
Estradiol levels were quantified using an ultra-sensitive radioimmunoassay kit (Diagnostic Systems Laboratories) that measures estradiol concentrations with a 5 pg/mL lower detection limit. Serum total IGF1 was measured using kits for the Siemens Immulite 1000 which provides chemiluminescent immunometric detection of IGF1 levels with a 25 ng/mL lower detection limit.
Statistical analysis
Therapeutic efficacy and safety were assessed based on an intention-to-treat principle. The primary outcome for this study was the CBR. The secondary outcomes included the incidence of grade 3 and higher toxicities or serious adverse events, progression-free survival (PFS), time to treatment failure (TTF), serum IGF1 and estradiol, tumor FDG uptake within 24 hours of treatment initiation (“metabolic flare”), and quality of life. The study was designed using Simon’s Minimax 2-stage design to detect, with 80% power at a one-sided 0.05 significance level, a minimum rate of interest on each arm—a 20% CBR— with a maximum expected rate of 40%. If both doses achieved this level of activity, then the best-tolerated dose would be recommended for further study (defined as the arm that has the lowest frequency of all ≥ grade 3 toxicities or serious adverse events, regardless of type). Demographic and clinical characteristics of the two arms were compared using the student t-test or Fisher’s exact test as appropriate. The CBR for each arm and the 95% exact binomial confidence intervals were calculated. The difference of grade 3 and higher toxicities or serious adverse events between two arms was compared by Cochran-Armitage two-sided trend test. PFS was defined as the time from treatment initiation to disease progression or death. Time of last observation for patients remaining on study and the time at which dose reductions, study drug termination and withdrawal of consent occurred were treated as censored data. TTF treated all events that led to termination of the assigned treatment as events, and time of last observation for patients remaining on study and time of withdrawal of consent as censored data. PFS and TTF were estimated using Kaplan-Meier product-limit method and the differences between two arms were compared by the log-rank test. To assess the ability of FDG-PET metabolic flare to predict response, positive predictive value (PPV, the proportion with clinical benefit among subjects with metabolic flare) and negative predictive value (NPV, the proportion of patients no clinical benefit without metabolic flare) were also calculated. Analysis of covariance (ANCOVA) was used to test the effect of response to therapy on total FACT-B scores at 28-day follow-up controlling for baseline FACT-B. For analysis of the estrogen side-effects as a grouping variable, the level of estrogen side effects at 28 days was reduced to a dichotomous variable (high or low) using the median value. Using a factorial ANCOVA controlling for baseline total FACT-B scores and response to therapy, we tested the main and interaction effects of treatment arm (6-mg vs. 30-mg) and severity of estrogen side effects at follow-up on 28-day follow-up FACT-B scores. Repeated-measures ANCOVA (RM-ANCOVA) was used to measure the significance of change in estrogen side effects after 28 days, grouping by treatment arm, and change in total FACT-B scores after 28 days, controlling for response to therapy and grouping by treatment arm and the dichotomous estrogen side-effects variable. A P value under.05 was taken to indicate significance, and all statistical tests were two-sided. All the analyses were performed using statistical package SAS version 9 (SAS Institute Inc., Cary, NC) or SPSS version 16.0 (SPSS, Inc., Chicago, IL).
Results
Study population and toxicity
Ninety-one (91) patients were screened for the study (details of the screening failures are listed in the supplementary Table A). The trial accrued 66 patients (self-reported 82% White, 15.0% Black, 3.0% other as required by the NIH funding mechanism; mean age 58.9, range 36.4–83.9 years), with 32 on the 30-mg arm and 34 on the 6-mg arm. There were no statistically significant differences in baseline patient and tumor characteristics on the two arms of the study (Table 1). The study population was dominated by patients with a late relapse pattern since the average time from diagnosis to relapse was over 7 years. The grade 3 or higher adverse events are summarized in Table 2A. Side effects were, in general, characteristic of estradiol therapy. Most notably there was less high-grade nausea and vomiting, electrolyte disturbances and problems with pleural effusion on the 6-mg arm. Consistent with these toxicity differences, the mean [standard deviation] trough levels of estradiol at one month were 302 [519] pg/mL on the 6-mg arm and 2403 [2268] pg/mL on the 30-mg arm (P <.001) (Table 3). Only one grade 3 tumor flare occurred (pain in a retro-orbital metastasis with diplopia on the 30-mg arm), and was managed by interruption of therapy, followed by retreatment at the 6-mg dose when flare symptoms had subsided. Grade 1 or 2 vaginal bleeding was seen in 17 patients and was associated with younger age (with mean age of 54 [9] vs. 61 [11] years; P =.04) and was well controlled with progestin therapy, either orally or as an intrauterine device. There was no evidence that the use of a progestin interacted with response. The rate of thrombosis was low with one event on each arm of the study. Overall there were significantly fewer grade 3+ adverse events on the 6-mg arm (4/34; 95% CI: 5%–22%) versus the 30-mg arm (11/32; 95% CI: 23%–47%; P =.03) (Table 2B).
