Abstract
Objective
To estimate the prognosis over 5 years of HIV-1-infected, treatment-naive patients starting HAART, taking into account the immunological and virological response to therapy.
Design
A collaborative analysis of data from 12 cohorts in Europe and north America on 20 379 adults who started HAART between 1995 and 2003.
Methods
Parametric survival models were used to predict the cumulative incidence at 5 years of a new AIDS-defining event or death, and death alone, first from the start of HAART and second from 6 months after the start of HAART. Data were analysed by intention-to-continue-treatment, ignoring treatment changes and interruptions.
Results
During 61 798 person-years of follow-up, 1005 patients died and an additional 1303 developed AIDS. A total of 10 046 (49%) patients started HAART either with a CD4 cell count of less than 200 cells/μl or with a diagnosis of AIDS. The 5-year risk of AIDS or death (death alone) from the start of HAART ranged from 5.6 to 77% (1.8–65%), depending on age, CD4 cell count, HIV-1-RNA level, clinical stage, and history of injection drug use. From 6 months the corresponding figures were 4.1–99% for AIDS or death and 1.3–96% for death alone.
Conclusion
On the basis of data collected routinely in HIV care, prognostic models with high discriminatory power over 5 years were developed for patients starting HAART in industrialized countries. A risk calculator that produces estimates for progression rates at years 1 to 5 after starting HAART is available from www.art-cohort-collaboration.org.
Keywords: AIDS, antiretroviral therapy, CD4 lymphocyte count, highly active, HIV infections, mortality, prognosis, risk factors, survival analysis, viral load
Introduction
Estimates of the probability of progression to AIDS or death are important for HIV-infected patients starting HAART and their treating physicians. Such estimates are also needed for a better understanding of the treated history of HIV infection, to monitor and predict the course of the epidemic, to develop treatment guidelines, and to plan health services.
The Antiretroviral Therapy (ART) Cohort Collaboration, an international collaboration between the investigators of cohort studies from Europe and north America, was established in 2000 to monitor disease progression among treatment-naive patients starting HAART [1,2]. Pre-HAART era probabilities of progression by viral load and CD4 cell count were estimated by Mellors and colleagues [3] using the Multicenter AIDS Cohort Study (MACS) of homosexual men. In that study, the probability of progression to AIDS within 3 years for those with a high viral load and CD4 cell count between 201 and 350 cells/μl was estimated to be 64%, rising to 89% at 6 years. Since the introduction of HAART, the incidence of AIDS in treated patients has declined markedly. Previous analyses from the ART Cohort Collaboration showed that for those aged under 50 years and starting HAART in the same viral load and CD4 cell count stratum, the 3-year probability of AIDS or death had fallen to 6% [1].
As treatment with HAART, once started, is likely to be lifelong, it is important that models for prognosis are updated as longer follow-up time becomes available. In previous analyses from the ART Cohort Collaboration, the probability of progression to AIDS or death, and to death alone, could be estimated up to 3 years after starting HAART, based on up to 80 different risk groups [1,2]. The collaborative database was updated in 2004 to include patients who had started HAART more recently, and to add the follow-up time and events observed since 2002. In this paper we describe updated prognostic models that estimate probabilities of clinical progression to AIDS or death and death alone up to 5 years after starting HAART, first according to prognostic factors measured at baseline, and second incorporating the initial response to treatment.
Patients and methods
The ART Cohort Collaboration has been described in detail elsewhere [1,2,4,5]. Briefly, prospective cohort studies were eligible if they had enrolled at least 100 patients with HIV-1 infection aged 16 years or older, who had not previously received antiretroviral treatment and who had started antiretroviral therapy with a combination of at least three drugs, including nucleoside reverse transcriptase inhibitors, protease inhibitors (PI), or non-nucleoside reverse transcriptase inhibitors (NNRTI), with a median duration of follow-up of at least one year. Patients with baseline HIV-1-RNA levels of less than 1000 copies/ml were excluded because they might not have been treatment naive. All cohorts provided anonymized data on a predefined set of demographic, laboratory and clinical variables.
Twelve cohorts contributed data: the French Hospital Database on HIV (FHDH) ANRS CO4 [6] and the Aquitaine Cohort ANRS CO3 (France) [7]; the AIDS Therapy Evaluation Project Netherlands (ATHENA) [8]; the Italian Cohort of Antiretroviral-Naive Patients (ICONA) [9]; the Swiss HIV Cohort Study (SHCS) [10]; the Frankfurt HIV Cohort [11] and Köln/Bonn Cohort (Germany) [12]; the EuroSIDA study (20 countries in Europe and Argentina) [13]; the Collaborations in HIV Outcomes Research US (CHORUS; USA) [14]; the Royal Free Hospital Cohort (UK) [15]; and the British Columbia Centre for Excellence in HIV/AIDS [16] and the South Alberta Clinic (Canada) [17]. At all sites, institutional review boards had approved the collection of data.
Statistical methods
We considered the probability of progression to a combined endpoint of an AIDS-defining disease or death and to death alone. In both definitions, we included deaths from all causes. We used the clinical part of the 1993 US Centers for Disease Control and Prevention revision of the AIDS case definition (i.e. individuals without an AIDS-defining disease but with a CD4 cell count below 200 cells/μl were not classified as having AIDS). The protocol stipulated 10 candidate prognostic variables measured at or immediately before the initiation of therapy: CD4 cell count, HIV-1-RNA level, age, sex, transmission group, AIDS diagnosis, year of starting HAART, number of drugs in the regimen, the inclusion of PI in the regimen, and the inclusion of NNRTI. A CD4 cell count and HIV-1-RNA measurement closest to 6 months after starting treatment, within 3–9 months, were also provided when available. In all analyses, we used an `intent-to-continue-treatment' approach and thus ignored subsequent changes to treatment, including treatment interruptions and terminations. We measured the time from the start of HAART to the date the endpoints first occurred. In analyses accounting for initial response to treatment, time was measured from the start of HAART, with delayed entry (i.e. patients entered the risk set) at the latest of either: 6 months after the start of HAART; the date of the 6-month CD4 cell measurement; or the date of the 6-month HIV-1-RNA measurement. For the combined endpoint, follow-up was censored according to information supplied by the cohorts (date followed up to, date of close of database) or on the date of the most recent follow-up visit. For mortality, follow-up was censored on the date the patient was last known to be alive.
We used parametric survival models based on the Weibull distribution with stratification to allow for the non-proportionality of hazards for prognosis from initiation of therapy, and models based on the loglogistic distribution (proportional odds model) for progression from 6 months. Full details of the methodology for the construction and validation of the prognostic models are published elsewhere [4,5]. Briefly, the process used to choose the prognostic models took into account the cohort structure of the dataset and used cross-validation to ensure that the model would generalize well to new patients. The chosen models were re-estimated on the pooled data, ignoring the cohort structure, and were used to estimate the probabilities of progression (with 95% confidence intervals) to the endpoint at yearly intervals up to 5 years after starting HAART.
Results
The combined database included 22 217 patients. We excluded 1838 patients (8%) with baseline HIV-1-RNA levels of less than 1000 copies/ml because they might not have been treatment naive. A further 4412 patients (19%) were excluded from the analyses of progression from 6 months, for the following reasons: 238 died before 6 months, 1277 had a follow-up shorter than 6 months, and 3135 had no CD4 cell count or viral load measurements between 3 and 9 months after the start of HAART. These patients were more likely to have been infected via injection drug use (IDU) and to have an AIDS diagnosis before starting HAART than those who were eligible for the study, but were similar in terms of CD4 cell count, viral load, and age at baseline. A total of 20 379 patients were therefore included in analyses from the start of HAART and 16 167 for analyses from 6 months. Table 1 shows patient characteristics at baseline. The median age of the patients was 36 years [interquartile range (IQR) 31–42], the median CD4 cell count was 224 cells/μl (IQR 91–368) and the median HIV-1-RNA level was 4.96 log copies/ml (IQR 4.45–5.43). A total of 17 976 patients (88%) started on a three-drug regimen and 13 822 (68%) were on a PI-based and 5361 (26%) on an NNRTI-based HAART regimen. At 6 months, the median CD4 cell count was 345 cells/μl (IQR 198–523) and the median HIV-1-RNA level was 2.30 log copies/ml (IQR 1.70–2.70).
Table 1.
Characteristics of 20 379 treatment-naive study patients at the start of HAART, person-years of follow-up, number of patients with clinical progression and hazard ratios for progression from baseline to AIDS or death and death alone, from Weibull models: Antiretroviral Therapy Cohort Collaboration, 2004.
| AIDS or death |
Death |
||||||
|---|---|---|---|---|---|---|---|
| Person-years | No. of events | Hazard ratio (95% CI) | Person-years | No. of deaths | Hazard ratio (95% CI) | ||
| Age (years) | |||||||
| 16–29 | 3814 (19%) | 9859 | 298 | 1 | 11 290 | 96 | 1 |
| 30–39 | 9553 (47%) | 24 594 | 1066 | 1.16 (1.02–1.32) | 29 515 | 428 | 1.26 (1.01–1.58) |
| 40–49 | 4596 (23%) | 11 138 | 578 | 1.32 (1.14–1.52) | 13 775 | 258 | 1.66 (1.31–2.11) |
| > 50 | 2416 (12%) | 5715 | 366 | 1.62 (1.39–1.90) | 7218 | 223 | 3.09 (2.41–3.95) |
| Sex | |||||||
| Male | 15 407 (76%) | 39 435 | 1811 | 1 | 47 994 | 812 | 1 |
| Female | 4972 (24%) | 11 871 | 497 | 1.00 (0.89–1.12) | 13 804 | 193 | 0.91 (0.77–1.08) |
| Risk factor for transmission | |||||||
| Male homosexual contact | 8143 (40%) | 22 558 | 872 | 1 | 27123 | 326 | 1 |
| Injection drug use | 3231 (16%) | 8006 | 484 | 1.56 (1.39–1.75) | 9623 | 293 | 3.05 (2.59–3.60) |
| Heterosexual contact | 7227 (35%) | 16 877 | 740 | 1.06 (0.95–1.18) | 19 999 | 278 | 1.12 (0.94–1.33) |
| Other | 1778 (9%) | 3866 | 212 | 1.13 (0.97–1.32) | 5054 | 108 | 1.41 (1.13–1.77) |
| Clinical CDC stage | |||||||
| A/B | 15 642 (77%) | 43 761 | 1354 | 1 | 48232 | 505 | 1 |
| C | 4737 (23%) | 7546 | 954 | 2.04 (1.85–2.24) | 13 565 | 500 | 2.33 (2.01–2.69) |
| CD4 cell count (cells ×106/l) | |||||||
| < 25 | 2034 (10%) | 3588 | 519 | 1 | 5776 | 222 | 1 |
| 25–49 | 1295 (6%) | 2484 | 277 | 0.85 (0.73–0.98) | 3766 | 111 | 0.82 (0.66–1.04) |
| 50–99 | 2059 (10%) | 4448 | 408 | 0.76 (0.66–0.87) | 6140 | 162 | 0.77 (0.63–0.95) |
| 100–199 | 3782 (18%) | 8921 | 445 | 0.49 (0.43–0.56) | 10 784 | 202 | 0.67 (0.55–0.82) |
| 200–349 | 5550 (27%) | 14 694 | 361 | 0.29 (0.25–0.33) | 16 380 | 178 | 0.48 (0.39–0.60) |
| ≥350 | 5659 (28%) | 17 171 | 298 | 0.23 (0.19–0.27) | 18 952 | 130 | 0.34 (0.27–0.44) |
| Plasma HIV-1-RNA level (log copies/ml) | |||||||
| ≥5 | 9734 (48%) | 23 004 | 1449 | 1 | 29 362 | 607 | 1 |
| 4–4.99 | 8391 (41%) | 22 431 | 701 | 0.80 (0.73–0.88) | 25 607 | 305 | 0.89 (0.77–1.02) |
| 3–3.99 | 2254 (11%) | 5871 | 158 | 0.80 (0.68–0.95) | 6830 | 93 | 1.11 (0.89–1.39) |
CDC, Centers for Disease Control and Prevention; CI, confidence interval. Analyses adjusted for all variables listed and stratified by cohort.
During 61 798 person-years of follow-up 1005 patients died and an additional 1303 developed AIDS. The duration of follow-up was less than 2 years for 6982 (34%), 2–5 years for 9899 (49%) and more than 5 years for 3498 (17%) of patients. Of the 656 patients who died with no new AIDS diagnosis, 307 (47%) had been diagnosed with AIDS before starting HAART; of the 1844 patients who developed AIDS, 1449 patients (79%) developed one AIDS event, 317 patients (17%) developed two events, whereas 66 (4%) developed three or more events.
Hazard ratios for progression to AIDS or death and death from Weibull models are shown in Table 1 from the start of HAARTand in Table 2 from 6 months. Five prognostic variables were included in the final model for progression to AIDS or death from the start of HAART: CD4 cell count (< 25, 25–49, 50–99, 100–199, 200–349, ≥350 cells/μl), HIV-1-RNA level (< 5 versus ≥5 log copies/ml), age (16–29, 30–39, 40–49 and ≥50 years), assumed transmission group (IDU versus non-IDU), and clinical AIDS. Kaplan–Meier plots of the probability of progression from the start of HAART to AIDS or death, and to death alone, for the five prognostic variables are available from the authors on request and are on the website (www.art-cohort-collaboration.org).
Table 2.
Characteristics at the start of HAART and 6-month measurements of 16 167 treatment-naive study patients, person-years of follow-up, number of patients with clinical progression, and hazard ratios for progression from 6 months to AIDS or death and death alone, from weibull models: Antiretroviral Therapy Cohort Collaboration, 2004.
| AIDS or death |
Death |
||||||
|---|---|---|---|---|---|---|---|
| Person-years | No of events | Hazard ratio (95% CI) | Person-years | No of deaths | Hazard ratio (95% CI) | ||
| Age (years) | |||||||
| 16–29 | 3004 (19%) | 6957 | 147 | 1 | 9549 | 57 | 1 |
| 30–39 | 7549 (47%) | 17 896 | 459 | 0.99 (0.82, 1.20) | 25 100 | 271 | 1.29 (0.97, 1.73) |
| 40–49 | 3657 (23%) | 8248 | 230 | 1.12 (0.91, 1.38) | 11 928 | 166 | 1.80 (1.33, 2.45) |
| >50 | 1957 (12%) | 4245 | 176 | 1.92 (1.53, 2.41) | 6328 | 131 | 3.29 (2.39, 4.54) |
| Sex | |||||||
| Male | 12 341 (76%) | 29 063 | 798 | 1 | 41 303 | 511 | 1 |
| Female | 3826 (24%) | 8282 | 214 | 0.91 (0.77, 1.08) | 11 601 | 114 | 0.83 (0.67, 1.04) |
| Risk factor for transmission | |||||||
| Male homosexual contact | 6674 (41%) | 17 029 | 370 | 1 | 23 558 | 204 | 1 |
| Injection drug use | 2438 (15%) | 5507 | 243 | 2.01 (1.68, 2.41) | 7901 | 196 | 2.56 (2.04, 3.20) |
| Heterosexual contact | 5693 (35%) | 12 049 | 314 | 1.07 (0.89, 1.27) | 17 154 | 166 | 1.05 (0.83, 1.32) |
| Other | 1362 (8%) | 2759 | 85 | 1.04 (0.82, 1.33) | 4292 | 59 | 1.20 (0.89, 1.62) |
| Clinical stage | |||||||
| A/B at 6 months | 12 122 (75%) | 31 221 | 561 | 1 | 40 105 | 302 | 1 |
| C at baseline | 3239 (20%) | 5040 | 330 | 3.09 (2.62, 3.64) | 10 376 | 223 | 1.79 (1.47, 2.17) |
| A/B at baseline and C at 6 months | 806 (5%) | 1083 | 121 | 5.06 (4.02, 6.37) | 2424 | 100 | 3.04 (2.37, 3.90) |
| 6-Month CD4 cell count (cells × 106/l) | |||||||
| <25 | 222 (1%) | 251 | 88 | 1 | 573 | 74 | 1 |
| 25–49 | 273 (2%) | 401 | 63 | 0.75 (0.54, 1.05) | 775 | 48 | 0.64 (0.44, 0.93) |
| 50–99 | 953 (5%) | 1735 | 153 | 0.54 (0.41, 0.71) | 3016 | 96 | 0.38 (0.28, 0.53) |
| 100–199 | 2606 (16%) | 5331 | 192 | 0.26 (0.20, 0.35) | 8395 | 127 | 0.21 (0.16, 0.29) |
| 200–349 | 4054 (25%) | 8889 | 228 | 0.25 (0.19, 0.32) | 12 821 | 124 | 0.17 (0.13, 0.24) |
| ≥350 | 8059 (50%) | 20 738 | 288 | 0.18 (0.13, 0.23) | 27 325 | 156 | 0.14 (0.10, 0.19) |
| 6-Month plasma HIV-1-RNA level (log copies/ml) | |||||||
| ≥5 | 609 (4%) | 1014 | 170 | 1 | 1852 | 111 | 1 |
| 4–4.99 | 1152 (7%) | 2406 | 129 | 0.53 (0.42, 0.67) | 3736 | 80 | 0.61 (0.45, 0.82) |
| 2.7–3.99 | 2119 (13%) | 5465 | 177 | 0.43 (0.34, 0.54) | 7703 | 107 | 0.49 (0.37, 0.66) |
| <2.7 | 12 287 (76%) | 28 460 | 536 | 0.26 (0.21, 0.31) | 39 614 | 327 | 0.31 (0.24, 0.40) |
CI, Confidence interval. Analyses adjusted for all variables listed and stratified by cohort.
In the model for progression from 6 months, 6-month HIV-1 RNA was categorized in four groups (< 2.7, ≥2.7–3.99, 4–4.99, ≥5 log copies/ml) and a further category of disease stage was defined as no AIDS at baseline and AIDS diagnosis in the first 6 months. Age was less prognostic for the combined endpoint of AIDS or death and was more prognostic in the model for death alone. Age was therefore dichotomized in the model for AIDS and death (< 50, ≥50), but was considered in four groups (16–29, 30–39, 40–49 and ≥50 years) in the model for death.
Table 3 gives 5-year probabilities of progression from start of HAART to the combined endpoint of AIDS or death estimated from a Weibull model. The corresponding table for the cumulative probability of death is available from the authors on request and on the website (www.art-cohort-collaboration.org). The lowest estimated probability of progression to AIDS or death at 5 years was 5.6% [95% confidence intervals (CI) 4.8–6.5] in patients less than 30 years of age, not infected through IDU, and who started HAART with a CD4 cell count greater than 350 cells/μl and an HIV-1-RNA level below 5 log copies/ml. At the other end of the spectrum, the risk was estimated at 77% (95% CI 70–82) at 5 years in patients aged 50 years and over infected through IDU who started therapy with a CD4 cell count less than 25 cells/μl and an HIV-1-RNA level equal to or greater than 5 log copies/ml and a previous AIDS diagnosis (Table 3). The corresponding estimates for death were respectively 1.8% (95% CI 1.4–2.3) and 65% (95% CI 56–73). The median predicted probability of progression to AIDS or death by 5 years was 12.3% (95% CI 11.0–13.9) and for death was 5.2% (95% CI 4.1–6.6).
Table 3.