Table 1.
The characteristics of the patients and tumor characteristics on the two arms of the study. There were no statistically significant differences in any of the parameters listed.
| Characteristics | 30mg (N=32) | 6mg (N=34) |
|---|---|---|
| Age (median, range) | 59.5 (39.4–77.7) | 54.7 (36.3–83.8) |
| Months from diagnosis | 82.4 (12.9–327.2) | 90.2 (16.6–381.5) |
| Race | ||
| Black | 6 (19%) | 4 (12%) |
| White | 24 (75%) | 29 (85%) |
| Others | 2 (6%) | 1 (3%) |
| Site | ||
| Bone/soft tissue | 13 (40%) | 18 (53%) |
| Visceral | 5 (16%) | 5 (15%) |
| Both | 14 (44%) | 11 (32%) |
| ER status | ||
| Negative | 0 (0%) | 1 (3%) |
| Positive | 32 (100%) | 33 (97%) |
| PgR status | ||
| Negative | 6 (19%) | 8 (24%) |
| Positive | 26 (81%) | 26 (76%) |
| Prior Systemic Therapy | ||
| AI (Adjuvant) | 4 (13%) | 3 (9%) |
| AI (Metastatic) | 29(91%) | 32 (94%) |
| Tamoxifen (Adjuvant) | 14 (44%) | 18 (53%) |
| Tamoxifen (Metastatic) | 3 (9%) | 6 (18%) |
| Chemo (Adjuvant) | 16 (50%) | 20 (59%) |
| Chemo (Metastatic) | 5 (16%) | 4 (12%) |
Table 2.
Table 2A, a summary of grade 3 and higher (G3+) toxicities on the two arms of the study. Table 2B, the incidence of serious adverse events (SAEs) on the two arms of the study.
| Table 2A | ||||
|---|---|---|---|---|
| Class | 6mg G3 |
6 mg G4/5 |
30mg G3 |
30mg G4/5 |
| Nausea/Vomiting | 0 | 0 | 5 (16%) | 0 |
| Low Na+ | 1 (3%) | 0 | 5 (16%) | 0 |
| Pleural Effusion | 0 | 0 | 4 (13%) | 0 |
| Pain | 6 (18%) | 0 | 4 (13%) | 0 |
| Thombosis/Embolism | 0 | 1 (3%) | 0 | 1 (3%) |
| CNS ischemia | 1 (3%) | 0 | 0 | 0 |
| Infection | 2 (6%) | 0 | 1 (3%) | 2 (6%) |
| Hypercalcemia | 0 | 0 | 2 (6%) | 0 |
| Others | 6 (18%) | 0 | 10 (31%) | 0 |
| Table 2B | |||||
|---|---|---|---|---|---|
| Arm | 0 SAE | G3 SAE | G4 SAE | G5 SAE | Total |
| 30 mg | 21 (66%) | 8 (25%) | 1 (3%) | 2 (6%) | 32 |
| 6 mg | 30 (88%) | 3 (9%) | 1 (3%) | 0 (0%) | 34 |
Table 3. Biomarker comparisons between the two arms of the study.