Cumulative incidence of AIDS or death at 5 years after starting HAART according to CD4 cell count, viral load and sociodemographic factors: Antiretroviral Therapy Cohort Collaboration, 2004.
| CD4 cell count (cells/μl) |
||||||
|---|---|---|---|---|---|---|
| < 25 | 25–49 | 50–99 | 100–199 | 200–349 | ≥ 350 | |
| Age 16–29 years | ||||||
| CDC stage A/B and no history of IDU | ||||||
| Viral load ≥5a | 23 (20–27) | 21 (18–24) | 19 (16–22) | 13 (11–15) | 8.3 (7.1–9.7) | 6.9 (5.9–8.1) |
| Viral load <5 | 19 (17–22) | 17 (14–20) | 16 (13–18) | 11 (9.3–13) | 6.7 (5.8–7.8) | 5.6 (4.8–6.5) |
| CDC stage C and no history of IDU | ||||||
| Viral load ≥5 | 42 (37–47) | 37 (33–43) | 35 (31–40) | 25 (22–29) | 16 (14–19) | 14 (11–16) |
| Viral load <5 | 35 (31–40) | 31 (27–36) | 29 (25–34) | 21 (18–24) | 13 (11–16) | 11 (9.3–13) |
| CDC stage A/B and history of IDU | ||||||
| Viral load ≥5 | 36 (31–41) | 31 (27–37) | 29 (25–34) | 21 (18–24) | 13 (11–16) | 11 (9.3–13) |
| Viral load <5 | 30 (25–35) | 26(22–31) | 24 (21–29) | 17 (15–20) | 11 (9.2–13) | 9.1 (7.6–11) |
| CDC stage C and history of IDU | ||||||
| Viral load ≥5 | 59 (53–65) | 54 (47–61) | 51 (45–57) | 38 (33–44) | 25 (21–30) | 21 (18–26) |
| Viral load <5 | 51 (45–58) | 46 (40–53) | 43 (37–50) | 32 (27–37) | 21 (17–25) | 18 (15–21) |
| Age 30–39 years | ||||||
| CDC stage A/B and no history of IDU | ||||||
| Viral load ≥5 | 26 (24–29) | 23 (20–26) | 22 (19–24) | 15 (13–17) | 9.5 (8.4–11) | 7.9 (6.9–9.1) |
| Viral load <5 | 22 (19–25) | 19 (17–22) | 18 (16–20) | 12 (11–14) | 7.7 (6.8–8.7) | 6.4 (5.7–7.3) |
| CDC stage C and no history of IDU | ||||||
| Viral load ≥5 | 46 (43–50) | 42 (37–46) | 39 (35–43) | 28 (25–32) | 18 (16–21) | 15 (13–18) |
| Viral load <5 | 40 (36–44) | 35 (31–40) | 33 (29–37) | 23 (21–27) | 15 (13–17) | 13 (11–15) |
| CDC stage A/B and history of IDU | ||||||
| Viral load ≥5 | 40 (35–44) | 35 (31–40) | 33 (29–37) | 24 (21–27) | 15 (13–17) | 13 (11–15) |
| Viral load <5 | 33 (29–38) | 30 (25–34) | 28 (24–31) | 19 (17–22) | 12 (11–14) | 10 (9.0–12) |
| CDC stage C and history of IDU | ||||||
| Viral load ≥5 | 64 (59–69) | 59 (53–65) | 56 (51–61) | 42 (37–47) | 28 (25–33) | 24 (21–28) |
| Viral load <5 | 56 (51–62) | 51 (45–57) | 48 (43–54) | 36 (31–40) | 24 (20–27) | 20 (17–23) |
| Age 40–49 years | ||||||
| CDC stage A/B and no history of IDU | ||||||
| Viral load ≥5 | 29 (26–33) | 26 (23–30) | 24 (21–27) | 17 (15–19) | 11 (9.4–12) | 9.0 (7.7–10) |
| Viral load <5 | 25 (21–28) | 22 (19–25) | 20 (17–23) | 14 (12–16) | 8.7 (7.7–10) | 7.3 (6.3–8.4) |
| CDC stage C and no history of IDU | ||||||
| Viral load ≥5 | 51 (47–55) | 46 (41–51) | 43 (39–48) | 31 (28–35) | 21 (18–24) | 17 (15–20) |
| Viral load <5 | 44 (39–48) | 39 (34–44) | 36 (32–41) | 26 (23–30) | 17 (15–20) | 14 (12–17) |
| CDC stage A/B and history of IDU | ||||||
| Viral load ≥5 | 44 (39–49) | 39 (34–45) | 37 (32–41) | 26 (23–30) | 17 (15–20) | 14 (12–17) |
| Viral load <5 | 37 (32–42) | 33 (28–38) | 31 (27–35) | 22 (19–25) | 14 (12–16) | 12 (10–14) |
| CDC stage C and history of IDU | ||||||
| Viral load ≥5 | 69 (63–74) | 63 (57–70) | 60 (55–66) | 46 (41–52) | 32 (27–37) | 27 (23–32) |
| Viral load <5 | 61 (55–67) | 56 (49–62) | 53 (47–59) | 39 (34–45) | 26 (22–31) | 22 (19–26) |
| Age 50+ years | ||||||
| CDC stage A/B and no history of IDU | ||||||
| Viral load ≥5 | 35 (31–40) | 31 (27–36) | 29 (26–33) | 21 (18–23) | 13 (11–15) | 11 (9.4–13) |
| Viral load <5 | 29 (26–34) | 26 (22–30) | 24 (21–28) | 17 (15–19) | 11 (9.3–12) | 8.9 (7.7–10) |
| CDC stage C and no history of IDU | ||||||
| Viral load ≥5 | 59 (54–63) | 53 (48–59) | 50 (45–55) | 37 (33–42) | 25 (21–29) | 21 (18–25) |
| Viral load <5 | 51 (46–56) | 46 (40–52) | 43 (38–48) | 31 (27–36) | 21 (18–24) | 17 (15–20) |
| CDC stage A/B and history of IDU | ||||||
| Viral load ≥5 | 51 (45–57) | 46 (40–53) | 43 (38–49) | 32 (27–36) | 21 (17–24) | 17 (15–21) |
| Viral load <5 | 44 (38–50) | 39 (33–46) | 37 (31–42) | 26 (23–31) | 17 (14–20) | 14 (12–17) |
| CDC stage C and history of IDU | ||||||
| Viral load ≥5 | 77 (70–82) | 71 (64–78) | 68 (62–75) | 54 (47–60) | 38 (32–44) | 32 (27–38) |
| Viral load <5 | 69 (62–75) | 63 (56–71) | 60 (53–67) | 46 (40–53) | 32 (27–37) | 27 (22–32) |
CDC, Centers for Disease Control and Prevention; IDU, injection drug use. Endpointof AIDS includes subsequent AIDS event for those diagnosed with AIDS at the start of HAART.
Log copies/ml.
Table 4 gives probabilities of progression from 6 months after the start of HAART to the combined endpoint of AIDS or death estimated from a loglogistic model. The corresponding table for the cumulative probability of death is available from the authors on request and on the website (www.art-cohort-collaboration.org). The lowest estimated probability of progression to AIDS or death at 5 years was 4.1% (95% CI 3.6–4.8) in patients less than 50 years of age, who were not infected through IDU, had a 6-month CD4 cell count greater than 350 cells/μl and a 6-month HIV-1-RNA level of less than 2.7 copies/ml. At the other end of the spectrum, the risk was estimated at 99% (95% CI 98–99) in patients aged 50 years who were infected through IDU, had a 6-month CD4 cell count less than 25 cells/μl, a 6-month HIV-1-RNA level equal to or greater than 5 log copies/ml and had a first AIDS diagnosis within 6 months of the start of HAART (Table 4). The corresponding estimates for death were, respectively, 1.3% (95% CI 0.9–1.7) and 96% (95% CI 93–97).
Table 4.
Cumulative incidence of AIDS or death at 5 years after starting HAART, according to CD4 cell count and viral load at 6 months and sociodemographic factors: Antiretroviral Therapy Cohort Collaboration, 2004.
| CD4 cell count at 6 months (cells/(μl) |
||||||
|---|---|---|---|---|---|---|
| <25 | 25–49 | 50–99 | 100–199 | 200–349 | ≥350 | |
| Age 16–49 years | ||||||
| CDC stage A/B and no history of IDU | ||||||
| Viral load ≥5a | 80 (70–87) | 65 (54–75) | 50 (42–58) | 30 (24–36) | 28 (22–34) | 22 (18–28) |
| Viral load 4–4.99 | 59 (46–71) | 40 (30–52) | 27 (21–33) | 13 (10–17) | 12 (9.8–16) | 9.5 (7.5–12) |
| Viral load 2.7–3.99 | 52 (39–65) | 34 (24–44) | 21 (17–27) | 10 (8.2–13) | 9.6 (7.8–12) | 7.3 (6.0–8.9) |
| Viral load <2.7 | 37 (26–50) | 22 (15–30) | 13 (10–16) | 5.9 (4.9–7.3) | 5.5 (4.6–6.5) | 4.1 (3.6–4.8) |
| CDC stage C and no history of IDU | ||||||
| Viral load ≥5 | 92 (87–95) | 84 (77–90) | 74 (67–80) | 55 (47–62) | 53 (45–61) | 45 (37–54) |
| Viral load 4–4.99 | 81 (71–88) | 66 (55–75) | 51 (43–59) | 31 (25–37) | 29 (23–35) | 23 (18–29) |
| Viral load 2.7–3.99 | 76 (65–84) | 59 (48–70) | 44 (37–51) | 25 (20–30) | 23 (19–28) | 18 (15–23) |
| Viral load <2.7 | 63 (51–74) | 44 (34–55) | 30 (25–35) | 15 (13–18) | 14 (12–17) | 11 (9.1–13) |
| CDC stage A/B at baseline and C at 6 months and no history of IDU | ||||||
| Viral load ≥5 | 95 (91–97) | 90 (84–94) | 83 (76–88) | 67 (58–75) | 65 (56–74) | 58 (48–68) |
| Viral load 4–4.99 | 87 (80–92) | 76 (66–84) | 63 (54–72) | 42 (34–52) | 41 (32–50) | 33 (26–42) |
| Viral load 2.7–3.99 | 84 (75–90) | 71 (60–80) | 57 (47–65) | 36 (28–44) | 34 (27–42) | 27 (21–35) |
| Viral load <2.7 | 74 (62–83) | 57 (46–68) | 42 (34–50) | 23 (18–29) | 22 (17–28) | 17 (13–22) |
| CDC stage A/B and history of IDU | ||||||
| Viral load ≥5 | 89 (82–93) | 79 (70–86) | 66 (58–74) | 46 (38–54) | 44 (36–52) | 36 (29–44) |
| Viral load 4–4.99 | 74 (63–83) | 57 (46–68) | 42 (34–50) | 23 (18–29) | 22 (17–27) | 17 (14–22) |
| Viral load 2.7–3.99 | 68 (56–79) | 50 (39–62) | 35 (28–43) | 19 (15–23) | 18 (14–22) | 14 (11–17) |
| Viral load <2.7 | 54 (41–67) | 36 (26–46) | 23 (18–28) | 11 (9.0–14) | 10 (8.5–13) | 7.9 (6.6–9.4) |
| CDC stage C and history of IDU | ||||||
| Viral load ≥5 | 96 (93–98) | 91 (87–95) | 85 (80–89) | 71 (63–77) | 69 (61–76) | 62 (53–70) |
| Viral load 4–4.99 | 89 (83–93) | 79 (70–86) | 67 (59–74) | 47 (39–54) | 45 (37–53) | 37 (30–45) |
| Viral load 2.7–3.99 | 86 (78–92) | 74 (64–82) | 61 (53–68) | 40 (33–47) | 38 (31–45) | 31 (25–38) |
| Viral load <2.7 | 77 (66–85) | 61 (50–71) | 46 (39–53) | 26 (22–32) | 25 (21–30) | 20 (16–24) |
| CDC stage A/B at baseline and C at 6 months and history of IDU | ||||||
| Viral load ≥5 | 97 (95–99) | 95 (91–97) | 90 (86–94) | 80 (73–86) | 79 (71–85) | 73 (64–81) |
| Viral load 4–4.99 | 93 (89–96) | 87 (79–92) | 78 (70–84) | 59 (50–68) | 58 (48–67) | 50 (40–60) |
| Viral load 2.7–3.99 | 91 (85–95) | 83 (74–89) | 72 (64–79) | 52 (43–61) | 50 (41–60) | 43 (34–52) |
| Viral load <2.7 | 85 (76–91) | 73 (62–81) | 59 (50–67) | 38 (30–46) | 36 (28–44) | 29 (23–37) |
| Age 50+ years | ||||||
| CDC stage A/B and no history of IDU | ||||||
| Viral load ≥5 | 90 (83–94) | 80 (71–87) | 68 (60–76) | 48 (39–56) | 46 (38–54) | 38 (31–47) |
| Viral load 4–4.99 | 76 (64–85) | 59 (47–70) | 44 (35–53) | 25 (19–31) | 23 (18–29) | 18 (14–23) |
| Viral load 2.7–3.99 | 70 (57–81) | 52 (40–64) | 37 (29–45) | 20 (16–25) | 19 (15–23) | 15 (11–18) |
| Viral load <2.7 | 56 (43–69) | 38 (28–48) | 24 (19–30) | 12 (9.6–15) | 11 (9.1–14) | 8.5 (7.0–10) |
| CDC stage C and no history of IDU | ||||||
| Viral load ≥5 | 96 (93–98) | 92 (87–95) | 86 (81–90) | 72 (65–79) | 71 (63–78) | 64 (55–72) |
| Viral load 4–4.99 | 90 (84–94) | 81 (72–87) | 69 (61–76) | 49 (41–57) | 47 (39–55) | 39 (32–48) |
| Viral load 2.7–3.99 | 87 (79–92) | 76 (66–84) | 63 (54–70) | 42 (35–49) | 40 (33–47) | 33 (26–40) |
| Viral load <2.7 | 79 (68–86) | 63 (52–73) | 48 (41–55) | 28 (23–34) | 27 (22–32) | 21 (17–26) |
| CDC stage A/B at baseline and C at 6 months and no history of IDU | ||||||
| Viral load ≥5 | 98 (96–99) | 95 (92–97) | 91 (87–94) | 81 (74–87) | 80 (72–86) | 75 (66–82) |
| Viral load 4–4.99 | 94 (89–96) | 88 (80–92) | 79 (71–85) | 61 (52–70) | 60 (49–69) | 52 (42–62) |
| Viral load 2.7–3.99 | 92 (86–95) | 84 (76–90) | 74 (65–81) | 55 (45–64) | 53 (43–62) | 45 (36–55) |
| Viral load <2.7 | 86 (78–92) | 74 (64–82) | 61 (52–69) | 40 (32–48) | 38 (30–46) | 31 (24–39) |
| CDC stage A/B and history of IDU | ||||||
| Viral load ≥5 | 94 (90–97) | 89 (83–93) | 81 (74–86) | 64 (55–73) | 63 (53–71) | 55 (46–64) |
| Viral load 4–4.99 | 86 (78–92) | 4 (63–83) | 61 (51–70) | 40 (31–49) | 38 (30–46) | 31 (24–39) |
| Viral load 2.7–3.99 | 82 (72–89) | 69 (57–78) | 54 (44–63) | 33 (26–41) | 31 (25–39) | 25 (20–32) |
| Viral load <2.7 | 72 (59–82) | 54 (43–66) | 39 (31–47) | 21 (17–27) | 20 (16–25) | 16 (12–20) |
| CDC stage C and history of IDU | ||||||
| Viral load ≥5 | 98 (96–99) | 96 (93–98) | 92 (89–95) | 84 (78–89) | 83 (76–88) | 78 (70–85) |
| Viral load 4–4.99 | 95 (91–97) | 89 (83–93) | 82 (75–87) | 65 (57–73) | 64 (54–72) | 56 (47–66) |
| Viral load 2.7–3.99 | 93 (88–96) | 86 (79–91) | 77 (69–83) | 59 (50–67) | 57 (48–65) | 49 (40–58) |
| Viral load <2.7 | 88 (80–93) | 77 (68–85) | 65 (57–72) | 44 (36–52) | 42 (34–50) | 35 (28–42) |
| CDC stage A/B at baseline and C at 6 months and history of IDU | ||||||
| Viral load ≥5 | 99 (98–99) | 97 (96–99) | 95 (93–97) | 90 (84–93) | 89 (83–93) | 86 (78–91) |
| Viral load 4–4.99 | 97 (94–98) | 93 (89–96) | 88 (82–92) | 76 (67–83) | 75 (65–82) | 68 (58–77) |
| Viral load 2.7–3.99 | 96 (92–98) | 91 (86–95) | 85 (78–90) | 70 (61–78) | 69 (59–77) | 62 (51–72) |
| Viral load <2.7 | 92 (87–96) | 85 (77–91) | 75 (67–82) | 57 (47–66) | 55 (45–64) | 47 (37–57) |
CDC, Centers for Disease Control and Prevention; IDU, injection drug use. Endpoint of AIDS includes subsequent AIDS event for those diagnosed with AIDS before or within 6 months after the start of HAART.
Log copies/ml.
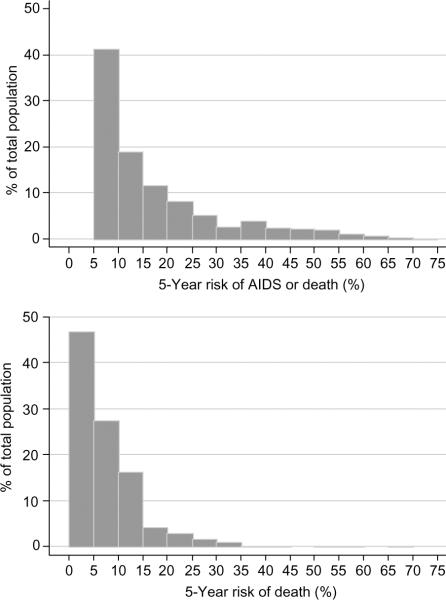
The absolute number of patients predicted to experience clinical progression is determined by the number of patients in each risk group. Additional tables giving these figures are available from the authors on request and on the website (www.art-cohort-collaboration.org). In order to illustrate the distribution of risk, the estimated 5-year risk was grouped in intervals of 5% and the proportion of patients in each interval was determined. Figure 1 shows the distribution of the estimated 5-year risk of AIDS or death and death alone from the start of HAART among the 20 379 treatment-naive study patients. The number (%) of patients with an estimated probability of AIDS or death of less than 10%, 10–29%, 30–49% and over 50% were 7628 (41%), 8101 (44%), 2059 (11%), and 669 (3.6%). Corresponding figures for death were 15 080 (74%), 5049 (25%), 237 (1.2%), and 13 (0.06%).
Fig. 1.
Distribution of estimated 5-year risk of AIDS or death (upper panel) and death alone (lower panel) from the start of HAART among 20 379 treatment-naive patients included in the Antiretroviral Therapy Cohort Collaboration, 2004.
A risk calculator based on the four prognostic models, which produces estimates for cumulative progression rates at years 1–5 after starting HAART, will be made available on the ART Cohort Collaboration's website (www.art-cohort-collaboration.org).
Discussion
Information on prognosis is required to counsel patients who start HAART, to investigate the treated history of HIV-1 infection, and to inform treatment guidelines. Such data are also needed to monitor and predict the progress of the HIV/AIDS epidemic, and to plan health services in the era of potent antiretroviral therapy. The recent update of the database of the Antiretroviral Therapy (ART) Cohort Collaboration made it possible to extend earlier analyses and to estimate the cumulative incidence of AIDS or death and death from all causes up to 5 years after starting HAART.