Mean difference between 28-day and baseline in IGF1 and Estradiol levels and estradiol-induced changes in FDG-PET SUV uptake in responding patients.
| Outcomes | Treatment groups |
n | Mean [SD] | P |
|---|---|---|---|---|
| IGF1 (ng/ml) | 6-mg | 29 | −61 [32] | .96 |
| 30-mg | 23 | −61 [41] | ||
| Estradiol (pg/ml) | 6-mg | 29 | 301 [519] | <.001 |
| 30-mg | 23 | 2403 [2268] | ||
| FDG-PET SUV (%) | 6-mg | 8 | 20.9 [21.7] | .92 |
| 30mg | 7 | 22.1 [11.7] |
Response
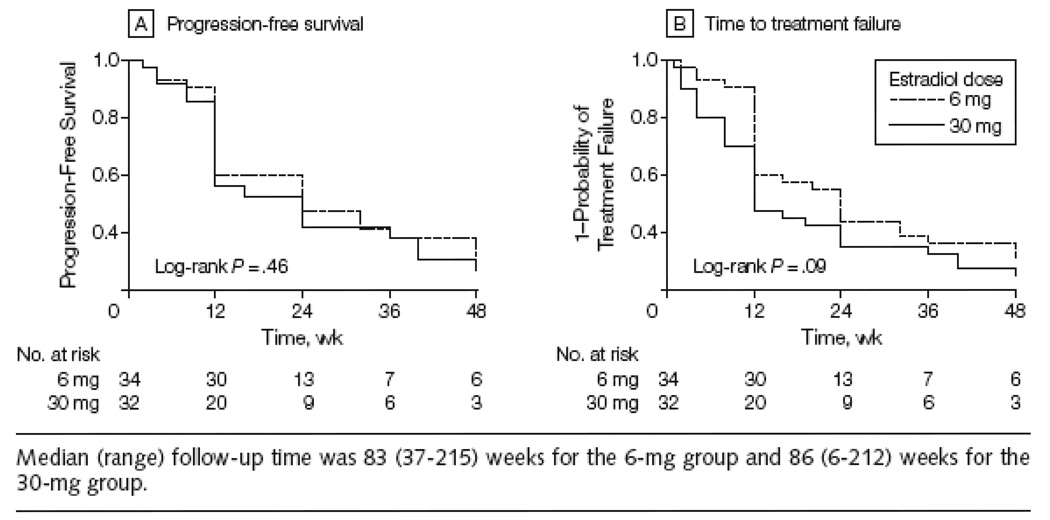
The slight imbalance in numbers assigned to the two arms (32 on the 30-mg arm and 34 on the 6-mg arm) was a consequence of yearly data and safety monitoring, which lead to early closure of the 30-mg arm for toxicity concerns after 32 patients had been enrolled, after which the study was completed by enrolling the remaining two patients onto the 6-mg arm. The primary endpoint, CBR, was 28% (9/32; 95% CI: 18%–41%) on the 30-mg arm and 29% (10/34; 95% CI: 19%–42%) on the 6-mg arm (Table 4A). There were relatively few RECIST responses (one on the 30-mg arm and three on the 6-mg arm). Two of the SD patients on the 30-mg arm were identified after a dose reduction to 6-mg because of a grade 3 or 4 adverse event. Only 7 patients entered the study who had relapsed while receiving adjuvant AI therapy (with one PR and one SD both on the 6-mg arm). There was no difference between the two arms in PFS (P =.46; Figure 2A) or TTF (P =.09; Figure 2B). After noting a significant number of patients responding to estradiol, the study was extended to address the hypothesis that the acquired AI resistance exhibited by the trial population might, in some instances, be reversed by an extended period of estradiol therapy. The protocol was therefore amended in 2005 to allow data collection on response to retreatment with the last AI received i.e. avoiding a change in the type of AI so that true reversal of resistance could be assessed. This approach was only offered to patients experiencing clinical benefit on estradiol. To date, seven patients have been retreated with an AI (Supplementary Table B). Three patients have experienced clinical benefit (two PRs and one SD, lasting 36, 36 and 28+ weeks respectively).
Table 4.