Consistent with previous analyses [1,2], the most strongly prognostic factor from the start of HAARTwas the CD4 cell count at baseline. Patients with IDU as the likely mode of transmission, or with an AIDS diagnosis at baseline were also at a substantially increased risk of progression. HIV-1 RNA was not prognostic for mortality. In analyses from 6 months, baseline CD4 cell count was no longer prognostic, but both 6-month CD4 cell count and 6-month HIV-1-RNA level were. Those with an AIDS diagnosis in the first 6 months after the start of HAARTwere also at an increased risk of a new AIDS diagnosis and death. The prognostic models have strong discriminatory power: the estimated 5-year probability of AIDS or death ranged from 5.6 to 77% at baseline, and from 4.1 to 99% when taking into account the response to therapy at 6 months. Of note is the fact that in the present analysis the effect of age on disease progression and mortality is more evident than previously [1,2]. Also, the predicted rates of progression from baseline tended to be somewhat higher for patients with HIV-1-RNA levels less than 5 log copies/ml. This could be expected as we omitted individuals with low HIV-1-RNA concentrations at baseline (< 3 log copies/ml) from the present analysis (8% of the total), who we suspected might not be antiretroviral naive. In the Multicenter AIDS Cohort Study natural history cohort [3], approximately 6% of individuals had HIV-1-RNA levels below 2.7 log copies/ml (the limit of detection of the assay for determining HIV-1 RNA), but the majority of these men had CD4 cell counts greater than 750 cells/μl. They would therefore probably not have been included in a treated cohort if treatment had been available at the time. For comparison, in the ART Cohort Collaboration database of treated patients, 6.9% had HIV-1-RNA levels below 2.7 log copies/ml. We decided to omit individuals with HIV-1-RNA levels below 3 log copies/ml because we wished to restrict the analysis to typical naive patients starting their first HAART regimen. We acknowledge that some of these individuals might in fact have been antiretroviral naive when starting HAART.
The large number of patients and events analysed is an important strength of this study. Also, because all patients were treatment naive, our results are not confounded by previous antiretroviral therapy. The collaborative dataset includes patients from many settings in industrialized countries in Europe and north America, with a wide variation in clinical characteristics. We chose models that generalized best to cohorts omitted from the estimation procedure [18]. Our results should therefore be applicable to many patients who started HAART or will start HAART in these settings.
The lack of information on cause-specific mortality is an important limitation of the current ART Cohort Collaboration database. By contrast with the pre-HAART era, when most deaths were associated with recent AIDS-defining events, the situation has become more complex in the era of HAART [19–21]. The current definition of AIDS is no longer a near-complete marker for overall progression. Infectious complications such as sepsis, pneumonia, or meningitis, and cancers whose incidence is increased in patients infected with HIVare not included in the definition of AIDS. The risk of Hodgkin's disease, for example, is increased approximately 10-fold, and Hodgkin's disease is among the most common non-AIDS-defining tumours in HIV-infected patients [22,23]. Unfortunately, these conditions, as well as adverse events associated with antiretroviral therapy, are currently not recorded in a standardized fashion in the different cohort studies. There is clearly a need for complete and standardized information on all events that affect patients infected with HIV-1, and on causes of death, whether or not they are directly related to HIV-1 infection. Several of the cohorts participating in the ART Cohort Collaboration are now implementing standardized, common protocols for the assessment of causes of death.
Approximately half of patients started HAART either with a CD4 cell count of less than 200 cells/μl or with a diagnosis of AIDS, despite the fact that the initiation of treatment is recommended above a CD4 cell count of 200 cells/μl. The start of HAART below this level is therefore probably caused by late patient presentation and diagnosis of HIV infection. In a separate analysis of the collaborative database, we recently showed that the CD4 cell count when starting HAART has declined in recent years [24]. This must be of concern: early diagnosis and timely treatment are of great importance to prevent clinical progression as well as the spread of the infection, in line with the public health principles of wide screening and treatment that have been successfully applied to the control of many infectious diseases [25]. The Centers for Disease Control and Prevention estimate that more than half of new HIV infections are spread by HIV-positive individuals who are unaware of their infection [26]. A survey of new HIV diagnoses in the United Kingdom and Ireland showed that many opportunities for earlier diagnosis are missed [27]. Our results indicate that this may be a common problem in many countries and settings, and support expanded, voluntary and cost–effective screening in healthcare settings [28]. The ART Cohort Collaboration will continue to monitor the characteristics and prognosis of HIV-infected patients starting HAART and update analyses at regular intervals.
Acknowledgements
The authors are grateful to all patients, doctors and study nurses who were involved in the participating cohort studies. They would also like to thank Paula Braitstein and Francois Dabis for helpful comments on a previous version of the manuscript.
Sponsorship: The ART Cohort Collaboration is supported by the UK Medical Research Council grant number G0100221 and GlaxoSmithKline. Sources of funding of individual cohorts include the Agence Nationale de la Recherche sur SIDA, the Institut National de la Santé et de la Recherche Meédicale, the French, Italian and Swiss Ministries of Health, Stichting HIV Monitoring, the European Commission, the British Columbia and Alberta Governments, the Michael Smith Foundation for Health Research, the Canadian Institutes of Health Research and unrestricted grants from GlaxoSmithKline, Roche and Boehringer-Ingelheim.
Writing committee: Margaret May, Jonathan A.C. Sterne, Caroline Sabin, Dominique Costagliola, Amy C. Justice, Rodolphe Thiébaut, John Gill, Andrew Phillips, Peter Reiss, Robert Hogg, Bruno Ledergerber, Antonella D'Arminio Monforte, Norbert Schmeisser, Schlomo Staszewski, Matthias Egger.
Steering committee: Jordi Casabona, Geneviève Chêne, Dominique Costagliola, François Dabis, Antonella D'Arminio Monforte, Frank de Wolf, Matthias Egger, John Gill, Robert Hogg, Amy Justice, Mari Kitahata, Bruno Ledergerber, Catherine Leport, Jens Lundgren, Margaret May, Andrew Phillips, Peter Reiss, Michael Saag, Caroline Sabin, Norbert Schmeisser, Schlomo Staszewski, Jonathan Sterne, Ian Weller.
Data managers: Margaret May, Brenda Beckthold, Benita Yip, Brenda Dauer, Jenifer Fusco, Emilie Lanoy, Martin Rickenbach, Valerie Lavignolle, Ard van Sighem, Edwige Pereira, Patrizio Pezzotti, Andrew Phillips, Caroline Sabin, Norbert Schmeisser.
The members of the 12 study groups were as follows: French Hospital Database on HIV (FHDH ANRS CO4) (61 sites): Scientific committee: Dr E. Billaud, Pr F. Boué, D. Costagliola, Dr X. Duval, Dr C. Duvivier, Dr P. Enel, Dr S. Fournier, Dr J. Gasnault, Dr C. Gaud, Dr J. Gilquin, Dr S. Grabar, Dr M.A. Khuong, Pr J.M. Lang, M. Mary-Krause, Pr S. Matheron, Pr M.C. Meyohas, Pr G. Pialoux, Dr I. Poizot-Martin, Dr C. Pradier, Pr E. Rouveix, Pr D. Salmon-Ceron, Pr A. Sobel, Dr P. Tattevin, Dr H. Tissot-Dupont, Dr Y. Yasdanpanah. DMI2 coordinating centre: French Ministry of Health: Dr E. Aronica, Dr V. Tirard-Fleury, I. Tortay. Statistical analysis centre: INSERM EMI 0214: Dr S. Abgrall, D. Costagliola, Dr S. Grabar, M. Guiguet, E. Lanoy, H. Leneman, L. Lièvre, M. Mary-Krause, V. Potard, Dr S. Saidi. CISIH: Paris area: CISIH de Bichat-Claude Bernard, Hôpital Bichat-Claude Bernard: Pr S. Matheron, Pr J.L. Vildé, Pr C. Leport, Pr P. Yeni, Pr E. Bouvet, C. Gaudebout, Pr B. Crickx, Dr C. Picard-Dahan. CISIH de Paris-Centre Ouest, Hôpital Européen Georges Pompidou: Pr L. Weiss, D. Tisne-Dessus. GH Tarnier-Cochin: Pr D. Sicard, Pr D. Salmon; Hôpital Saint-Joseph: Dr J. Gilquin, Dr I. Auperin; Hôpital Necker Adultes: Dr J.P. Viard, Dr L. Roudière. CISIH de Paris-Sud, Hôpital Antoine Béclère: Pr F. Boué, Dr R. Fior; Hôpital de Bicêtre: Pr J.F. Delfraissy, Dr C. Goujard; Hôpital Henri Mondor: Dr Ph. Lesprit, C.Jung; Hôpital Paul Brousse. CISIH de Paris-Est, Hôpital Saint-Antoine: Pr M.C. Meyohas, Dr J.L. Meynard, Dr O. Picard, N. Desplanque; Hôpital Tenon: Pr J. Cadranel, Pr C. Mayaud, Pr G. Pialoux, Pr W. Rozenbaum. CISIH de Pitié-Salpétrière, GH Pitié-Salpétrière: Pr F. Bricaire, Pr C. Katlama, Pr S. Herson, Dr A. Simon. CISIH de Saint-Louis, Hôpital Saint-Louis: Pr J.M. Decazes, Pr J.M. Molina, Pr J.P. Clauvel, Dr L. Gerard; GH Lariboisière-Fernand Widal: Dr P. Sellier, Dr M. Diemer. CISIH 92, Hôpital Ambroise Paré: Dr C. Dupont, H. Berthé, Pr P. Saïag; Hôpital Louis Mourier: Dr E. Mortier, C. Chandemerle; Hôpital Raymond Poincaré: Dr P. de Truchis. CISIH 93, Hôpital Avicenne: Dr M. Bentata, P. Honoré; Hôpital Jean Verdier: S. Tassi, Dr V. Jeantils; Hôpital Delafontaine: Dr D. Mechali, B. Taverne. Outside Paris area: CISIH Auvergne-Loire, CHU de Clermont-Ferrand: Dr H. Laurichesse, Dr F. Gourdon; CHRU de Saint-Etienne: Pr F. Lucht, Dr A. Fresard; CISIH de Bourgogne-Franche Comté, CHRU de Besançon; CHRU de Dijon; CH de Belfort: Dr J.P. Faller, P. Eglinger; CHRU de Reims. CISIH de Caen, CHRU de Caen: Pr C. Bazin, Dr R. Verdon. CISIH de Grenoble, CHU de Grenoble; CISIH de Lyon, Hôpital de la Croix-Rousse: Pr D. Peyramond, Dr A. Boibieux; Hôpital Edouard Herriot: Pr J.L. Touraine, Dr J.M. Livrozet; Hôtel-Dieu: Pr C. Trepo, Dr L. Cotte. CISIH de Marseille, Hôpital de la Conception: Dr I. Ravaux, Dr H. Tissot-Dupont; Hôpital Houphouët-Boigny: Pr J.P. Delmont, Dr J. Moreau; Institut Paoli Calmettes: Pr J.A. Gastaut; Hôpital Sainte-Marguerite: Dr I. Poizot-Martin, Pr J. Soubeyrand, Dr F. Retornaz; CHG d'Aix-En-Provence: Dr P.A. Blanc, Dr T. Allegre; Centre pénitentiaire des Baumettes: Dr A. Galinier, Dr J.M. Ruiz; CH d'Arles; CH d'Avignon: Dr G. Lepeu; CH de Digne Les Bains: Dr P. Granet-Brunello; CH de Gap: Dr L. Pelissier, Dr J.P. Esterni; CH de Martigues: Dr M. Nezri, Dr R. Cohen-Valensi; CHI de Toulon: Dr A. Laffeuillade, Dr S. Chadapaud. CISIH de Montpellier, CHU de Montpellier: Pr J. Reynes; CHG de Nîmes. CISIH de Nancy, Hôpital de Brabois: Pr T. May, Dr C. Rabaud. CISIH de Nantes, CHRU de Nantes: Pr F. Raffi, Dr E. Billaud. CISIH de Nice, Hôpital Archet 1: Dr C. Pradier, Dr P. Pugliese; CHG Antibes Juan les Pins. CISIH de Rennes, CHU de Rennes: Pr C. Michelet, Dr C. Arvieux. CISIH de Rouen, CHRU de Rouen: Pr F. Caron, Dr F. Borsa-Lebas. CISIH de Strasbourg, CHRU de Strasbourg: Pr J.M. Lang, Dr D. Rey, Dr P. Fraisse; CH de Mulhouse. CISIH de Toulouse, CHU Purpan: Pr P. Massip, Dr L. Cuzin, Pr E. Arlet-Suau, Dr M.F. Thiercelin Legrand; Hôpital la Grave; CHU Rangueil. CISIH de Tourcoing-Lille, CH Gustave Dron; CH de Tourcoing: Dr Y. Yasdanpanah. CISIH de Tours, CHRU de Tours; CHU Trousseau. Overseas: CISIH de Guadeloupe, CHRU de Pointe-à-Pitre. CISIH de Guyane, CHG de Cayenne: Dr M. Sobesky, Dr R. Pradinaud. CISIH de Martinique, CHRU de Fort-de-France. CISIH de La Réunion, CHD Félix Guyon: Dr C. Gaud, Dr M. Contant.
Italian Cohort of Antiretroviral-Naive Patients (ICONA) (47 sites): Ancona: M. Montroni, G. Scalise, M.C. Braschi, A. Riva. Aviano (PN): U. Tirelli, R. Cinelli. Bari: G. Pastore, N. Ladisa, G. Minafra. Bergamo: F. Suter, C. Arici. Bologna: F. Chiodo, V. Colangeli, C. Fiorini, O. Coronado. Brescia: G. Carosi, G.P. Cadeo, C. Torti, C. Minardi, D. Bertelli. Busto Arsizio: G. Rizzardini, S. Melzi. Cagliari: P.E. Manconi, P. Piano. Catanzaro: L. Cosco, A. Scerbo. Chieti: J. Vecchiet, M. D'Alessandro. Como: D. Santoro, L. Pusterla. Cremona: G. Carnevale, P. Citterio. Cuggiono: P. Viganò, M. Mena. Ferrara: F. Ghinelli, L. Sighinolfi. Firenze: F. Leoncini, F. Mazzotta, M. Pozzi, S. Lo Caputo. Foggia: G. Angarano, B. Grisorio, A. Saracino, S. Ferrara. Galatina (LE): P. Grima, P. Tundo. Genova: G. Pagano, G. Cassola, A. Alessandrini, R. Piscopo. Grosseto: M. Toti, S. Chigiotti. Latina: F. Soscia, L. Tacconi. Lecco: A. Orani, P. Perini. Lucca: A. Scasso, A. Vincenti. Macerata: F. Chiodera, P. Castelli. Mantova: A. Scalzini, L. Palvarini. Milano: M. Moroni, A. Lazzarin, A. Cargnel, G.M. Vigevani, L. Caggese, A. d'Arminio Monforte, D. Repetto, A. Galli, S. Merli, C. Pastecchia, M.C. Moioli. Modena: R. Esposito, C. Mussini. Napoli: N. Abrescia, A. Chirianni, C.M. Izzo, M. Piazza, M. De Marco, R. Viglietti, E. Manzillo, S. Nappa. Palermo: A. Colomba, V. Abbadessa, T. Prestileo, S. Mancuso. Parma: C. Ferrari, P. Pizzaferri. Pavia: G. Filice, L. Minoli, R. Bruno, S. Novati. Perugia: F. Baldelli, M. Tinca. Pesaro: E. Petrelli, A. Cioppi. Piacenza: F. Alberici, A. Ruggieri. Pisa: F. Menichetti, C. Martinelli. Potenza: C. De Stefano, A. La Gala. Ravenna: G. Ballardini, E. Rizzo. Reggio Emilia: G. Magnani, M.A. Ursitti. Rimini: M. Arlotti, P. Ortolani. Roma: R. Cauda, F. Dianzani, G. Ippolito, A. Antinori, G. Antonucci, S. D'Elia, P. Narciso, N. Petrosillo, V. Vullo, A. De Luca, A. Bacarelli, M. Zaccarelli, R. Acinapura, P. De Longis, A. Brandi, M.P. Trotta, P. Noto, M. Lichtner, M.R. Capobianchi, F. Carletti, E. Girardi, P. Pezzotti, G. Rezza. Sassari: M.S. Mura, M. Mannazzu. Torino: P. Caramello, G. Di Perri, M.L. Soranzo, G.C. Orofino, I. Arnaudo, M. Bonasso. Varese: P.A. Grossi, C. Basilico. Verbania: A. Poggio, G. Bottari. Venezia: E. Raise, F. Ebo. Vicenza: F. De Lalla, G. Tositti. Taranto: F. Resta, K. Loso. London, UK: A. Cozzi Lepri.
Swiss HIV Cohort Study (SHCS) (7 sites): M. Battegay, E. Bernasconi, J. Böni, H. Bucher, Ph. Bürgisser, S. Cattacin, M. Cavassini, R. Dubs, M. Egger, L. Elzi, P. Erb, K. Fantelli, M. Fischer, M. Flepp, A. Fontana, P. Francioli (President of the SHCS, Centre Hospitalier Universitaire Vaudois, CH-1011 Lausanne), H. Furrer (Chairman of the Clinical and Laboratory Committee), M. Gorgievski, H. Günthard, B. Hirschel, L. Kaiser, C. Kind, Th. Klimkait, U. Lauper, B. Ledergerber, M. Opravil, F. Paccaud, G. Pantaleo, L. Perrin, J.-C. Piffaretti, M. Rickenbach (Head of Data Centre), C. Rudin (Chairman of the Mother and Child Substudy), P. Schmid, J. Schüpbach, R. Speck, A. Telenti, A. Trkola, P. Vernazza (Chairman of the Scientific Board), R. Weber, S. Yerly.