Table 4A, the responses on the two arms of the study. Table 4B, a contingency table for the interaction between the presence of a positive FDG-PET estradiol stimulation test and response (PR+SD) to estradiol treatment (combined data from both arms, P=.0001).
| Table 4A | ||
|---|---|---|
|
Response |
30 mg (N=32) | 6 mg (N=34) |
| Complete Remission | 0 (0%) | 0 (0%) |
| Partial Response | 1 (3%) | 3 (9%) |
| Stable Disease | 8 (25%) | 7 (20%) |
| Progression Disease | 16 (50%) | 21 (62%) |
| Not Assessable | 7 (22%) | 3 (9%) |
| Table 4B | |||
|---|---|---|---|
| Response Status |
Metabolic Flare on FDG-PET/CT |
||
| Yes | No | Total | |
| Partial Response | 3 | 0 | 3 |
| Stable Disease | 9 | 4 | 13 |
| Progression Disease | 3 | 27 | 30 |
| Total | 15 | 31 | 46 |
Figure 2.
Progression-Free Survival and Probability of Treatment Failure by Study Group
Pharmacodynamic analysis
To compare the systemic endocrine effects of the two doses of estradiol, serum IGF1 levels were assessed. IGF1 decreased from baseline in the 6-mg arm by an average of 61 [32] ng/mL and on the 30-mg arm by 61 [41] ng/mL (Table 3). These decreases from baseline were highly significant (P <.001), but did not differ between the two arms (P =.96). The FDG PET/CT data allowed a direct comparison the two doses of estradiol at the level of the metastatic tumor. No differences in the change in FDG uptake were detected on the two treatment arms in responding patients (with mean changes of 20.9 [21.7]% and 22.1 [11.7]% in 6-mg and 30-mg arms, respectively; P=.92), indicating 6-mg daily stimulated glucose uptake to a similar degree as the higher dose (Table 3).
The predictive value of FDG-PET metabolic flare
The relationship between metabolic flare assessed by FDG-PET/CT and response, combining the two arms could be conducted in 46 patients (Table 4B). Ten patients were not evaluable for response because early toxicity prevented response assessment (Figure 1); the PET data were considered technically inadequate or were not available in another 8 patients. The presence of a metabolic flare was a highly significant predictor of response (P<.001). With a ≥12% increase in FDG uptake prospectively defined as a metabolic flare, the positive predictive value (PPV) for response was 12/15=80% (95% CI: 61–92%) and the negative predictive value (NPV) for non-response was 27/31=87% (95% CI: 76–94%).
Quality-of-life analysis
The scores from the six estrogen side-effect items were combined to produce a single score (Cronbach’s alpha=.61 at baseline and.72 at 28-day follow-up). A significant increase in severity of side effects from baseline to follow-up was observed overall (0.47 to 0.80; P <.001), but the change was not significantly different by treatment arm (0.47–0.70 in 6-mg arm vs. 0.46–0.92 in 30-mg arm; P =.10). However, the study underestimated the negative impact of treatment on QOL on the 30-mg arm because patients with the most severe side effects were dose-reduced or withdrew before the 28-day QOL follow-up (Figure 1). In the factorial ANCOVA, FACT-B scores at follow-up differed significantly by the dichotomous estrogen side-effects measure (low side effects: 114.8 vs. high side effects: 99.8; P =.003) but not by treatment arm (6-mg: 109.5 vs. 30-mg: 106.9; P =.52) after controlling for baseline FACT-B and response to therapy. The difference in QOL by estradiol side-effects intensity met the criterion of a minimally important difference of 7–8 points 12. A significant interaction between estrogen side effects and treatment arm on FACT-B at follow-up (P=.03) indicated that the poorest QOL was reported by patients in the 30-mg arm who had more severe side effects.
Discussion
The CBR rates of 28% (30mg) and 29% (6 mg) reported in this study were just below our prespecified expectations for clinical activity because the lower boundaries of the 95% confidence intervals crossed 20% (Table 4A). However at the time the study was powered there was only limited information on the activity of further endocrine therapy in patients who had progressed on an aromatase inhibitor. Recent data from a large Phase 3 double-blind randomized clinical trial that compared fulvestrant and exemestane in patients with disease progression after a non-steroidal aromatase inhibitor produced outcomes very similar to our experience with estradiol (CBR of 32.2% and 31.5% respectively) 13 On this basis it is reasonable to conclude that the activity of estradiol is sufficient to warrant further investigation.
In further studies of estradiol treatment, the 6-mg dose should be favored because it was significantly safer, with a lower serious adverse event rate. We also observed that intense estradiol side effects have an adverse effect on QOL which are mitigated by lowering the estradiol dose. Thus, in women with advanced breast cancer and acquired resistance to AI, an estradiol dose of 6-mg daily provided a similar CBR as 30-mg daily, with fewer serious adverse events and side effects that impact on QOL. We express caution regarding safety, and emphasize that patients must continue to be excluded from further investigations on the basis of risk for serious adverse estrogen side effects. These exclusion criteria probably accounted for the low rate of thrombosis in the study. The low rate of hypercalcemia (there were no cases on the 6-mg arm), historically a major problem with estrogen treatment 5, almost certainly reflects the uniform use of an i.v. bisphosphonate in patients with bone metastasis. The enhanced tolerability of the 6-mg dose in terms of nausea and vomiting is reflected in the serum estradiol measurements, which achieved the goal of an average concentration typical for the first trimester of pregnancy with the 30-mg dose, and the preovulatory phase of the menstrual cycle with the 6-mg dose.
Biomarker analysis contributed evidence that 6-mg of estradiol is a “biologically” effective dose in the post-aromatase-inhibitor setting. Serum IGF1 suppression was equivalent on the two arms of the study and, more directly, so was estradiol stimulation of tumor FDG uptake in responding patients (Table 3). Thus the 6-mg dose should be also favored when conducting the FDG-PET estradiol stimulation test.
Given that only a minority of patients will respond, the validation of the FDG-PET estradiol stimulation test as a predictive biomarker for estradiol therapy is a major finding of this study (Table 4B). The 12% increase in FDG uptake as the threshold for a “positive estradiol stimulation test” was prespecified on the basis of an earlier study10 . We have therefore validated the 12% threshold and broadened the spectrum of agents for which the test predicts activity, i.e. a positive PET-based estradiol stimulation test predicts sensitivity to fulvestrant, aromatase inhibitors and estradiol (with the exception of tamoxifen since, in our original study 14, tamoxifen itself used as the agonist to induce metabolic flare). The estradiol stimulation test therefore differentiates between hormone-receptor-positive patients in whom serial endocrine therapy with a number of different agents is likely to be an effective approach and patients in whom a change to non-endocrine treatment approaches is likely to be necessary earlier in the treatment course. In the group with a positive estradiol challenge test, the order with which endocrine therapies are applied is an important consideration. For example, patients with a positive test would be a reasonable population to further investigate retreatment with an AI after estradiol progression, as our limited experience suggests that estradiol therapy may, in some cases, resensitize metastatic ER+ breast cancer to estrogen deprivation therapy.
In conclusion, 6-mg estradiol daily, which produces estradiol levels similar to those in ovulating premenopausal women, is an active low cost treatment for postmenopausal women with advanced breast cancer and acquired resistance to aromatase inhibitor treatment and should be further investigated. The activity of other endocrine agents after successful treatment with estradiol, including AI retreatment, should be explored further. Finally, investigation of the mechanism of estradiol efficacy is critical for progress since the use of this treatment in earlier disease settings will require a robust tissue-based predictive biomarker that identifies the subset of tumors susceptible to this paradoxical treatment.
Acknowledgments
Specific Contributions of Each Author.
Matthew J. Ellis: conception and design, data acquisition, analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content; statistical analysis, obtaining funding, supervision; Feng Gao: analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, statistical analysis; Farrokh Dehdashti: conception and design, data acquisition, analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content; administrative technical or material support, supervision. Donna B. Jeffe: conception and design, analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content; statistical analysis; P. Kelly Marcom: conception and design, acquisition of the data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content; obtaining funding, administrative technical or material support: Lisa A. Carey; conception and design, acquisition of the data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content; obtaining funding, administrative technical or material support; Maura N. Dickler: acquisition of the data, administrative technical or material support; Paula Silverman: acquisition of the data, administrative technical or material support; Gini F. Fleming: acquisition of the data, critical revision of the manuscript for important intellectual content; obtaining funding, administrative technical or material support; Aruna Kommareddy: conception and design, data acquisition, drafting of the manuscript, obtaining funding; Shohreh Jamalabadi-Majidi: acquisition of the data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content; administrative technical or material support; Robert Crowder: acquisition of the data, drafting of the manuscript; administrative technical or material support; Barry A Siegel; concept and Design, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content.