AIDS Therapy Evaluation Project Netherlands (ATHENA) (25 sites): Treating physicians (*site coordinating physicians): Dr W. Bronsveld*, Drs M.E. Hillebrand-Haverkort, (Alkmaar); Dr J.M. Prins*, Drs J.C. Bos, Dr J.K.M. Eeftinck Schattenkerk, Dr S.E. Geerlings, Dr M.H. Godfried, Prof Dr J.M.A. Lange, Drs F.C. van Leth, Drs S.H. Lowe, Dr J.T.M. van der Meer, Drs F.J.B. Nellen, Drs K. Pogány, Dr T. van der Poll, Dr P. Reiss, Drs Th.A. Ruys, Drs S. Sankatsing, Dr R. Steingrover, Drs G. van Twillert, Drs M. van der Valk, Drs M.G.A. van Vonderen, Dr S.M.E Vrouenraets, Dr M. van Vugt, Dr F.W.M.N. Wit, (Amsterdam); Prof Dr T.W. Kuijpers, Drs D. Pajkrt, Drs H.J. Scherpbier, Emmakinderziekenhuis (Amsterdam); Drs A. van Eeden*, Onze Lieve Vrouwe Gasthuis, (Amsterdam); Dr J.H. ten Veen*, Dr P.S. van Dam, Dr J.C. Roos, Onze Lieve Vrouwe Gasthuis, (Amsterdam); Dr K. Brinkman*, Dr P.H.J. Frissen, Dr H.M. Weigel, Onze Lieve Vrouwe Gasthuis, (Amsterdam); Dr J.W. Mulder*, Dr E.C.M. van Gorp, Dr P.L. Meenhorst, Dr A.T.A. Mairuhu, Slotervaart Ziekenhuis (Amsterdam); Dr J. Veenstra* (Amsterdam); Prof Dr S.A. Danner*, Dr M.A. Van Agtmael, Drs F.A.P. Claessen, Dr R.M. Perenboom, Drs A. Rijkeboer, Dr M. van Vonderen, (Amsterdam); Dr C. Richter*, Dr J. van der Berg, Dr R. van Leusen, (Arnhem); Dr R. Vriesendorp*, Dr F.J.F. Jeurissen, (Westeinde-Den Haag); Dr R.H. Kauffmann*, Dr E.L.W. Koger, (Leyenburg-Den Haag); Dr B. Bravenboer*, Catharina Ziekenhuis, Eindhoven; Dr C.H.H. ten Napel*, Dr G.J. Kootstra, Medisch Spectrum Twente-Enschede; Dr H.G. Sprenger*, Dr W.M.A.J. Miesen, Dr R. Doedens, Dr E.H. Scholvinck, (Groningen); Dr R.W. ten Kate*, Kennemer Gasthuis-Haarlem; Dr D.P.F. van Houte*, Dr M. Polee, (Zuid); Dr F.P. Kroon*, Prof Dr van den Broek, Prof Dr J.T. van Dissel, Dr E.F. Schippers, (Leiden); Dr G. Schreij*, Drs S. van de Geest, Dr A. Verbon, (Maastricht); Dr P.P. Koopmans*, Dr M. Keuter, Dr F. Post, Dr A.J.A.M. van der Ven, (Nijmegen); Dr M.E. van der Ende*, Dr I.C. Gyssens, Drs M. van der Feltz, Dr J.G. den Hollander, Dr S. de Marie, Drs J.L Nouwen, Drs B.J.A. Rijnders, Dr T.E.M.S. de Vries, (Rotterdam); Dr G. Driessen, Prof Dr R. de Groot, Dr N. Hartwig (Rotterdam); Dr J.R. Juttmann*, Dr C. van de Heul, Dr M.E.E. van Kasteren, St Elisabeth (Tilburg); Dr M.M.E. Schneider* (until October 2004), Dr M.J.M. Bonten, Dr J.C.C. Borleffs, Dr P.M. Ellerbroek, Prof Dr I.M. Hoepelman*, Drs C.A.J.J. Jaspers, Drs I. Schouten, Drs C.A.M. Schurink, (Utrecht); Dr S.P.M. Geelen, Dr T.F.W. Wolfs, (Utrecht); Dr W.L. Blok*, Dr A.A. Tanis, (Vlissingen); Dr P.H.P. Groeneveld*, Isala Klinieken-Zwolle. Virologists: Dr N.K.T. Back, Dr M.E.G. Bakker, Prof Dr B. Berkhout, Dr S. Jurriaans, (Amsterdam); Dr Th. Cuijpers, (Amsterdam); Dr P.J.G.M. Rietra, Dr K.J. Roozendaal, (Amsterdam); Drs W. Pauw, Dr A.P. van Zanten, Dhr P.H.M. (Amsterdam); Dr B.M.E. von Blomberg, Dr P. Savelkoul, (Amsterdam); Dr C.M.A. Swanink, (Arnhem); Dr P.F.H. Franck, Dr A.S. Lampe, HAGA, (Leyenburg, Den Haag); Dhr C.L. (Westeinde-Den Haag); Dr R. Hendriks, Streeklaboratorium Twente-Enschede; Dr J. Schirm, Dhr Benne, (Groningen); Dr D. Veenendaal, (Haarlem); Dr H. Storm, Drs J. Weel, Drs J.H. van Zeijl, (Leeuwarden); Prof Dr A.C.M. Kroes, Dr H.C.J. Claas, (Leiden); Prof Dr C.A.M.V.A. Bruggeman, Drs V.J. Goossens, (Maastricht); Prof Dr J.M.D. Galama, Dr W.J.G. Melchers, Mevr Y.A.G. Poort, (Nijmegen); Dr G.J.J. Doornum, Dr M.G. Niesters, Prof Dr A.D.M.E. Osterhaus, Dr M. Schutten, (Rotterdam); Dr A.G.M. Buiting, Mevr C.A.M. Swaans, (Tilburg); Dr C.A.B. Boucher, Dr R. Schuurman, (Utrecht); Dr E. Boel, Dr A.F. Jansz, (Veldhoven).
The Multicenter Study Group on EuroSIDA (national coordinators in parenthesis, 73 sites): Argentina: (M. Losso), A. Duran, Buenos Aires. Austria: (N. Vetter), Vienna. Belarus: (I. Karpov), A. Vassilenko, Minsk. Belgium: (N. Clumeck) S. De Wit, B. Poll, Brussels; R. Colebunders, Antwerp. Czech Republic: (L. Machala) H. Rozsypal, Prague; D. Sedlacek, Plzen. Denmark: (J. Nielsen), J. Lundgren, T. Benfield, O. Kirk, Copenhagen; J. Gerstoft, T. Katzenstein, A.-B. E. Hansen, P. Skinhøj, Copenhagen; C. Pedersen, Odense. Estonia: (K. Zilmer), Tallinn. France: (C. Katlama), J.-P. Viard, P.-M. Girard, Paris; T. Saint-Marc, P. Vanhems, Lyon; C. Pradier, Nice; F. Dabis, Bordeaux. Germany: M. Dietrich, C. Manegold, Hamburg; J. van Lunzen, H.-J. Stellbrink, Hamburg; S. Staszewski, M. Bickel, Frankfurt; F.-D. Goebel, Munich; G. Fätkenheuer, Cologne; J. Rockstroh, Bonn; R. Schmidt, Hannover. Greece: (J. Kosmidis), P. Gargalianos, H. Sambatakou, J. Perdios, G. Panos, A. Filandras, E. Karabatsaki, Athens. Hungary: (D. Banhegyi), Budapest. Ireland: (F. Mulcahy), Dublin. Israel: (I. Yust) D. Turner, M. Burke, Tel Aviv; S. Pollack, G. Hassoun, Haifa: Z. Sthoeger, Rehovot; S. Maayan, Jerusalem. Italy: (A. Chiesi), Rome; R. Esposito, R. Borghi, Modena; C. Arici, Bergamo; R. Pristera, Bolzano; F. Mazzotta, A. Gabbuti, Firenze; Vullo, M. Lichtner, Rome; A. Chirianni, E. Montesarchio, Presidio Ospedaliero AD. Cotugno, Napoli; Antonucci, F. Iacomi, Narciso, Zaccarelli, Rome; A. Lazzarin, R. Finazzi, A. D'Arminio Monforte, Milan. Latvia: (L. Viksna), Riga. Lithuania: (S. Chaplinskas), Vilnius. Luxembourg: (R. Hemmer), T. Staub, Luxembourg. Netherlands: (P. Reiss), Amsterdam. Norway: (J. Bruun) A. Maeland, V. Ormaasen, Oslo. Poland: (B. Knysz) J. Gasiorowski, Medical University, Wroclaw; A. Horban, Warsaw; D. Prokopowicz, A. Wiercinska-Drapalo, Bialystok; A. Boron-Kaczmarska, M. Pynka, Szczecin; M. Beniowski, E. Mularska, Chorzow; H. Trocha, Gdansk. Portugal: (F. Antunes) E. Valadas, Lisbon; K. Mansinho, Lisbon; F. Matez, Lisbon. Romania: (D. Duiculescu), Dr Victor Babes, Bucarest; A. Streinu-Cercel, Bucarest. Russia: E. Vinogradova, A. Rakhmanova, St Petersburg. Serbia: and Montenegro (D. Jevtovic), Belgrade. Slovakia: (M. Mokráš) D. Staneková, Bratislava. Spain: (J. González-Lahoz) M. Sánchez-Conde, T. Garćıa-Benayas, L. Martin-Carbonero, V. Soriano, Madrid; B. Clotet, A. Jou, J. Conejero, C. Tural, Badalona; J.M. Gatell, J.M. Miró, Barcelona. Sweden: (A. Blaxhult), Solna; A. Karlsson, Stockholm; P. Pehrson, Huddinge. Switzerland: (B. Ledergerber) R. Weber, Zürich; P. Francioli, A. Telenti, Lausanne; B. Hirschel, V. Soravia-Dunand, Geneve; H. Furrer, Bern. Ukraine: (E. Kravchenko) N. Chentsova, Kyiv. United Kingdom: (S. Barton), London; A.M. Johnson, D. Mercey, London; A. Phillips, M.A. Johnson, A. Mocroft, London; M. Murphy, London; J. Weber, G. Scullard, London; M. Fisher, Brighton; R. Brettle, Edinburgh. Virology group: C. Loveday, B. Clotet (Central Coordinators) plus ad hoc virologists from participating sites in the EuroSIDA Study. Steering Committee: Francisco Antunes; Anders Blaxhult; Nathan Clumeck; Jose Gatell; Andrzej Horban; Anne Johnson; Christine Katlama; Bruno Ledergerber (chair); Clive Loveday; Andrew Phillips; Peter Reiss; Stefano Vella. Coordinating centre staff: J. Lundgren (project leader), I. Gjørup, O. Kirk, N. Friis-Moeller, A. Mocroft, A. Cozzi-Lepri, W. Bannister, D. Mollerup, D. Podlevkareva, C. Holkmann Olsen, J. Kjær.
Collaborations in HIV Outcomes Research US (CHORUS) (4 sites): Stephen Raffanti, Douglas Dieterich, Amy Justice, Stephen Becker, Anthony Scarsella, Gregory Fusco, Bernard Most, Rukmini Balu, Rashida Rana, Robin Beckerman, Theodore Ising, Jennifer Fusco, Renae Irek, Bernadette Johnson, Ashwin Hirani, Edwin DeJesus, Gerald Pierone, Philip Lackey, Chip Irek, Alison Johnson, John Burdick, Saul Leon, Joseph Arch.