Drs Ellis and Gao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosures: Dr Siegel reports stock ownership, being a medical advisory board member, and receiving lecture honoraria from Radiology Corporation of America, and receiving lecture honoraria from Cardinal Health, Inc., DMS Imaging, Inc., GE Healthcare, Inc., PETNET Solutions, Philips Medical Systems Puerto Rico, Inc, Siemens Canada Ltd., and Siemens Medical Solutions, Inc.
The sponsors (the National Cancer Institute and the AVON Foundation) had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
We would like to thanks the patients and their families and the following individuals who assisted in the conduct of study and who received salary support from the grant: clinical research associates Helen Kaemmerer, Fennifer Frye, Matthew Paulson, Sonali Rege, Viju John, Bernadette Libao, M. Leda Dumadag, Vlayka Liotcheva, and radiologists Lina Mehta, MD, Heiko Schoder, MD, Terence Wong, MD, Amir Khandani, MD, Yonglin Pu, MD, PhD, and Daniel Appelbaum, MD.
Supported as Individual Cancer Center Supplements from an Avon Foundation and National Cancer Institute Partners for Progress Award to the following cancer centers (Siteman Cancer Center [coordinating center] P30 CA91842, Duke Comprehensive Cancer Center P30 CA014236, Lineberger Cancer Center, University of North Carolina P30 CA016086, Memorial Sloan-Kettering Cancer Center P30 CA008748, Case Comprehensive Cancer Center P30 CA043703 and the University of Chicago Cancer Research Center P30 CA14599).
We acknowledge use of the Siteman Cancer Center Health Behavior and Outreach Core, the Imaging Response Assessment Team, the Tissue Banking Core and the Biostatistics Core for data management and statistical services which are all supported by the Center Support Grant (P30 CA91842) to the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri. Serum estradiol and IGF1 concentrations were measured by the Washington University Core Laboratory for Clinical Studies.
Footnotes
The other authors report no conflicts of interest relevant to this study of generic estradiol therapy.
Aspects of this paper were presented at the San Antonio Breast Cancer Symposium in December 2008.
References
- 1.Dodds EC, Goldberg L, Lawson W, Robinson R. Estrogenic activity of certain synthetic compounds. Nature. 1938;141(3562):247. [Google Scholar]
- 2.Haddow A, Watkinson JM, Patterson E. Influence of synthetic oestrogens upon advanced malignant disease. BMJ. 1944;2:393–398. doi: 10.1136/bmj.2.4368.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter AC, Sedransk N, Kelley RM, et al. Diethylstilbestrol: recommended dosages for different categories of breast cancer patients. Report of the Cooperative Breast Cancer Group. JAMA. 1977 May 9;237(19):2079–2078. [PubMed] [Google Scholar]
- 4.Stoll BA. Hypothesis: breast cancer regression under oestrogen therapy. Br Med J. 1973 Aug 25;3(5877):446–450. doi: 10.1136/bmj.3.5877.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingle JN, Ahmann DL, Green SJ, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981 Jan 1;304(1):16–21. doi: 10.1056/NEJM198101013040104. [DOI] [PubMed] [Google Scholar]
- 6.Lonning PE, Taylor PD, Anker G, et al. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001 May;67(2):111–116. doi: 10.1023/a:1010619225209. [DOI] [PubMed] [Google Scholar]
- 7.Song RX, Mor G, Naftolin F, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001 Nov 21;93(22):1714–1723. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 8.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 9.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004 Jul 15;22(14):2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 10.Dehdashti F, Mortimer JE, Trinkaus K, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 2009 Feb;113(3):509–517. doi: 10.1007/s10549-008-9953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997 Mar;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 12.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004 Sep;57(9):898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008 Apr 1;26(10):1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 14.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001 Jun 1;19(11):2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]