Frankfurt HIV Cohort (1 site): Schlomo Staszewski, Eilke B. Helm, Amina Carlebach, Axel Müller, Annette Haberl, Gabi Nisius, Tessa Lennemann, Carsten Rottmann, Timo Wolf, Christoph Stephan, Markus Bickel, Manfred Mösch, Peter Gute, Leo Locher, Thomas Lutz, Stephan Klauke, Gabi Knecht (Clinical Group); Hans W. Doerr, Martin Stürmer (Virology Group); Brenda Dauer (Scientific Advisor); Nils von Hentig (Pharmacology Group); Beverly Jennings (Data Management).
Aquitaine Cohort ANRS CO3 (6 sites): Scientific committee: J. Beylot, G. Chêne, F. Dabis, M. Dupon, M. Longy-Boursier, J.L. Pellegrin, J.M. Ragnaud, R. Salamon. Methodological coordination: F. Dabis, G. Chêne, R. Thiébaut, C. Lewden, S. Lawson-Ayayi. Medical coordination: M. Dupon, P. Mercié, J.F. Moreau, P. Morlat, J.L. Pellegrin, J.M. Ragnaud, N. Bernard, D. Lacoste, D. Malvy, D. Neau. Data Management and Analysis: M.J. Blaizeau, M. Decoin, S. Delveaux, C. Hannapier, S. Labarrère, V. Lavignolle-Aurillac, B. Uwamaliya-Nziyumvira, G. Palmer, D. Touchard, E. Balestre, A. Alioum, H. Jacqmin-Gadda, R. Thiébaut. Participating physicians: Bordeaux University Hospital: J. Beylot, P. Morlat, N. Bernard, M. Bonarek, F. Bonnet, B. Coadou, P. Gellie, D. Lacoste, C. Nouts; M. Dupon, F. Bocquentin, H. Dutronc, S. Lafarie; M. Longy-Boursier, P. Mercié, A. Aslan, D. Malvy, T. Pistonne, P. Thibaut, R. Vatan; J.M. Ragnaud, D. Chambon, C. De La Taille, C. Cazorla, D. Neau, A. Ocho; J.L. Pellegrin, J.F. Viallard, O. Caubet, C. Cipriano, E. Lazaro; P. Couzigou, L. Castera; H. Fleury, M.E. Lafon, B. Masquelier, I. Pellegrin; D. Breilh; J.F. Moreau, P. Blanco. Dax Hospital: P. Loste, L. Caunègre. Bayonne Hospital: F. Bonnal, S. Farbos, M. Ferrand. Libourne Hospital: J.Ceccaldi, S. Tchamgoué. Mont de Marsan Hospital: S. De Witte Villeneuve sur Lot Hospital: E. Buy.
HAART Observational Medical Evaluation and Research (HOMER), British Columbia Centre for Excellence in HIV/AIDS (96 sites): Chris Alexander, Rolando Barrios, Paula Braitstein, Zabrina Brumme, Keith Chan, Helen Cote, Nada Gataric, Josie Geller, Silvia Guillemi, P. Richard Harrigan, Marianne Harris, Robert Hogg, Ruth Joy, Adrian Levy, Julio Montaner, Val Montessori, Anita Palepu, Elizabeth Phillips, Peter Phillips, Natasha Press, Mark Tyndall, Evan Wood, Benita Yip.
Royal Free Hospital Cohort (1 site): Jayne Ballinger, Sanjay Bhagani, Ronan Breen, Pat Byrne, Anne Carroll, Ian Cropley, Zoë Cuthbertson, Tony Drinkwater, Tom Fernandez, Anna Maria Geretti, Gabrielle Murphy, Dan Ivens, Margaret Johnson, Sabine Kinloch-de Loes, Marc Lipman, Sara Madge, Beth Prinz, Diane Robertson Bell, Sapna Shah, Leonie Swaden, Mervyn Tyrer, Mike Youle (Clinical); Clinton Chaloner, Helen Gumley, Jackie Holloway, Dewi Puradiredja, Joyce Sweeney, Robert Tsintas (Data Management); Wendy Bannister, Loveleen Bansi, Alessandro Cozzi-Lepri, Zoë Fox, Fiona Lampe, Amanda Mocroft, Andrew Phillips, Caroline Sabin, Colette Smith (Epidemiology/Biostatistics); Eric Amoah, Gillian Clewley, Louise Dann, Brendon Gregory, Ilesh Jani, George Janossy, Mel Kahan, Clive Loveday, Mike Thomas (Laboratory).
South Alberta Clinic (1 site): John Gill, Ron Read.
Köln/Bonn Cohort (2 sites): G. Fatkenheuer, J. Rockstroh, N. Schmeisser, K. Voigt, J.C. Wasmuth, A. Wohrmann.
References
- 1.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 2.Chene G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 3.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4R lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 4.May M, Royston P, Egger M, Justice AC, Sterne JA, ART Cohort Collaboration Development and validation of a prognostic model for survival time data: application to prognosis of HIV positive patients treated with antiretroviral therapy. Stat Med. 2003;23:2375–2398. doi: 10.1002/sim.1825. [DOI] [PubMed] [Google Scholar]
- 5.May M, Porter K, Sterne J, Royston P, Egger M. Prognostic model for HIV-1 disease progression in patients starting antiretroviral therapy was validated using independent data. J Clin Epidemiol. 2005;58:1033–1041. doi: 10.1016/j.jclinepi.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Grabar S, Le MV, Goujard C, Leport C, Kazatchkine MD, Costagliola D, et al. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 7.Binquet C, Chene G, Jacqmin-Gadda H, Journot VV, Saves M, Lacoste D, et al. Modeling changes in CD4-positive T-lymphocyte counts after the start of highly active antiretroviral therapy and the relation with risk of opportunistic infections the: Aquitaine Cohort, 1996–1997. Am J Epidemiol. 2001;153:386–393. doi: 10.1093/aje/153.4.386. [DOI] [PubMed] [Google Scholar]
- 8.Wit FW, van Leeuwen R, Weverling GJ, Jurriaans S, Nauta K, Steingrover R, et al. Outcome and predictors of failure of highly active antiretroviral therapy: one-year follow-up of a cohort of human immunodeficiency virus type 1-infected persons. J Infect Dis. 1999;179:790–798. doi: 10.1086/314675. [DOI] [PubMed] [Google Scholar]
- 9.D'Arminio MA, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 10.Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodt HR, Kamps BS, Gute P, Knupp B, Staszewski S, Helm EB. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS. 1997;11:1731–1738. doi: 10.1097/00002030-199714000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Fatkenheuer G, Theisen A, Rockstroh J, Grabow T, Wicke C, Becker K, et al. Virological treatment failure of protease inhibitor therapy in an unselected cohort of HIV-infected patients. AIDS. 1997;11:F113–F116. doi: 10.1097/00002030-199714000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Lundgren JD, Phillips AN, Vella S, Katlama C, Ledergerber B, Johnson AM, et al. Regional differences in use of antiretroviral agents and primary prophylaxis in 3122 European HIV-infected patients. EuroSIDA Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:153–160. doi: 10.1097/00042560-199711010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Becker SL, Raffanti SR, Hansen NI, Fusco JS, Fusco GP, Slatko GH, et al. Zidovudine and stavudine sequencing in HIV treatment planning: findings from the CHORUS HIV cohort. J Acquir Immune Defic Syndr. 2001;26:72–81. doi: 10.1097/00126334-200101010-00011. [DOI] [PubMed] [Google Scholar]
- 15.Mocroft A, Barry S, Sabin CA, Lepri AC, Kinloch S, Drinkwater A, et al. The changing pattern of admissions to a London hospital of patients with HIV: 1988–1997. Royal Free Centre for HIV Medicine. AIDS. 1999;13:1255–1261. doi: 10.1097/00002030-199907090-00016. [DOI] [PubMed] [Google Scholar]
- 16.Rhone SA, Hogg RS, Yip B, Sherlock C, Conway B, Schechter MT, et al. The antiviral effect of ritonavir and saquinavir in combination amongst HIV-infected adults: results from a community-based study. AIDS. 1998;12:619–624. doi: 10.1097/00002030-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Mocroft A, Gill MJ, Davidson W, Phillips AN. Predictors of a viral response and subsequent virological treatment failure in patients with HIV starting a protease inhibitor. AIDS. 1998;12:2161–2167. doi: 10.1097/00002030-199816000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Wolf K, Young J, Rickenbach M, Vernazza P, Flepp M, Furrer H, et al. Prevalence of Unsafe Sexual Behavior Among HIV-Infected Individuals: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2003;33:494–499. doi: 10.1097/00126334-200308010-00010. [DOI] [PubMed] [Google Scholar]
- 19.Lewden C, Salmon D, Morlat P, Bevilacqua S, Jougla E, Bonnet F, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–130. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet F, Lewden C, May T, Heripret L, Jougla E, Bevilacqua S, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101:317–324. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- 21.Salmon-Ceron D, Lewden C, Morlat P, Bevilacqua S, Jougla E, Bonnet F, et al. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 23.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 24.May MT, Sterne JA, Costagliola D, Sabin CA, Phillips AN, Justice AC, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–458. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 25.Frieden TR, Das-Douglas M, Kellerman SE, Henning KJ. Applying public health principles to the HIV epidemic. N Engl J Med. 2005;353:2397–2402. doi: 10.1056/NEJMsb053133. [DOI] [PubMed] [Google Scholar]
- 26.Advancing HIV prevention: new strategies for a changing epidemic – United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:329–332. [PubMed] [Google Scholar]
- 27.Sullivan AK, Curtis H, Sabin CA, Johnson MA. Newly diagnosed HIV infections: review in UK and Ireland. BMJ. 2005;330:1301–1302. doi: 10.1136/bmj.38398.590602.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paltiel AD, Weinstein MC, Kimmel AD, Seage GR, III, Losina E, Zhang H, et al. Expanded screening for HIV in the United States – an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]